Congresoscare.com.co2
Propofol Protects Against Focal Cerebral Ischemia via
Inhibition of Microglia-Mediated Proinflammatory
Cytokines in a Rat Model of Experimental Stroke
Rong Zhou, Zailiang Yang, Xiaofeng Wu
1 Department of Operating Room, Children's Hospital, Chongqing Medical University, Chongqing, China,
2 Ministry of Education Key Laboratory of Child
Development and Disorders, Children's Hospital, Chongqing Medical University, Chongqing, China,
3 Hematopoietic Stem Cell Transplantation and Gene
Therapy Center, Affiliated Hospital of Academy of Military Medical Sciences, Beijing, China,
4 State Key Laboratory of Trauma, Burn and Combined Injury,
Daping Hospital, Third Military Medical University, Chongqing, China,
5 Research Institute of Surgery, Daping Hospital, Third Military Medical University,
Chongqing, China,
6 Department of Anesthesiology, Children's Hospital, Chongqing Medical University, Chongqing, China
Ischemic stroke induces microglial activation and release of proinflammatory cytokines, contributing to the expansion
of brain injury and poor clinical outcome. Propofol has been shown to ameliorate neuronal injury in a number of
experimental studies, but the precise mechanisms involved in its neuroprotective effects remain unclear. We tested
the hypothesis that propofol confers neuroprotection against focal ischemia by inhibiting microglia-mediated
inflammatory response in a rat model of ischemic stroke. Sprague-Dawley rats were subjected to middle cerebral
artery occlusion (MCAO) for 2 h followed by 24 h of reperfusion. Propofol (50 mg/kg/h) or vehicle was infused
intravenously at the onset of reperfusion for 30 minutes. In vehicle-treated rats, MCAO resulted in significant cerebral
infarction, higher neurological deficit scores and decreased time on the rotarod compared with sham-operated rats.
Propofol treatment reduced infarct volume and improved the neurological functions. In addition, molecular studies
demonstrated that mRNA expression of microglial marker Cd68 and Emr1 was significantly increased, and mRNA
and protein expressions of proinflammatory cytokines tumor necrosis factor-α, interleukin-1β and interleukin-6 were
augmented in the peri-infarct cortical regions of vehicle-treated rats 24 h after MCAO. Immunohistochemical study
revealed that number of total microglia and proportion of activated microglia in the peri-infarct cortical regions were
markedly elevated. All of these findings were ameliorated in propofol-treated rats. Furthermore, vehicle-treated rats
had higher plasma levels of interleukin-6 and C-reactive protein 24 h after MCAO, which were decreased after
treatment with propofol. These results suggest that propofol protects against focal cerebral ischemia via inhibition of
microglia-mediated proinflammatory cytokines. Propofol may be a promising therapeutic agent for the treatment of
ischemic stroke and other neurodegenerative diseases associated with microglial activation.
Citation: Zhou R, Yang Z, Tang X, Tan Y, Wu X, et al. (2013) Propofol Protects Against Focal Cerebral Ischemia via Inhibition of Microglia-Mediated
Proinflammatory Cytokines in a Rat Model of Experimental Stroke. PLoS ONE 8(12): e82729. doi:10.1371/journal.pone.0082729
Editor: Tobias Eckle, University of Colorado Denver, United States of America
Received September 24, 2013;
Accepted November 5, 2013;
Published December 9, 2013
Copyright:, which permits
unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The work was supported by Chongqing Medical University Research Foundation. The funders had no role in study design, data collection and
analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
* E-mail:
[email protected]
Microglia are major immune cells in the central nervous
system, which are activated rapidly in response to brain injury
Stroke is the leading cause of death and the most frequent
or during neurodegenerative processes and produce
cause of long-term disability in the adult population worldwide
proinflammatory cytokines, growth factors, reactive oxygen
]. Ischemic strokes are the most common type of stroke,
species, nitric oxide, and glutamate []. Although activation of
representing about 87% of all strokes ]. Cerebral ischemia
microglia is necessary and crucial for host defense, the over-
induces acute inflammation by triggering excessive production
activation of microglia results in deleterious and neurotoxic
of proinflammatory cytokines in the brain as well as in
consequences. Experimental studies have shown that resident
peripheral blood, which exacerbate brain damage and are
microglia in the brain are activated within minutes of ischemia
related to poor clinical outcome in patients with ischemic stroke
onset and release multiple proinflammatory cytokines, such astumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and
interleukin-6 (IL-6), which play a crucial role in the progression
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
of neuronal loss and brain injury following ischemic stroke
Induction of MCAO
Thus, development of agents that reduce microglial activation
Transient MCAO was induced by the intraluminal suture
in the brain and inhibit the release of proinflammatory cytokines
method as previously described [Briefly, rats were
is considered to be an important therapeutic strategy for
anesthetized with an intraperitoneal (i.p.) injection of
ischemic stroke.
pentobarbital sodium (50 mg/kg). Body core temperature was
Propofol (2,6-diisopropylphenol) is an intravenous hypnotic
maintained within a normothermic range (37°C to 38°C) with a
agent widely used for induction and maintenance of anesthesia
temperature-controlled heating pad. A 4/0 surgical nylon
during surgeries. In addition, Propofol has antiinflammatory
monofilament with a silicone-beaded tip was introduced into the
properties, reducing production of proinflammatory cytokines,
right internal carotid artery through the external carotid artery to
altering expression of nitric oxide, and inhibiting neutrophil
occlude the origin of the middle cerebral artery. After 2 h of
function An
in vitro study recently showed that propofol
occlusion, the monofilament was removed to allow reperfusion
for 24 h. In addition, the left femoral artery was cannulated for
activation of microglia and the production of proinflammatory
monitoring blood pressure (BP) and heart rate (HR) and for
cytokines [A number of experimental studies have reported
arterial blood gas measurements. The left femoral vein was
that propofol ameliorates neuronal injury in animal models of
cannulated for the administration of drugs. BP and HR were
ischemic stroke []. However, the precise mechanisms
continuously recorded on a computer using the PowerLab
involved in its neuroprotective effects remain unclear. In this
software (PowerLab/8SP, Chart 5.0; ADInstruments Pty, Ltd.,
study, we tested the hypothesis that propofol attenuates
Castle Hill, Australia). Blood gas measurements were
cerebral ischemic injury by inhibiting microglia-mediated
performed 15 min after the onset of ischemia or reperfusion
inflammatory response in a rat model of ischemic stroke.
using a blood gas analyzer (Compact 3, AVL Medizintechnik).
