Diabetes.diabetesjournals.org
Tetracycline Treatment Retards the Onset and Slows the
Progression of Diabetes in Human Amylin/Islet Amyloid
Polypeptide Transgenic Mice
Jacqueline F. Aitken,1,2 Kerry M. Loomes,1,2 David W. Scott,1,3 Shivanand Reddy,1
Anthony R.J. Phillips,1,2,4 Gordana Prijic,1 Chathurini Fernando,1 Shaoping Zhang,1,2
Ric Broadhurst,5 Phil L'Huillier,5 and Garth J.S. Cooper1,2,3,6
polypeptide (hA/hIAPP) into small soluble -sheet– containingoligomers is linked to islet -cell degeneration and the pathogen-esis of type 2 diabetes. Here, we used tetracycline, whichmodifies
der caused by defective action and/or secretionof insulin, which manifests with complications
whereby hA/hIAPP causes diabetes in hemizygous hA/hIAPP-
that ultimately cause most of its morbidity and
transgenic mice.
mortality. It is also an amyloidosis, since it is accompanied
by amyloid deposits in regions of tissue degeneration and
treated hemizygous hA/hIAPP transgenic mice with oral tetracy-
-cell loss in the islets of Langerhans (1,2). These deposits
cline to determine its effects on rates of diabetes initiation,
(3) comprise mainly fibrillar aggregates of a 37–amino acid
progression, and survival.
monomer, human amylin (hA)/islet amyloid polypeptide
RESULTS—Homozygous mice developed severe spontaneous
(hIAPP) (4,5) (the term hA/hIAPP has been used to reflect
diabetes due to islet -cell loss. Hemizygous transgenic animals
the two names commonly used for this peptide hormone),
also developed spontaneous diabetes, although severity was less
which is secreted from the -cells.
and progression rates slower. Pathogenesis was characterized by
Islet amyloid is associated with substantial reductions in
initial islet -cell dysfunction followed by progressive -cell loss.
relative -cell mass in type 2 diabetes (on average ⬃60%),
Islet amyloid was absent from hemizygous animals with early-
probably due to increased apoptosis compared with obese
onset diabetes and correlated positively with longevity. Somelong-lived nondiabetic hemizygous animals also had large islet-
and lean nondiabetic humans (2). An inability to adap-
amyloid areas, showing that amyloid itself was not intrinsically
tively compensate -cell mass in type 2 diabetes has been
cytotoxic. Administration of tetracycline dose-dependently ame-
postulated to lead to or cause an absolute insulin defi-
liorated hyperglycemia and polydipsia, delayed rates of diabetes
ciency over time with a resulting requirement for insulin
initiation and progression, and increased longevity compared
replacement therapy in affected subjects (1).
with water-treated controls.
Several lines of evidence now provide compelling sup-
CONCLUSIONS—This is the first report to show that treating
port for the idea that processes associated with hA/hIAPP
hA/hIAPP transgenic mice with a modifier of hA/hIAPP misfold-
aggregation contribute to -cell degeneration. First, in
ing can ameliorate their diabetic phenotype. Fibrillar amyloid
vitro studies with synthetic hA/hIAPP preparations show
was neither necessary nor sufficient to cause diabetes and indeed
that fibrillar structures are generated spontaneously
was positively correlated with longevity therein, whereas early-
through self-association of monomers into protofibrils and
to mid-stage diabetes was associated with islet -cell dysfunction
higher-order fibrils (6). Cytotoxic hA/hIAPP preparations
followed by -cell loss. Interventions capable of suppressingmisfolding in soluble hA/hIAPP oligomers rather than mature
contain few preformed fibrils but undergo time-dependent
fibrils may have potential for treating or preventing type 2
aggregation into soluble -conformers (7). -Cell toxicity
diabetes.
