Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone
Population-Level Incidence and Risk Factors for Pulmonary
Toxicity Associated With Amiodarone
Cynthia Anne Jackevicius, BScPhm, PharmD, MSca,b,c,d, Albert Tom, PharmDa,
Vidal Essebag, MD, PhDe, Mark J. Eisenberg, MD, MPHe, Elham Rahme, PhDe,
Jack Ven Tu, MD, PhDb,c, Karin Humphries, MBA, DScf, Hassan Behlouli, PhDe, and
Louise Pilote, MD, MPH, PhDe,*
Estimates from clinical trials and small observational studies of the incidence of pulmonary
toxicity (PT) associated with amiodarone range from 1% to 10%. We report a unique study
of the population-based incidence and potential predictors of PT in a real-world atrial
fibrillation (AF) population. We conducted a retrospective cohort study of patients >
65
years old discharged with AF using linked administrative databases from Quebec, Canada
from 1999 to 2007. "Users" and "nonusers" of amiodarone were identified by prescriptions
dispensed within 7 days after hospital discharge. PT was defined through International
Classification of Diseases, Ninth Revision and Tenth Revision codes for pulmonary fibrosis,
alveolar/interstitial lung disease, and adult respiratory distress syndrome. Potential risk
factors for PT were identified using multivariable Cox regression. PT occurred in 250 of 6,460
amiodarone users (3.87%) and 676 of 50,993 nonusers (1.33%). Age-standardized PT incidences
were 28.30 and 16.02 per 1,000 person-years in men and women users, respectively, and 14.05
and 8.82 per 1,000 person-years in nonusers, respectively. It was associated with amiodarone
exposure at all doses (<
200 mg/day, hazard ratio 1.62, 1.35 to 1.96; >200 mg/day, 1.46, 1.22 to
1.75). Other predictors of PT included increasing age (1.01 per year, 1.00 to 1.02), male gender
(1.37, 1.19 to 1.57), chronic obstructive pulmonary disease (2.53, 2.21 to 2.89), and renal disease
(1.26, 1.06 to 1.50). In conclusion, the population-based incidence of amiodarone PT is in the
lower range of what has been previously reported. However, patients with AF who use
amiodarone have an approximately 50% higher risk of PT than nonusers. Clinicians may be
able to use the present results to identify patients at higher risk for PT and implement strategies
to increase monitoring or select alternative therapy.
2011 Elsevier Inc. All rights reserved.
(Am J Cardiol 2011;108:705–710)
Amiodarone is a highly effective Its
pulmonary PT secondary to amiodarone has
long 1/2 life causes long-lasting major adverse effects (pul-
nonspecific diagnostics; however, impairment of diffusion
monary toxicity [PT], thyroid dysfunction, hepatotoxicity,
capacity for carbon monoxide, total lung capacity, and
and skin Amiodarone PT, first reported in
forced vital capacity are Increased age, pre-
1980, is 1 of the most serious adverse effects, thus limiting
existing lung disease, dose, and duration of therapy are
its The incidence of amiodarone PT is reported to be
potential risk Although higher doses tend to
0% to 10% (0% to 8% in randomized controlled trials, 2%
be more toxic, low doses of amiodarone can cause serious
to 8% in prospective cohorts and case series, and ⬍2% in
Meta-analyses have provided estimates of
Mortality is estimated at 1% to
amiodarone However, participants in clinical trials
Onset of amiodarone PT is unpredictable and in-
are usually healthier than the general population. Although
sidious, often remaining a diagnosis of exclusion, after
many case series have described the nature of the presen-
consideration of heart failure, pulmonary embolism, and
tation of amiodarone PT, the samples were small or thestudies were limited to a single center. Given these limita-tions, there is a need to estimate the population-level inci-
dence of and risk factors for amiodarone PT. Furthermore,
Western University of Health Sciences, Pomona, California; bInstitute
as new drug and nondrug therapies for rhythm control in
for Clinical Evaluative Sciences, Toronto, Ontario, Canada; cUniversity of
atrial fibrillation (AF) become available, there is a need to
Toronto, Toronto, Ontario, Canada; dUniversity Health Network, Toronto,Ontario, Canada; eMcGill University, Montreal, Quebec, Canada; fProvin-
revisit the risks of existing therapies in a contemporary
cial Health Services Authority, Vancouver, British Columbia, Canada.
patient cohort.