Assessment of neurological outcome
Eight rats from each group were used for assessment of
neurological outcome. Neurological deficit scores were
Male Sprague-Dawley rats weighing 250-300 g were
evaluated 24 h after ischemia using an eight-point scale as
purchased from Beijing Laboratory Animal Research Center
described previously The score was 0 for no apparent
(Beijing, China). Animals were housed and cared for in the
deficits; 1 for failure to extend left forepaw fully; 2 for decreased
Animal Resource Center and allowed free access to food and
grip of the left forelimb; 3 for spontaneous movement in all
water. All procedures were reviewed and approved by the
directions, contralateral circling only if pulled by the tail; 4 for
Institutional Animal Care and Use Committee at the Chongqing
circling or walking to the left; 5 for walking only if stimulated; 6
Medical University and were performed in accordance with the
for unresponsiveness to stimulation and with depressed level of
"Guiding Principles for Research Involving Animals and Human
consciousness; and 7 for death.
Measurement of motor coordination was performed 24 h
before and after ischemia, respectively. The experimental
procedure was described previously []. Briefly, the time that
The animals were randomly assigned to 3 groups (n=20 for
the rats stayed on a rotating rod was recorded automatically in
each group) as follows: (1) middle cerebral artery occlusion
each case for up to 3 minutes. The trial was conducted five
(MCAO) group treated with propofol (MCAO+PRO). Rats were
times for each rat, and the mean riding time was used as the
subjected to MCAO for 2 h followed by 24 h of reperfusion and
mean value for this test. When the time of riding was over 3
infused intravenously with propofol (50 mg/kg/h) using syringe
minutes, the rat was released from the rod, and the riding time
pump at the onset of reperfusion for 30 minutes; (2) MCAO
was recorded as 3 minutes.
group treated with vehicle (saline) (MCAO+VEH). Same as
At the end of the observation period, these rats were
group (1), but these rats were infused intravenously with saline
euthanized with an overdose of anesthesia and brains were
at the onset of reperfusion for 30 minutes; (3) sham-operated
quickly removed for assessment of infarct volume, as
group (SHAM). Rats were subjected to sham MCAO without
previously described ]. Briefly, brains were sectioned at 2-
treatment. The dose for intravenous infusion of propofol was
mm intervals throughout the rostrocaudal axis of the striatum.
derived from a previous study in which such dose of propofol
Slices were then staining with 2% 2,3,5 triphenyltetrazolium
significantly reduced infarct size 24 h after MCAO in rats
chloride (TTC) for 15 min at 37°C. Slice images were
Based on a formula for dose translation from animal to human
digitalized and infarct areas were analyzed using NIH Image
], a dose of 50 mg/kg/h of propofol in rats is roughly
1.60. The Complete lack of staining with TTC was defined as
equivalent to a dose of 8.1 mg/kg/h in human, which is within
the infarct lesion. The infarct volume was expressed as a
the infusion rates of propofol for clinical use in human. At the
percentage of the contralateral hemisphere.
end of the protocol (24 h after MCAO and reperfusion),neurological deficit scores and motor coordination were
Real-time PCR analysis
evaluated. Rats were then sacrificed, the blood samples were
The mRNA expression of microglial markers (CD68 and
collected for biochemical measurements and the brains were
Emr1) and proinflammatory cytokines (TNF-α, IL-1β and IL-6)
removed for infarct volume assessment, molecular analysis or
in the peri-infarct cortical tissue was measured with real-time
PCR. Rats (n=8 for each group) were euthanized 24 h after
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
Table 1. Primer sequences for real-time PCR.
Primer name
Forward primer (5'→3')
Reverse primer (5'→3')
MCAO, and the brains were removed and cut into seven serial
antibody penetration. Subsequently, the sections were
2-mm-thick coronal sections. The peri-infarct cortical tissue
incubated for 72 h with a mouse monoclonal primary antibody
was dissected from the coronal brain sections for extraction of
directed against CD11b (clone OX-42) (1:100, Chemicon,
total RNA and protein using an operating microscope as
Temecula, USA) in 2% normal horse serum and 0.2% Triton
described previously ]. The total RNA was extracted using
X-100 in phosphate buffered saline. This was followed by
TRI Reagent (Molecular Research Center, Inc) and reverse
incubations in a biotinylated antimouse secondary antibody
transcribed into cDNA. mRNA levels for CD68, Emr1, TNF-α,
raised in horse (1:100, Vector Laboratories, Burlingame, USA)
IL-1β, IL-6 and GAPDH were measured with SYBR green real-
for 2 h. The sections were exposed to DAB reagent (Vector
time PCR. The sequences for primers used were summarized
in . Real-time PCR was performed using the ABI prism
hematoxylin, dehydrated in ethanol, cleared with xylene, and
7000 Sequence Detection System (Applied Biosystems,
coverslipped with mounting medium.
Carlsbad, CA). The values were normalized to GAPDH and
Morphological analysis and quantification of microglia were
expressed as a fold change relative to the SHAM group.
performed with a light microscope as described ]. Non-activated microglia were distinguished by their small soma from
Western blot analysis
which there emanated extensive, highly branched, long, thin
The protein levels of proinflammatory cytokines TNF-α, IL-1β
processes, a morphology termed ramified. Activated microglia
and IL-6 in the peri-infarct cortical tissue were measured by
immunohistochemical staining for the marker CD11b (clone
Western blot. The peri-infarct cortical tissue was dissected from
OX-42), the presence of a clearly enlarged soma and marked
the coronal brain sections and homogenized in lysis buffer. The
changes in the appearance of the processes which were now
protein concentration in the supernatant was measured with
reduced in number, but considerably thicker and shorter giving
the BCA protein assay Kit (Pierce, Rockford, IL, USA).
a stubby appearance. The number of activated and non-
Equivalent amounts of protein were separated on 12% SDS-
activated microglia was counted in several 0.2 × 0.2 mm
polyacrylamide gels and transferred to polyvinylidene difluoride
squares and the average was calculated.
membranes (Millipore Corporation, Bedford, MA, USA). Themembranes were blocked with 3% nonfat dry milk and thenincubated using primary antibody to TNF-α, IL-1β, IL-6 and β-
actin (Santa Cruz Biotechnology Inc, Santa Cruz, CA) at 4°C
Blood samples were collected 24 h after MCAO for
overnight. After three washing, the membranes were incubated
measurements of plasma proinflammatory cytokines (TNF-α,
with horseradish peroxidase-conjugated second antibody
IL-1β, IL-6 and C-reactive protein) by ELISA kits (Biosource
(Santa Cruz Biotechnology Inc, Santa Cruz, CA) for 1 h at
International Inc, Camarillo, CA or R&D Systems Inc,
room temperature. The signal was visualized using the
Minneapolis, MN).
enhanced chemiluminescence (ECL) detection system(Amersham) and the densities of the immunobands were
quantitated. All data were corrected and normalized to β-actin.
