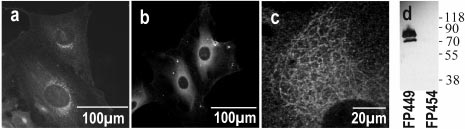
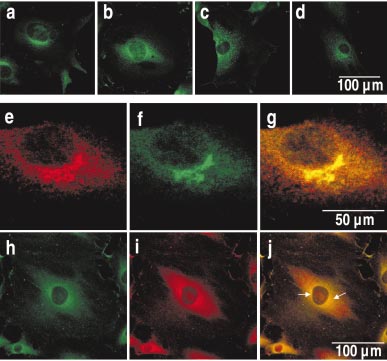
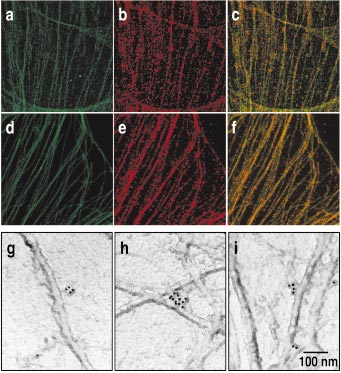
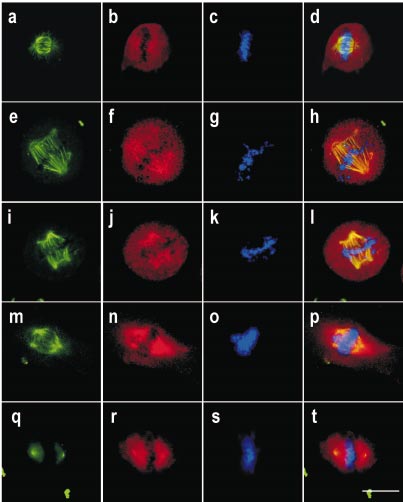
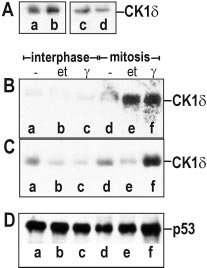
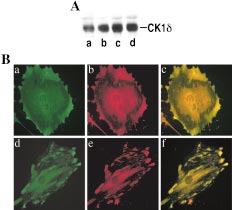
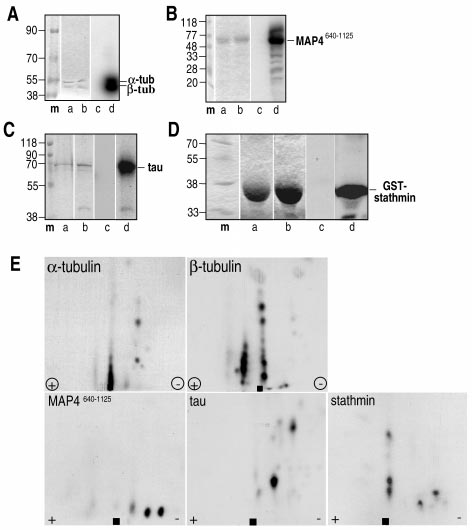
Detecting bacillus spores by raman and surface-enhanced raman spectroscopy (sers)
Detecting Bacillus Spores by Raman and Surface-Enhanced Raman Spectroscopy (SERS) Raman spectroscopy has been employed to detect Bacillus cereusspores, an anthrax surrogate, collected from a letter as it passed Intensity (arbitrary units) through a mail sorting system. Raman spectroscopy also has the capability to identify many common substances used as hoaxes. A