Manuscript received January 12, 2011; revised manuscript received andaccepted April 21, 2011.
This work was funded by an operating grant from the Canadian Insti-
We conducted a retrospective observational cohort study
tutes for Health Research, Ottawa, Ontario, Canada.
of patients with AF using linked administrative data. For
*Corresponding author: Tel: 514-934-1934, ext 34667; fax: 514-843-
patient identification, we used the hospital discharge ab-
E-mail address: (L. Pilote).
stract database Maintenance et Exploitation des Données
0002-9149/11/$ – see front matter 2011 Elsevier Inc. All rights reserved.
The American Journal of Cardiology (www.ajconline.org)
pour l'Étude de la Clientèle Hospitalière, which contains
inpatient diagnostic and therapeutic procedures codes. Med-
Baseline characteristics of patients with atrial fibrillation with and
ications were identified by the drug identification number
without amiodarone exposure
in the provincial prescription claims database Régie de
l'Assurance Maladie du Québec for information on medi-
cation use and duration of therapy, which is available only
(n ⫽ 6,460) (n ⫽ 50,933)
for those ⱖ65 years old. Survival data were obtained fromthe Maintenance et Exploitation des Données pour l'Étude
de la Clientèle Hospitalière and Régie de l'Assurance Mala-
die du Québec databases.
Chronic obstructive pulmonary
Subjects were included if they were ⱖ65 years old and
discharged with a primary or secondary diagnosis of AF
from January 1, 1999 through March 31, 2007 identified
Diabetes mellitus
according to
International Classification of Diseases, Ninth
Coronary artery disease
Revision and
Tenth Revision (ICD-9/10) codes 427.3,
Stroke (including transient
427.31, and 427.32/I48. We conducted an internal valida-
tion using AF diagnoses in the hospital discharge abstract
database and the physician billing database. To ensure pa-
Chronic kidney disease
tients had nontransient AF and for patients without a pri-
mary diagnosis of AF, 2 secondary diagnoses of AF were
Median CHADS2 score
required. For patients with ⬎1 AF diagnosis code, the first
Concurrent use of nonamiodarone
hospitalization discharge date was the cohort entry date.
Concurrent use of rate-control
Patients were excluded if they had AF coded as a compli-
cation; a history of valvular disease/surgery; AF within 30
Concurrent use of strong
days of coronary artery bypass surgery, pericardial surgery,
cytochrome P450 3A4
or structural cardiac repair; history of pulmonary fibrosis
within 12 months; or were residents of long-term care fa-
Concurrent use of corticosteroids
Concurrent use of medications
Exposure was determined based on amiodarone prescrip-
associated with pulmonary
tions filled within 7 days of index hospital discharge. A
sensitivity analysis was conducted by extending this period
CHADS2 ⫽ congestive heart failure, hypertension, age ⱖ75 years,
to 30 days. Those without an amiodarone prescription were
diabetes mellitus, stroke.
considered nonusers. Discontinuation of an amiodarone pre-scription was defined as the end date of the last filledprescription plus 60 days or end of follow-up, whichever
came first. The 60-day period was used because of the long
Pulmonary toxicity events by diagnosis category*
1/2 life of amiodarone and to avoid miscoding patients who
Postinflammatory pulmonary fibrosis
were late in refilling their prescription as unexposed. To
Other pulmonary insufficiency
calculate incidence, quantity, tablet strength, and days of
Pulmonary insufficiency after trauma and surgery
supply of each amiodarone prescription were used to create
Other interstitial pulmonary disorders
an average daily dose (milligrams per day). Patients were
Other specified alveolar and parietoalveolar
categorized into low-dose (ⱕ200 mg/day) or high-dose
Idiopathic fibrosing alveolitis
(⬎200 mg/day) groups.