Data are expressed as mean±SEM. The significance of
differences in mean values was analyzed by one-way or two-
way repeated-measure ANOVA followed by Fisher's
post hoc
Twenty-four hours after MCAO, four rats from each group
test.
P<0.05 was considered statistically significant.
were perfused transcardially with heparinized saline followedby ice-cold 4% paraformaldehyde in phosphate buffered saline.
Brains were removed and fixed overnight in 4%paraformaldehyde at 4°C and then immersed in 30% sucrose.
Hemodynamic and physiological variables
Brain tissue was sliced into 20-μm serial coronal sections using
To eliminate potential confounding factors on neurological
a cryostat. Standard immunohistochemical procedures were
outcomes, hemodynamic and physiological variables, including
performed according to a previous study ]. Briefly, the
BP, HR and arterial blood gases, were monitored and
sections were blocked by 0.5% H O for 30 min and then
controlled before, during and after MCAO. As shown in
incubated in 10% normal horse serum for 60 min to facilitate
and , no significant differences among groups in mean BP,
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
Table 2. Systemic hemodynamic variables during MCAO and reperfusion.
Ischemia 15 min
Ischemia 35 min
Reperfusion 5 min
Reperfusion 35 min
MBP (mmHg)
HR (beats/min)
Table 3. Physiological variables during MCAO and reperfusion.
HR, arterial pH, carbon dioxide tension (Pco ) and arterial
sham rats, but there were few microglia with an activated
oxygen tension (Po ) were observed at each time point before,
morphology (). The average number of total microglia
during MCAO and during reperfusion.
and the proportion of activated microglia counted in the peri-infarct cortical tissue were significantly
Propofol ameliorated MCAO-induced neuronal injury
increased in vehicle-treated rats 24 h after MCAO compared
A 2-h MCAO followed by 24 h reperfusion induced an infarct
with those in sham rats. In contrast, both the number of total
volume of 23 ± 2% in vehicle-treated rats
microglia and the proportion of activated microglia in the peri-
In contrast, treatment with propofol after MCAO reduced infarct
infarct cortical tissue were reduced in rats treated with propofol.
volume significantly by approximately 35% (p < 0.05).
The time spent on rotarod among three groups was similar
Propofol attenuated MCAO-induced proinflammatory
before MCAO (average time on rotarod was 154 ± 17 sec).
Twenty-four hours after MCAO, vehicle-treated rats exhibited
Multiple proinflammatory cytokines play an important role in
markedly higher neurological deficit scores () and
the regulation of inflammation. TNF-α, IL-1β and IL-6 are major
reduced time on rotarod (than sham rats. Whereas
early response cytokines that trigger a cascade of inflammatory
rats treated with propofol after MCAO demonstrated significant
mediators, including other cytokines, chemokines, reactive
decrease in neurological deficit scores and improvement in
nitrogen or oxygen intermediates In the brain, microglia
rotarod performance compared with vehicle-treated rats.
produce all 3 cytokines. C-reactive protein is an exquisitelysensitive systemic marker of inflammation and tissue damage.
In the present study, the neuroprotective actions of propofol
Propofol inhibited MCAO-induced microglial activation
administrated after MCAO could be due to its inhibitory effects
Real-time PCR showed that mRNA expression of Cd68 and
on microglia and subsequent production of proinflammatory
Emr1, two microglia specific markers, markedly increased by
cytokines in the brain and periphery. To test this hypothesis,
178% and 290%, respectively, in the peri-infarct cortical tissue
we measured the levels of above proinflammatory cytokines in
in vehicle-treated rats 24 h after MCAO as compared to those
the brain and plasma. The mRNA () and protein
in sham rats ). Compared with vehicle-treated rats,
) expressions of the proinflammatory cytokines TNF-α,
propofol-treated rats had significantly decreased mRNA
IL-1β and IL-6 were significantly augmented in the peri-infarct
expression of Cd68 and Emr1 in the peri-infarct cortical tissue
cortical tissue of vehicle-treated rats compared with sham rats.
24 h after MCAO.
After treatment with propofol, mRNA and protein expressions of
OX42 antibody is a specific microglial marker and stains all
IL-1β and IL-6 were significantly reduced, and mRNA and
microglia. Activated microglia were defined as cells that exhibit
protein expressions of TNF-α were normalized in the peri-
strong OX-42 immunoreactivity, an enlarged soma, fewer and
infarct cortical tissue of rats at 24 h following MCAO.