Acute respiratory distress syndrome
Patients were followed from AF diagnosis until occur-
Other (each ⬍1% in frequency)
rence of the study outcome of first principal or secondary
PT diagnosis (pulmonary fibrosis ICD-9/10 515/J84, J84.1,J84.8, J84.9, J70.2, J70.3, J70.4, J70.8, J70.9; idiopathic
*Some patients had ⬎1 diagnosis type coded; therefore, coding diagno-
pulmonary fibrosis ICD-9 516.3; or other alveolar/intersti-
sis events ⫽ 948, whereas actual patient events ⫽ 926.
tial disease ICD-9 516; adult respiratory distress syndromeICD-9/10 518.5, 518.82/J80) or March 31, 2007, whichever
Potential risk factors for the development of PT that we
came first. Idiopathic pulmonary fibrosis was included be-
evaluated included patient demographics (age, gender), co-
cause PT is more likely a result of amiodarone-associated
morbidities within previous 12 months (chronic obstructive
pulmonary fibrosis rather than idiopathic pulmonary fibro-
pulmonary disease [COPD], heart failure, diabetes mellitus,
sis. We considered a broad definition of PT because it may
hypertension, coronary artery disease, stroke, chronic kid-
be diagnosed as pulmonary fibrosis, interstitial pneumonitis,
ney disease [CKD], hypothyroidism, liver disease), AF
pulmonary alveolitis, or acute respiratory distress syn-
medications (nonamiodarone antiarrhythmics, rate-con-
Death was documented if it occurred before
trol medications [ blockers, verapamil, diltiazem,
the end of the follow-up period (in the absence of the PT
digoxin]), amiodarone dose (ⱕ200 or ⬎200 mg/day, non-
outcome). Follow-up for each subject was censored after the
users 0 mg), and strong cytochrome 3A4 inhibitors
first PT diagnosis, drug discontinuation, end of follow-up,
(erythromycin, clarithromycin, ketoconazole, itracona-
or death, whichever came first.
zole, nelfinavir, or ritonavir).
Arrhythmias and Conduction Disturbances/Pulmonary Toxicity Associated With Amiodarone
rson-y
pe 20
Amiodarone ADD ≤ 200 mg
Amiodarone ADD > 200 mg
Figure 1. Age-adjusted incidence rate (per 1,000 person-years) of pulmonary toxicity by gender and dose. ADD ⫽ average daily dose.
Time to Development of Pulmonary Toxicity
tion Fr 0.4
ropor 0.3
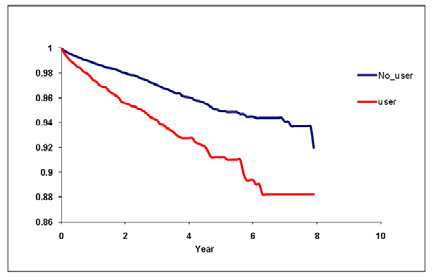
Figure 2. Time to development of pulmonary toxicity.
Descriptive statistics were used to compare baseline char-
any amiodarone. The crude mortality rate for those who de-
acteristics between groups. Crude and age-standardized inci-
veloped PT was estimated. Multivariable Cox proportional
dence rates of PT overall and stratified by gender, amiodarone
hazards models were used to estimate hazard ratios with 95%
dose, and significant risk factors for PT (number per 1,000
confidence intervals for outcome of PT between exposed and
person-years) were estimated for users and nonusers. The ref-
unexposed subjects and to identify potential predictors of PT.
erence group for age standardization was all patients in the AF
Sensitivity analyses included dose– gender and dose–age inter-
cohort for fiscal year 2002. Crude incidence of PT in users was
action terms and exposure based on prescriptions filled within
calculated by dividing the number of subjects with PT by the
30 days (instead of 7 days) of index hospital discharge. All p
sum of observation times of all subjects who were exposed to
values were 2-sided. Data were analyzed using SAS 9.1 (SAS
The American Journal of Cardiology (www.ajconline.org)
Amiodarone was associated with a significantly in-
Adjusted estimate of factors associated with pulmonary toxicity
creased risk of PT when adjusting for potential confounders
at doses ⱕ200 mg/day (hazard ratio 1.62, 95% confidenceinterval 1.35 to 1.96) and ⬎200 mg/day (hazard ratio 1.46,
Amiodarone ⱕ200 vs 0 mg/day
1.22 to 1.75). A significantly increased risk of PT was also
Amiodarone ⬎200 vs 0 mg/day
associated with male gender, increased age, COPD, and
Gender (male gender is reference)
CKD. Patients with COPD had the highest risk of develop-
ing PT (2.53, 2.21 to 2.89). Although patients who were
Chronic obstructive pulmonary
taking strong cytochrome P450 3A4 inhibitors had a trend
toward increased risk of PT, it was not significant (hazard
ratio 1.53, 0.96 to 2.44), whereas subjects who were taking
concurrent rate-control medications had a significantly
Diabetes mellitus
lower risk of PT (hazard ratio 0.85, 0.75 to 0.97;
Coronary artery disease
Sensitivity analyses including dose– gender and dose–age
Stroke (including transient
interaction terms found no evidence of interaction. Sensi-
tivity analyses that defined use of amiodarone within 30
Chronic kidney disease
days instead of 7 days of index AF diagnosis revealed
consistent estimates for risk of amiodarone PT (hazard ratio
Concurrent use of nonamiodarone
1.84, 1.58 to 2.14; 1.79, 1.56 to 2.07, respectively).
Concurrent use of rate-control
Concurrent use of strong cytochrome
We found a nearly threefold higher crude incidence of
P450 3A4 inhibitors
PT in amiodarone users compared to nonusers. Our inci-
CI ⫽ confidence interval; HR ⫽ hazard ratio.
dence of almost 4% in users confirms results from previousstudies reporting an incidence from 0% to Afteradjustment for potential confounders, risk of PT remained
Institute, Cary, North Carolina). This study was approved by
⬎50% higher in amiodarone users than nonusers. However,
the ethics review board of McGill University, Montreal, Que-
our crude incidence rates were approximately 2 times those
bec, Canada and Western University of Health Sciences,
found in previous meta-analyses and possi-
Pomona, California.
bly because our cohort is population based, whereas patientsenrolled in randomized controlled trials were younger with
fewer co-morbidities, and we defined PT in a broader sense
In total 57,393 patients were included in the cohort:
by ICD-9/10 codes to ensure we captured the various diag-
6,460 users and 50,933 nonusers. Significantly more men
noses under which PT might be coded. Definitions of PT in
were in the user than in the nonuser group. COPD, heart
randomized controlled trials would likely be more stringent,
failure, diabetes, coronary artery disease, hypothyroidism,
which could account for the lower incidence of PT.
and CKD were significantly higher in the user group. Con-
We did not find a dose–response relation with amioda-
versely, age, stroke/transient ischemic attack, and liver dis-
rone use and development of PT using adjusted analysis in
ease were significantly higher in nonusers. As expected, users
contrast to previous studies reporting increased risk at
were less likely to be receiving rate-control and nonamioda-
higher amiodarone Although previous studies
rone antiarrhythmic medications than nonusers
have reported that PT is related to amiodarone dose, it is
Crude incidences of PT were 3.87% (250 of 6,460) for
important to note that lower amiodarone doses have also
users and 1.33% (676 of 50,993) for nonusers. The most
been associated with serious Our findings con-
common PT diagnosis was ICD-9 code 515.9 (postinflam-
firm this by showing a crude incidence rate of PT with
matory pulmonary fibrosis; A higher age-stan-
ⱕ200 mg/day nearly 2 times that of nonusers. We would
dardized incidence of PT was found in users than nonusers
expect 11 more men users and 7 more women users to have
and in men than women Men and women users
PT for every 1,000 person-years treated with the lower-
had incidences of 28.30 and 16.02 cases per 1,000 person-
range dose of ⱕ200 mg/day compared to nonusers using our
years, respectively, whereas men and women nonusers had
age-standardized incidence rates.