shorter processes. Using immunohistochemical study, we
There were no differences in plasma levels of TNF-α and
found that microglia were presented in the cortical tissue of
IL-1β across the 3 experimental groups
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
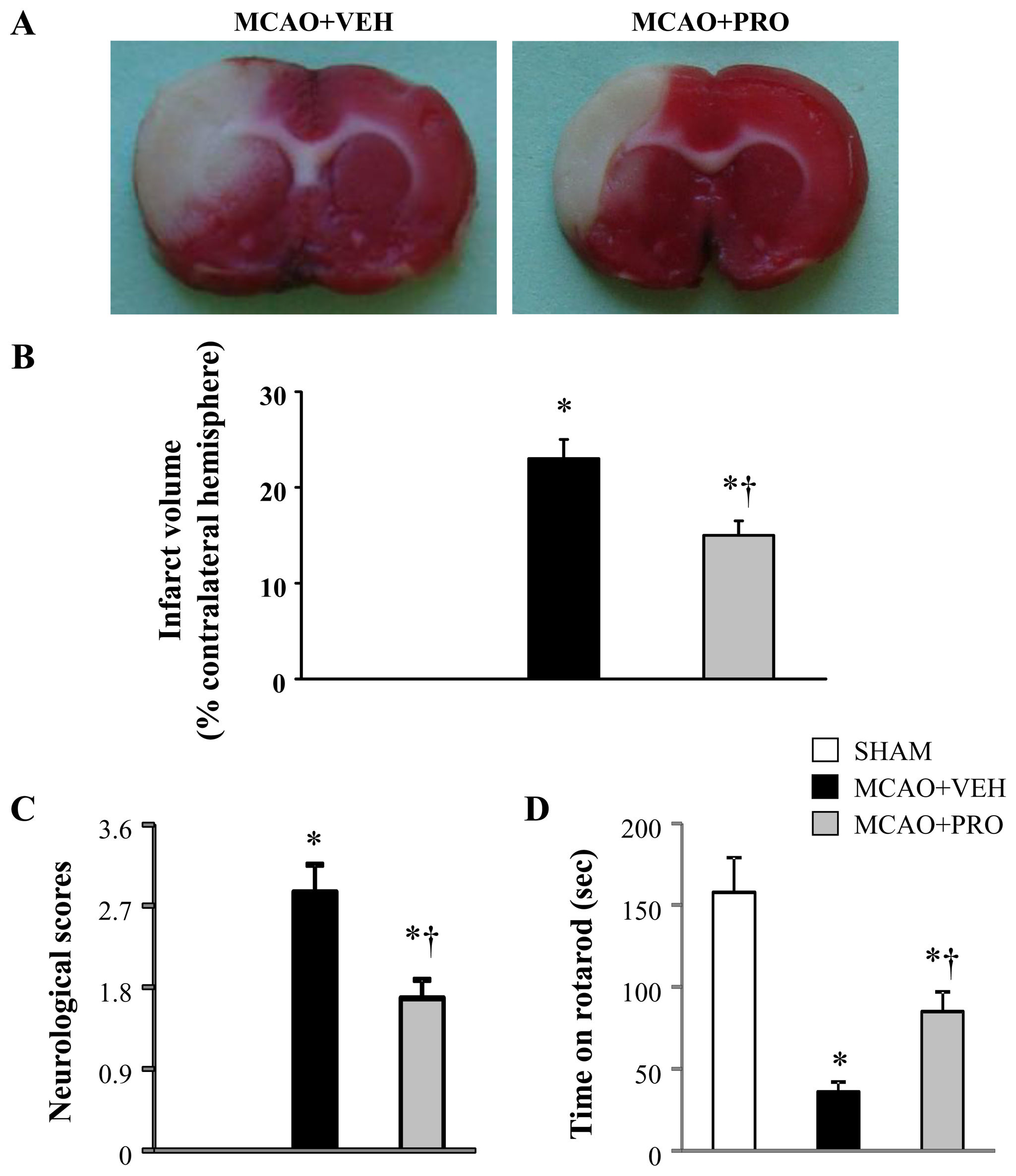
Propofol and Neuroprotection
Figure 1. Representative coronal brain slices stained with TTC (A), infarct volume (B), neurological deficit scores (C) and
motor coordination (D) 24 h after middle cerebral artery occlusion (MCAO) in rats treated with vehicle (VEH) or propofol
(PRO). Sham-operated rats (SHAM) without treatment were used as control. Values are mean ± SEM (n = 8 for each group). *P<
0.05 vs. SHAM, †P< 0.05 MCAO+PRO vs. MCAO+VEH.
doi: 10.1371/journal.pone.0082729.g001
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
Figure 2. mRNA expression for microglia specific markers Cd68 (A) and Emr1 (B) in the peri-infarct cortical tissue 24 h
after MCAO in rats treated with VEH or PRO. SHAM rats without treatment were used as control. Values are mean ± SEM (n = 8
for each group) and expressed as a fold change relative to SHAM. *P< 0.05 vs. SHAM, †P< 0.05 MCAO+PRO vs. MCAO+VEH.
doi: 10.1371/journal.pone.0082729.g002
However, vehicle-treated rats had higher plasma levels of IL-6
], but the underlying mechanisms remain unclear. In the
and C-reactive protein, which were significantly reduced after
present study, we found that a 2-h MCAO followed by 24 h
treatment with propofol (
reperfusion elicited large brain infarct in the frontoparietalcortex. Administration of propofol early after MCAO reduced
infarct volume, improved neurological outcome as evidencedby decrease neurological deficit scores and increased time in
The novel finding of this study is that treatment with propofol
rotarod performance. These results are consistent with
early after ischemic stroke suppressed microglia activation and
previous studies, suggesting a protective effect of propofol on
proliferation in the peri-infarct cortical regions, reduced the
ischemic brain injury. More importantly, our data extend
production of proinflammatory cytokines in the brain as well as
previous findings by revealing that the beneficial effects of
in peripheral blood, and improved neurological outcome. To our
propofol on ischemic brain injury are associated with
knowledge, this is the first study in vivo to demonstrate that the
suppression of microglial activation and proliferation in the peri-
propofol confers neuroprotection against ischemic brain injury
by modulating microglial function. Our finding provides new
understanding of the protective mechanisms of anesthetic
The inflammatory responses in the brain to ischemic stroke
propofol, which may be applicable in the immediate aftermath
are characterized by a rapid activation and proliferation of
of stroke as well as to patients with a stroke history undergoing
microglial cells, followed by the infiltration of circulating
surgery, patients in the intensive care unit under sedation, and
inflammatory cells, including neutrophils, T cells, monocyte/
patients undergoing neurosurgery.
macrophages, and other cells in the ischemic brain region, as
Propofol has become the most widely used anesthetics in
demonstrated in animal models and in stroke patients
neurosurgery. More recently, the anti-inflammatory functions of
]. The microglia are activated within minutes after onset
propofol have been received much attention because this
of focal cerebral ischemia and may last for several weeks after
agent has been shown to exert protective effects during acute
initial injury ]. Activated microglia produce a plethora of
inflammatory in neurologic and cardiovascular diseases
proinflammatory mediators in the brain, including TNF-α, IL-1β
For example, experimental studies in animals showed
and IL-6, which contribute to the expansion of brain injury and
that propofol inhibits cytokine release during sepsis
the delayed loss of neurons It has been shown that
neutrophil-mediated
intraventricular injection of IL-1 and TNF-α increases infarct
pulmonary injury ]. Clinical studies revealed that propofol
volume and brain edema after MCAO in rats, whereas the
attenuates myocardial reperfusion injury and pulmonary
injection of microglial inhibitor minocycline [or PPAR-γ
dysfunction following cardiopulmonary bypass by reducing free
agonist pioglitazone that suppresses microglial activation and
radical release and modulating the inflammatory process
expression of proinflammatory cytokines [], or administration
In addition, a number of studies have reported that
of antibodies against IL-1 and TNF-α [reduces brain
propofol protects against ischemic brain injury in animal models
injury. The data of present study showed that, at 24 h after
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
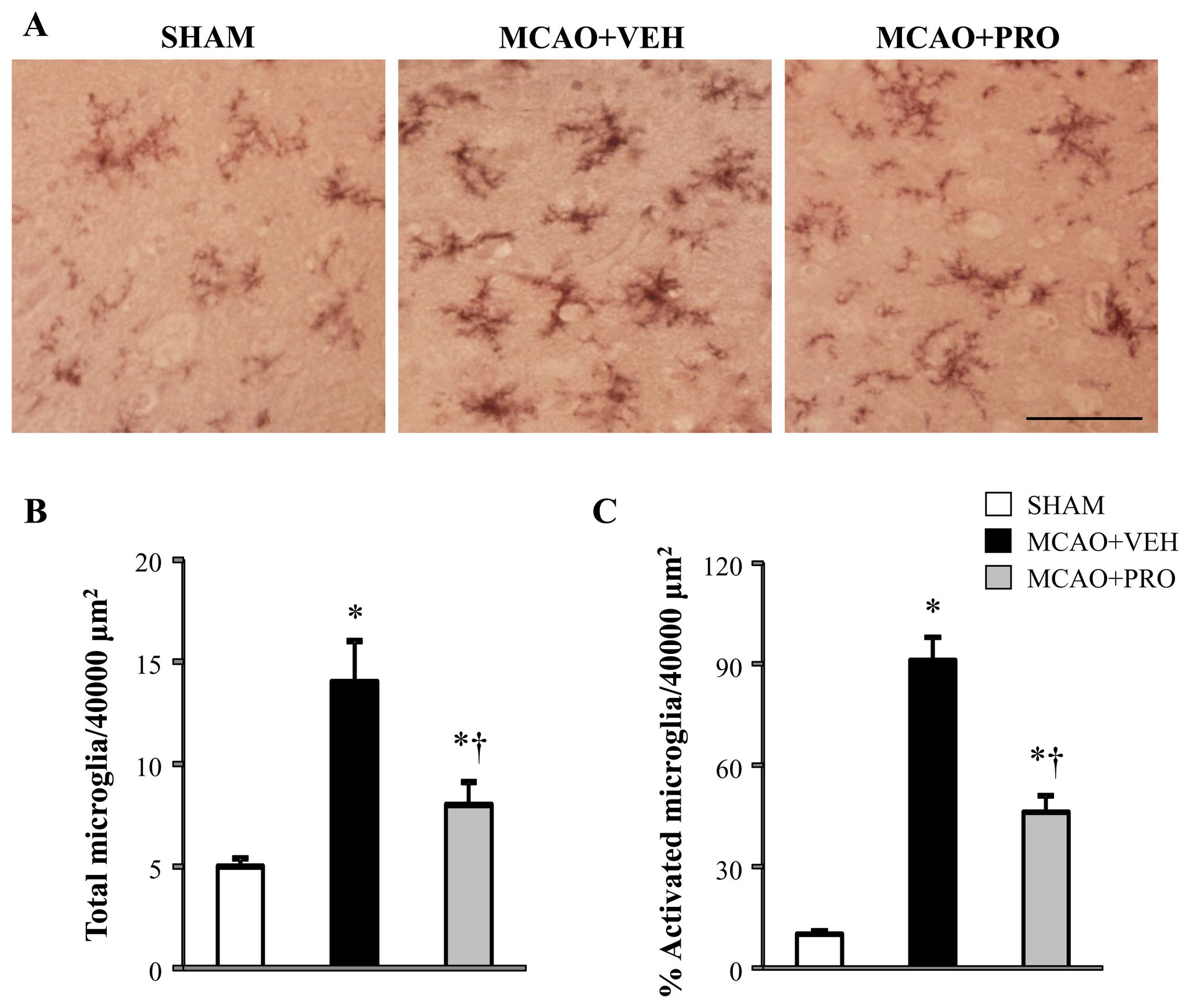
Propofol and Neuroprotection
Figure 3. Representative photomicrographs of microglia stained with CD11b (A), total number of microglia (B) and the
proportion of activated microglia (expressed as percent of total microglia) (C) in the peri-infarct cortical tissue 24 h after
MCAO in rats treated with VEH or PRO. SHAM rats without treatment were used as control. Activated microglia were defined as
strong CD11b immunoreactivity, an enlarged soma, and fewer and shorter processes. Scale bar, 200 μm. Values are mean ± SEM
(n = 4 for each group). *P< 0.05 vs. SHAM, †P< 0.05 MCAO+PRO vs. MCAO+VEH.
doi: 10.1371/journal.pone.0082729.g003
MCAO, mRNA expression of microglial markers Cd68 and
cytokines is mostly co-localized with activated microglia
Emr1 in the peri-infarct cortical tissue was augmented, and the
indicating that activated microglial cells are the main
number of total microglia and the proportion of activated
source of proinflammatory cytokines in the brain after ischemic
microglia were increased, suggesting that ischemic stroke
stroke. Furthermore, we found that early treatment with
results in microglial activation and proliferation in this brain
propofol reduced mRNA expression of Cd68 and Emr1,
area. In addition, expression of proinflammatory cytokines
decreased the number of total microglia and the proportion of
TNF-α, IL-1β and IL-6 in this brain area was also increased.
activated microglia in the peri-infarct cortical tissue,
These results are consistent with previous findings showing
accompanied by decreased mRNA and protein expressions of
that cerebral ischemia substantially activates microglia and
proinflammatory cytokines 24 h after MCAO. These findings
increases expression of proinflammatory cytokines in the
provided evidence for suppressive effects of propofol on
frontoparietal cortex adjacent to the ischemic core 24 h after
microglial activation and release of proinflammatory cytokines
MCAO, and increased immunoreactivity for proinflammatory
in vivo in rats after ischemic stroke. Our current data are
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
Figure 4. mRNA expression for proinflammatory cytokines TNF-α (A), IL-1β (B) and IL-6 (C) in the peri-infarct cortical
tissue 24 h after MCAO in rats treated with VEH or PRO. SHAM rats without treatment were used as control. Values are mean ±
SEM (n = 8 for each group) and expressed as a fold change relative to SHAM. *P< 0.05 vs. SHAM, †P< 0.05 MCAO+PRO vs.