estimates of 14.05 and 8.82 cases per 1,000 person-years,
Risk of PT was greater in men, older patients, and those
respectively. Age-standardized incidence of PT had a slight
with COPD and CKD. We found a nearly 40% increased
dose response, with an increase in incidence with doses
risk of PT in men versus women. Although previous studies
ⱖ200 mg/day Incidences in users with and
did not show male gender to be a risk factor for amiodarone
without COPD were 35.63 and 15.36 cases per 1,000 per-
PT, men have rates of idiopathic pulmonary fibrosis 50% to
son-years, respectively, whereas in nonusers with and with-
80% higher than Certain PT risk factors that
out COPD incidences were 21.80 and 7.59 cases per 1,000
we cannot examine with administrative data such as smok-
person-years, respectively. PT events continued to cumulate
ing may be higher in men than in women, increasing their
over the follow-up period The crude mortality
risk. Patients were 1% more likely to have PT for each
rate for those who developed PT was 75.7%.
incremental year of age. Results of previous studies have
Arrhythmias and Conduction Disturbances/Pulmonary Toxicity Associated With Amiodarone
not been entirely consistent with age as a risk factor for
in a younger population. However, because AF mainly
affects elderly patients, the age restriction of our data may
Pre-existing COPD more than doubled the risk of PT, the
be less concerning. Many baseline characteristics differed
highest risk of all characteristics examined, which warrants
between amiodarone users and nonusers. Although we ad-
caution with amiodarone use in patients with COPD. Risk of
justed for these differences and all known confounders in
PT in amiodarone users with previous lung disease has been
the Cox model, unknown confounders may be associated
inconsistent in previous Several studies
with PT. We defined PT by ICD coding that was more rather
have found an increased risk of acute respiratory distress
than less inclusive. Although this may have increased the
syndrome and acute respiratory failure postoperatively in
overall incidence of PT, differential misclassification bias of
patients after cardiac and pulmonary surgery who were
PT would not be expected. The nonusers' PT incidence rate
amiodarone Patients with a history of COPD
of 1.33% may partly represent the underlying rate of idio-
who take amiodarone and present with nonspecific symptoms
pathic pulmonary fibrosis in the general population and
of respiratory distress may be assumed to have COPD exac-
misclassification of nonusers who during follow-up subse-
erbation but in fact may have undiagnosed amiodarone PT.
quently became amiodarone users.
Although CKD was associated with a moderately higher
risk of PT in our study, no previous studies have found this
1. Amiodarone Trialists' Meta-Analysis Investigators. Effect of prophy-
association. Amiodarone is eliminated primarily by hepatic
lactic amiodarone on mortality after acute myocardial infarction and in
metabolism with negligible urinary excretion of amiodarone
congestive heart failure: meta-analysis of individual data from 6500patients in randomized trials. Lancet 2007;350:1417–1424.
or its main active metabolite, Renal
2. Mason JW. Amiodarone. N Engl J Med 1987;316:455– 466.
impairment has been associated with a potential decrease in
3. Martin WJ, Rosenow EC. Amiodarone pulmonary toxicity. Recogni-
hepatic drug metabolic activity, including the cytochrome
tion and pathogenesis (part I). Chest 1988;93:1067–1075.