MCAO+VEH.
doi: 10.1371/journal.pone.0082729.g004
supported by recent in vitro studies showing that propofol
important, as they may be valuable tools in the search for an
dramatically reduced levels of proinflammatory cytokines TNF-
optimal management of stroke patients. It is notable that
α, IL-1β and IL-6, and activation of microglia induced by
cerebral ischemia did not change the levels of plasma
lipopolysaccharide [or extracellular pressure []. Taken
proinflammatory cytokines TNF-α and IL-1β, but significantly
together, these observations demonstrate that the beneficial
increased the levels of plasma IL-6 and C-reactive protein,
effects of propofol on infarct volume and neurological outcome
which were attenuated by propofol treatment. Inflammation in
are associated with inhibition of microglia activation and
the brain is known to modulate inflammation in the periphery in
suppression of the exaggerated production of proinflammatory
ischemic stroke, and measurement of peripheral inflammatory
cytokines in ischemic brain early after ischemic stroke.
response has been suggested to be a far more practical
The use of biochemical markers as predictors of stroke
lesion evolution and prognosis is becoming increasingly
proinflammatory cytokines and C-reactive protein in plasma
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
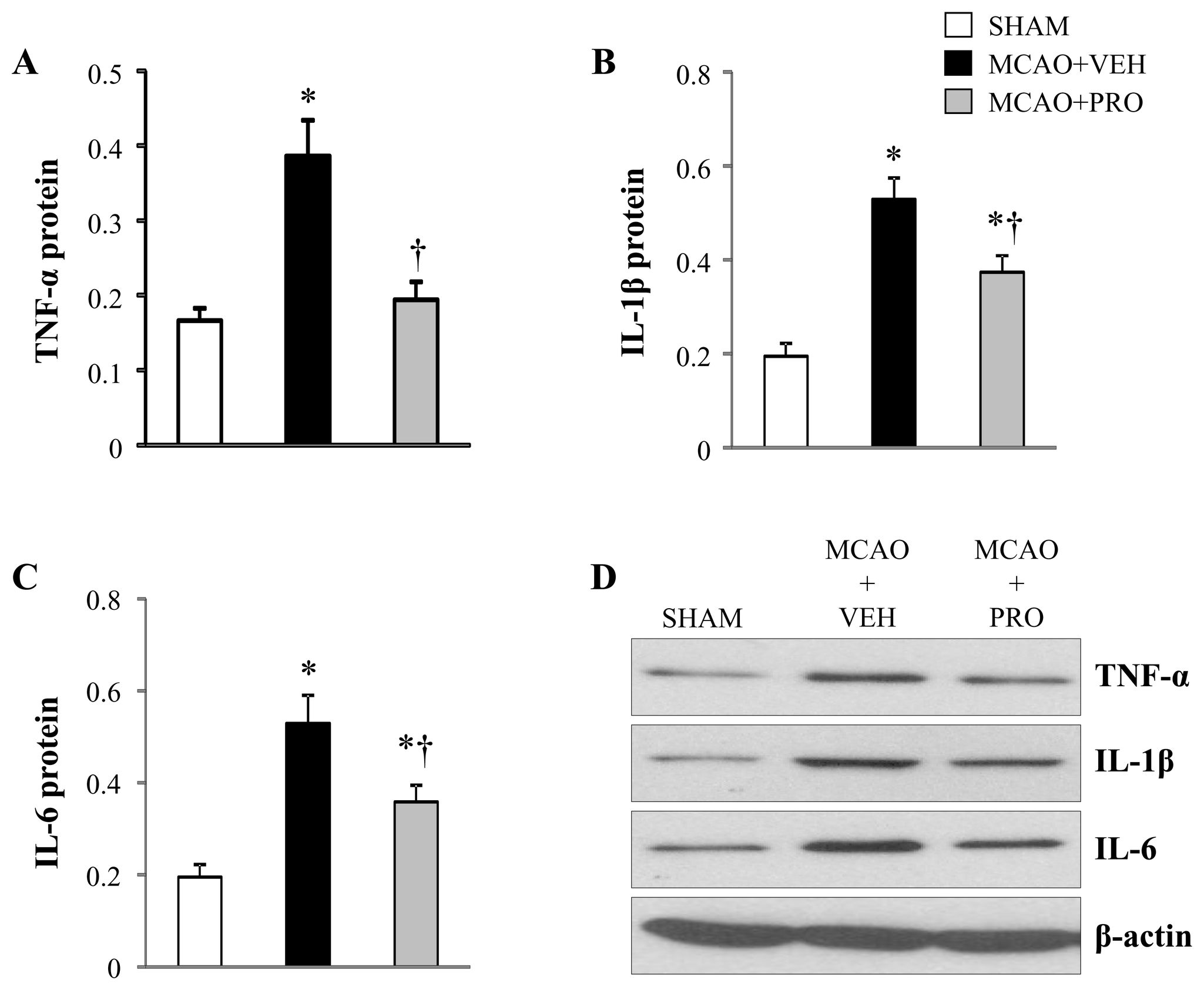
Propofol and Neuroprotection
Figure 5. Protein levels for proinflammatory cytokines TNF-α (A), IL-1β (B) and IL-6 (C) in the peri-infarct cortical tissue 24
h after MCAO in rats treated with VEH or PRO. SHAM rats without treatment were used as control. Representative Western blots
are shown in figure D. Values are expressed as mean ± SEM (n= 8 for each group) and corrected by β-actin. *P< 0.05 vs. SHAM,
†P< 0.05 MCAO+PRO vs. MCAO+VEH.