P450 3A4 pathway, which is the main metabolic pathway of
4. Rotmensch HH, Liron M, Tupilski M, Laniado S. Possible association
of pneumonitis with amiodarone therapy. Am Heart J 1980;100:412–
Therefore, a plausible mechanism for de-
creased metabolism of amiodarone and the potential for
5. Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects
increased toxicity exists in patients with CKD.
of low dose amiodarone: a meta-analysis. J Am Coll Cardiol 1997;30:
Amiodarone is a known cytochrome P450 3A4 substrate
and inhibitor, and concurrent use of other cytochrome P450
6. Tisdale JE, Follin SL, Ordelova A, Webb CR. Risk factors for the
development of specific noncardiovascular adverse effects associated
3A4 inhibitors may potentially increase amiodarone and
with amiodarone. J Clin Pharmacol 1995;35:351–356.
desethylamiodarone levels, potentially increasing the risk of
7. Olshansky B, Sami M, Rubin A, Kostis J, Shorofsky S, Slee A, Greene
PT with amiodarone We saw this in our results, with
HL; NHLBI AFFIRM Investigators. Use of amiodarone for atrial
a trend toward an increased risk of PT of ⬎50%. Previous
fibrillation in patients with preexisting pulmonary disease in the AF-FIRM study. Am J Cardiol 2005;95:404 – 405.
studies have not addressed this drug interaction potential;
8. Dusman RE, Stanton MS, Miles WM, Klein LS, Zipes DP, Fineberg
further research is needed to verify and assess the extent of
NS, Heger JJ. Clinical features of amiodarone-induced pulmonary
this association. We found a modest decrease in risk of PT
with those concurrently using rate-control medications at
9. Kerin NZ, Aragon E, Faitel K, Frumin H, Rubenfire M. Long-term
baseline. Patients using rate-control medications may re-
efficacy and toxicity of high- and low-dose amiodarone regimens.
J Clin Pharmacol 1989;29:418 – 423.
quire lower doses or shorter duration of amiodarone, poten-
10. Magro SA, Lawrence EC, Wheeler SH, Krafchek J, Lin HT,
tially lessening the risk for PT. This novel theoretical ex-
Wyndham CR. Amiodarone pulmonary toxicity: prospective evalua-
planation requires further investigation.
tion of serial pulmonary function tests. J Am Coll Cardiol 1988;12:
Guidelines recommend that patients taking amiodarone
11. Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of
receive a chest x-ray at baseline and then yearly and pul-
outcome after myocardial infarction in patients with frequent or repet-
monary function tests at baseline and then for unexplained
itive ventricular premature depolarisations: CAMIAT. Canadian Ami-
Because we found that events continued to occur
odarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet
over time, clinicians must continue to be vigilant long term
in monitoring for PT. Identification of potential risk factors
12. Greene HL for the CASCADE Investigators. The CASCADE study:
randomized antiarrhythmic drug therapy in survivors of cardiac arrest
for PT may assist clinicians in targeting surveillance to
in Seattle. CASCADE Investigators. Am J Cardiol 1993;72(suppl):
those at higher risk. In patients considered at very high risk
of PT, some clinicians may consider alternative long-term
13. Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R.
antiarrhythmics, (sotalol, dronedarone), although these
Randomised trial of low-dose amiodarone in severe congestive heartfailure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca
agents are less effective than Dronedarone
en Argentina (GESICA). Lancet 1994;344:493– 498.
is a noniodinated benzofuran derivative of amiodarone that
14. Yamada Y, Shiga T, Matsuda N, Hagiwara N, Kasanuki H. Incidence
appears to have fewer adverse effects than amiodarone
and predictors of pulmonary toxicity in Japanese patients receiving
including less However, it is a less effective antiar-
low-dose amiodarone. Circ J 2007;71:1610 –1616.
rhythmic than amiodarone for AF and is contraindicated in
15. Rakita L, Sobol SM, Mostow N, Vrobel T. Amiodarone pulmonary
toxicity. Am Heart J 1983;106:906 –916.
patients with severe or recently decompensated heart fail-
16. Kennedy JI, Myers JL, Plumb VJ, Fulmer JD. Amiodarone pulmonary
ure, unlike amiodarone, which can be safely used in patients
toxicity. Clinical, radiologic, and pathologic correlations. Arch Intern
Med 1987;147:50 –55.
Although we believe our study provides new insight into
17. Ashrafian H, Davey P. Is amiodarone an underrecognized cause of
acute respiratory failure in the ICU? Chest 2001;120:275–282.
the incidence and associated risk factors for amiodarone PT,
18. Sobol SM, Rakita L. Pneumonitis and pulmonary fibrosis associated
it has inherent limitations. Our study was restricted to el-
with amiodarone treatment: a possible complication of a new antiar-
derly patients; incidence and risk factors for PT may differ
rhythmic drug. Circulation 1982;65:819 – 824.
The American Journal of Cardiology (www.ajconline.org)
19. Myers JL, Kennedy JI, Plumb VJ. Amiodarone lung: pathologic find-
26. Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B.
ings in clinically toxic patients. Hum Pathol 1987;18:349 –354.
Practical guidelines for clinicians who treat patients with amioda-
20. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence
rone. Practice Guidelines Subcommittee, North American Society
and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit
of Pacing and Electrophysiology. Arch Intern Med 2000;160:
Care Med 2006;174:810 – 816.
21. Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, Fletcher
27. Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL,
RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD Jr, Raisch DW,
Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD Jr,
Ezekowitz MD. Sotalol Amiodarone Atrial Fibrillation Efficacy Trial
Raisch DW, Ezekowitz MD. Amiodarone versus sotalol for atrial
(SAFE-T) Investigators. Pulmonary effect of amiodarone in patients with
fibrillation. N Engl J Med 2005;352:1861–1872.
heart failure. J Am Coll Cardiol 1997;30:514 –517.
28. Sanofi-Aventis. DIONYSOS study results showed the respective pro-
22. Greenspon AJ, Kidwell GA, Hurley W, Mannion J. Amiodarone-
files of dronedarone and amiodarone, December 23 2008. Available at:
related postoperative adult respiratory distress syndrome. Circulation
23. Van Mieghem W, Coolen L, Malysse I, Lacquet LM, Deneffe GJ,
Accessed on December 28, 2009.
Demedts MG. Amiodarone and the development of ARDS after lung
29. Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM, Kong
surgery. Chest 1994;105:1642–1645.
DF. Comparative efficacy of dronedarone and amiodarone for the
24. Cordarone (Amiodarone) Prescribing Information. Ambares, France:
maintenance of sinus rhythm in patients with atrial fibrillation. J Am
Sanofi Winthrop Industrie, 2008.
Coll Cardiol 2009;54:1089 –1095.
25. Dreisbach AW, Lertora JJ. The effect of chronic renal failure on
hepatic drug metabolism and drug disposition. Semin Dial 2003;16:
France: Sanofi Winthrop Industrie, 2009. Available at:
Accessed on December 28, 2009.
Source: http://02b6616.netsolhost.com/journalclub/Sept2011/Amiodarone%20toxicity_HS.pdf
Isabell Hensel, Gunther Teubner Matrix Reloaded. Critica dell'effetto orizzontale dei diritti fondamentali centrato sullo Stato sull'esempio del publication bias (errore sistematico di pubblicazione) Versione in tedesco: http://www.jura.uni-frankfurt.de/49069887/KJ_Teubner_Hensel.pdf Matrix Reloaded Critica dell'effetto orizzontale dei diritti fondamentali centrato sullo Stato sull'esempio del
Trichuris suis therapy in Crohn's disease R W Summers, D E Elliott, J F Urban, Jr, R Thompson and J V Weinstock Updated information and services can be found at: These include: This article cites 16 articles, 7 of which can be accessed free at: 5 online articles that cite this article can be accessed at: You can respond to this article at: Receive free email alerts when new articles cite this article - sign up in the box at the