doi: 10.1371/journal.pone.0082729.g005
after ischemic stroke have been reported in both clinical and
ischemia also induces activation of astrocytes, another resident
experimental studies [The acute-phase response,
cells in the brain, which can produce the proinflammatory
characterized by elevated plasma concentrations of IL-6, C-
cytokines including TNF-α, IL-1β and IL-6. Intervention to
reactive protein and neutrophil leukocytosis, is induced within
inhibit astrocyte activation has been shown to enhance
hours of ischemic stroke []. Parameters of the acute-phase
neuronal survival and improve outcome following cerebral
response, particularly plasma IL-6 and C-reactive protein
ischemia [However, the present study focused only on
concentrations, are positively associated with stroke severity
the role of microglia activation in ischemic brain injury, further
and infarct volume, and predict a higher risk of early clinical
studies are needed to determine whether neuroprotective
worsening ].Thus, reductions in plasma proinflammatory
effects of propofol observed in this study are partially due to
cytokines IL-6 and C-reactive protein after treatment with
inhibition of astrocyte activation. Second, although propofol
propofol in our study are likely to reflect decreased risk of early
treatment suppressed microglia activation and reduced the
production of proinflammatory cytokines in the brain as well as
Two major limitation of the present study should be
in peripheral blood, accompanied by decreased infarct size and
acknowledged. First, it has been reported that cerebral
improved neurological outcome, but the finding of decreases in
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
Figure 6. Plasma levels of proinflammatory cytokines TNF-α (A), IL-1β (B), IL-6 (C) and C-reactive protein (D) 24 h after
MCAO in rats treated with VEH or PRO. SHAM rats without treatment were used as control. Values are mean ± SEM (n = 8 for
each group). *P< 0.05 vs. SHAM, †P< 0.05 MCAO+PRO vs. MCAO+VEH.
doi: 10.1371/journal.pone.0082729.g006
these inflammatory markers does not prove that these cause
direct relationship between the levels of these proinflammatory
the decrease in infarct size. Other important mediators
cytokines and infarct size after ischemic stroke.
associated with ischemic neuronal injury might also be reduced
In conclusion, the present study demonstrates that
by propofol and contributed to the decrease in infarct size.
administration of propofol early after cerebral ischemia reduces
Further research is necessary to determine whether there is a
infarct volume and improves neurological function by inhibition
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
of microglia activation and proinflammatory cytokine release in
the brain. Propofol may be a promising therapeutic agent forthe prevention and/or treatment of ischemic brain injury and
Conceived and designed the experiments: RZ FL. Performed
other neurodegenerative diseases associated with microglial
the experiments: RZ ZY XT YT XW FL. Analyzed the data: RZZY XT FL. Contributed reagents/materials/analysis tools: RZ
ZY XT YT XW. Wrote the manuscript: RZ FL.
1. Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet
19. Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Tabira T (2002)
371: 1612-1623. PubMed:
Chronic stress impairs rotarod performance in rats: implications for
depressive state. Pharmacol Biochem Behav 71: 79-84. doi:
2. Schellinger PD, Kaste M, Hacke W (2004) An update on thrombolytic
therapy for acute stroke. Curr Opin Neurol 17: 69-77. doi:
20. Arrick DM, Sun H, Mayhan WG (2012) Influence of exercise training on
ischemic brain injury in type 1 diabetic rats. J Appl Physiol (1985) 113:
3. Vila N, Castillo J, Dávalos A, Chamorro A (2000) Proinflammatory
1121-1127. doi:.
cytokines and early neurological worsening in ischemic stroke. Stroke
21. Patzer A, Zhao Y, Stöck I, Gohlke P, Herdegen T et al. (2008)
Peroxisome proliferator-activated receptorsgamma (PPARgamma)
4. Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic
differently modulate the interleukin-6 expression in the peri-infarct
stroke: role of inflammatory cells. J Leukoc Biol 87: 779-789. doi:
cortical tissue in the acute and delayed phases of cerebral ischaemia.
Eur J Neurosci 28: 1786-1794. doi:
5. Block ML, Hong JS (2005) Microglia and inflammation-mediated
neurodegeneration: multiple triggers with a common mechanism. Prog
22. Rana I, Stebbing M, Kompa A, Kelly DJ, Krum H et al. (2010) Microglia
Neurobiol 76: 77-98. doi:PubMed:
activation in the hypothalamic PVN following myocardial infarction.
Brain Res 1326: 96-104. PubMed:
6. Stolp HB, Dziegielewska KM (2009) Review: Role of developmental
23. Dinarello CA (2000) Proinflammatory cytokines. Chest 118: 503-508.
neurodevelopmental and neurodegenerative diseases. Neuropathol
Appl Neurobiol 35: 132-146. doi:
24. Yang SC, Chung PJ, Ho CM, Kuo CY, Hung MF et al. (2013) Propofol
inhibits superoxide production, elastase release, and chemotaxis in
7. Banati RB, Gehrmann J, Schubert P, Kreutzberg GW (1993)
formyl peptide-activated human neutrophils by blocking formyl peptide
Cytotoxicity of microglia. Glia 7: 111-118.
receptor 1. J Immunol 190: 6511-6519. doi:
8. Barone FC, Arvin B, White RF, Miller A, Webb CL et al. (1997) Tumor
25. Taniguchi T, Yamamoto K, Ohmoto N, Ohta K, Kobayashi T (2000)
necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke
Effects of propofol on hemodynamic and inflammatory responses to
endotoxemia in rats. Crit Care Med 28: 1101-1106. doi:
9. Rothwell N, Allan S, Toulmond S (1997) The role of interleukin 1 in
acute neurodegeneration and stroke: pathophysiological and
26. Taniguchi T, Kanakura H, Yamamoto K (2002) Effects of posttreatment
therapeutic implications. J Clin Invest 100: 2648-2652. doi:
with propofol on mortality and cytokine responses to endotoxin-induced
immunomodulating
27. Chen HI, Hsieh NK, Kao SJ, Su CF (2008) Protective effects of
propofol on acute lung injury induced by oleic acid in conscious rats.
11. Ye X, Lian Q, Eckenhoff MF, Eckenhoff RG, Pan JZ (2013) Differential
Crit Care Med 36: 1214-1221. doi:
general anesthetic effects on microglial cytokine expression. PLOS
28. Corcoran TB, Engel A, Sakamoto H, O'Callaghan-Enright S, O'Donnell
A et al. (2004) The effects of propofol on lipid peroxidation and
12. Zhao XC, Zhang LM, Tong DY, An P, Jiang C et al. (2013) Propofol
inflammatory response in elective coronary artery bypass grafting. J
increases expression of basic fibroblast growth factor after transient
Cardiothorac Vasc Anesth 18: 592-604. doi:
cerebral ischemia in rats. Neurochem Res 38: 530-537. doi:
29. An K, Shu H, Huang W, Huang X, Xu M et al. (2008) Effects of propofol
13. Liang C, Cang J, Wang H, Xue Z (2013) Propofol attenuates cerebral
on pulmonary inflammatory response and dysfunction induced by
ischemia/reperfusion injury partially using heme oxygenase-1. J
cardiopulmonary bypass. Anaesthesia 63: 1187-1192.
30. Wang X (2005) Investigational anti-inflammatory agents for the
14. Wang H, Luo M, Li C, Wang G (2011) Propofol post-conditioning
treatment of ischaemic brain injury. Expert Opin Investig Drugs 14:
induced long-term neuroprotection and reduced internalization of
AMPAR GluR2 subunit in a rat model of focal cerebral ischemia/
31. Yilmaz G, Granger DN (2008) Cell adhesion molecules and ischemic
stroke. Neurol Res 30: 783-793.
15. Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from
32. Zhou H, Chen S, Wang W, Wang Z, Wu X et al. (2012) Nanog inhibits
animal to human studies revisited. FASEB J 22: 659-661. PubMed:
lipopolysaccharide-induced expression of pro-inflammatory cytokines
by blocking NF-kappaB transcriptional activity in rat primary microglial
16. Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible
cells. Mol Med Rep 5: 842-846. PubMed:
middle cerebral artery occlusion without craniectomy in rats. Stroke 20:
33. Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J (1998)
Tetracyclines inhibit microglial activation and are neuroprotective in
17. Li D, Huang B, Liu J, Li L, Li X (2013) Decreased brain KATP channel
global brain ischemia. Proc Natl Acad Sci U S A 95: 15769-15774. doi:
contributes to exacerbating ischemic brain injury and the failure of
neuroprotection by sevoflurane post-conditioning in diabetic rats. PLOS
34. Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001)
Minocycline, a tetracycline derivative, is neuroprotective against
excitotoxicity by inhibiting activation and proliferation of microglia. J
18. Chen Y, Wu X, Yu S, Lin X, Wu J et al. (2012) Neuroprotection of
Neurosci 21: 2580-2588. PubMed: .
tanshinone IIA against cerebral ischemia/reperfusion injury through
35. Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J (2005) The
inhibition of macrophage migration inhibitory factor in rats. PLOS ONE
intracerebral application of the PPARgamma-ligand pioglitazone
confers neuroprotection against focal ischaemia in the rat brain. Eur J
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Propofol and Neuroprotection
translation to experimental research. J Neuroimmune Pharmacol 8:
36. Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y et al.
41. Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M et al. (1999)
(1995) Interleukin-1 as a pathogenetic mediator of ischemic brain
Increased cytokine release from peripheral blood cells after acute
damage in rats. Stroke 26: 676-680; discussion:
42. Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A et al. (2003) An
37. Wei Z, Chigurupati S, Arumugam TV, Jo DG, Li H et al. (2011) Notch
early and sustained peripheral inflammatory response in acute
activation enhances the microglia-mediated inflammatory response
ischaemic stroke: relationships with infection and atherosclerosis. J
associated with focal cerebral ischemia. Stroke 42: 2589-2594. doi:
Neuroimmunol 139: 93-101.
43. Wang YY, Chen CJ, Lin SY, Chuang YH, Sheu WH et al. (2013)
38. Gui B, Su M, Chen J, Jin L, Wan R et al. (2012) Neuroprotective effects
Hyperglycemia is associated with enhanced gluconeogenesis in a rat
of pretreatment with propofol in LPS-induced BV-2 microglia cells: role
model of permanent cerebral ischemia. Mol Cell Endocrinol 367: 50-56.
of TLR4 and GSK-3beta. Inflammation 35: 1632-1640. doi:
44. Barreto G, White RE, Ouyang Y, Xu L, Giffard RG (2011) Astrocytes:
39. Yu G, Dymond M, Yuan L, Chaturvedi LS, Shiratsuchi H et al. (2011)
targets for neuroprotection in stroke 11. Cent Nerv Syst Agents Med
Propofol's effects on phagocytosis, proliferation, nitrate production, and
Chem. pp. 164-173.
cytokine secretion in pressure-stimulated microglial cells. Surgery 150:
45. Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB et al. (2010) Age
exaggerates proinflammatory cytokine signaling and truncates signal
40. Smith CJ, Lawrence CB, Rodriguez-Grande B, Kovacs KJ, Pradillo JM
transducers and activators of transcription 3 signaling following
et al. (2013) The immune system in stroke: clinical challenges and their
ischemic stroke in the rat. Neuroscience 170: 633-644. doi:.
PLOS ONE www.plosone.org
December 2013 Volume 8 Issue 12 e82729
Induction of MCAO
Assessment of neurological outcome
Real-time PCR analysis
Western blot analysis
Hemodynamic and physiological variables
Propofol ameliorated MCAO-induced neuronal injury
Propofol inhibited MCAO-induced microglial activation
Propofol attenuated MCAO-induced proinflammatory cytokines
Source: http://congresoscare.com.co/cali/concursos/documentos-adicionales-juicio-del-siglo.html?download=105:propofol-protects-against-focal-cerebral-ischemia-via-inhibition-of-microglia-mediated-proinflammatory-cytokines-in-a-rat-model-of-experimental-stroke&start=100
M. Weidmann A novel dermal filler with lidocaine and its applicationMichael Weidmann1 1Dermatologist, Klinik am Forsterpark Augsburg, Germany Methods and materials – how and what was performed Background – a brief discussion of the subject Patients, 18 to 80 years old, were treated and Hyaluronic acid (HA) is a polysaccharide (glycosamino-
Temporal and Spatial Manifestations of Exercise-induced Hypoalgesia and Conditioned Pain Modulation Henrik Bjarke Vægter, MSc, PT PhD Thesis Pain Center South Department of Anesthesiology and Intensive Care Medicine University Hospital Odense Heden 9, Entrance 201