Diabetes 59:161–171, 2010
evoked by aggregating extracellular hA/hIAPP occursthrough an apoptotic mechanism (8,9) mediated via apathway comprising initial activation of a membrane-
From the 1School of Biological Sciences, Faculty of Medical and Health
bound Fas/FasL/FADD/caspase-8 complex (10) followed
Sciences, University of Auckland, Auckland, New Zealand; the 2Maurice
by a three-pronged downstream cascade comprising
Wilkins Centre for Molecular Biodiscovery, Faculty of Science, University
c-Jun NH -terminal protein kinase 1/cJun (11), activat-
of Auckland, Auckland, New Zealand; the 3Department of Medicine, Facultyof Medical and Health Sciences, University of Auckland, Auckland, New
ing transcription factor 2/p38 mitogen-activated protein
Zealand; the 4Department of Surgery, Faculty of Medical and Health
kinase (12), and p53/p21WAF1/CIP1 (9) that leads ulti-
Sciences, University of Auckland, Auckland, New Zealand; 5AgResearch,
mately to activation of caspase-3 (13). In addition,
Ruakura, Hamilton, New Zealand; and the 6Department of Pharmacology,Medical Sciences Division, University of Oxford, Oxford, U.K.
parallel amylin-mediated activation of endoplasmic re-
Corresponding author: Garth J.S. Cooper,
[email protected].
ticulum stress–related pathways may contribute to islet
Received 15 April 2009 and accepted 4 September 2009. Published ahead of
-cell degeneration (14).
print at http://diabetes.diabetesjournals.org on 30 September 2009. DOI:10.2337/db09-0548.
Second, associations between hA/hIAPP aggregation
J.F.A., K.M.L., and D.W.S. contributed equally to this article.
and decreased -cell mass have been reported from in vivo
2010 by the American Diabetes Association. Readers may use this article as
studies (15–19) in several murine transgenic models of
long as the work is properly cited, the use is educational and not for profit,
hA/hIAPP-mediated diabetes. By contrast, mA/mIAPP mol-
and the work is not altered. See http://creativecommons.org/licenses/by-nc-nd/3.0/ for details.
ecules do not aggregate, so diabetic phenotypes in hA/
The costs of publication of this article were defrayed in part by the payment of page
hIAPP transgenic mice develop in a background devoid of
charges. This article must therefore be hereby marked "advertisement" in accordancewith 18 U.S.C. Section 1734 solely to indicate this fact.
amyloid formed by mA/mIAPP. Obese hA/hIAPP trans-
DIABETES, VOL. 59, JANUARY 2010
TETRACYCLINE CURBS DIABETES IN hA/hIAPP MICE
genic mice have been reported to replicate pathological
(16 –18 h) with free access to water. Glucose was administered (1 mg
findings of human type 2 diabetes, showing nonketotic
glucose/g body wt) followed by tail blood sampling.
Blood and tissue extraction for hormone measurements. Cardiac punc-
hyperglycemia, amyloid deposition, and decreased -cell
ture blood (EDTA) was separated (3,000
g, 4°C, 15 min). Pancreata were
mass, possibly via increased apoptosis (18).
excised and snap frozen (liquid nitrogen) and peptides extracted (homogeni-
These data support a hypothesis that hA/hIAPP aggre-
zation, acid/ethanol) (27).
gation could mediate -cell failure in type 2 diabetes.
Hormone measurements. Murine insulin was determined in plasma, serum,
However, the significance of mature amyloid fibrils in the
or pancreatic extracts by Ultrasensitive Mouse Insulin enzyme-linked immu-nosorbent assay (ELISA) (Mercodia, Uppsala, Sweden) or rat/mouse insulin
pathogenesis of this process is still uncertain, as is
ELISA (Linco). Pancreatic extracts were diluted (1:500 and 1:5,000) in PBS
whether hA/hIAPP-mediated cytotoxicity can be abro-
(pH 7.4). Plasma hA/hIAPP was determined by ELISA (Linco) in plasma and
gated by in vivo treatment with amylin-binding com-
pounds. Peptide-based analogs that bind amyloid-forming
structural motifs within hA/hIAPP have reportedly inhib-
Pancreatic histochemistry and quantitative islet histomorphometry.
Pancreatic tissue was paraffin embedded, serially sectioned (5 m), and
ited aggregation of synthetic hormone in vitro, with con-
stained with hematoxylin and eosin. On adjacent sections, Congo red (1%;
comitant suppression of cytotoxicity in cultured -cells
15–20 min, then saturated lithium carbonate, 30 s) and hematoxylin staining
(20), but in vivo efficacy of this therapeutic approach has
were used to measure frequency and extent of islet amyloid (polarization
yet to be reported.
microscopy). Islet morphology and amyloid content were analyzed by a
Here, we have generated lines of hA/hIAPP transgenic
single-blinded histologist who scored nine or more islets/animal. Pancreata
mice that spontaneously develop diabetes. Phenotypes
were sectioned at sufficient levels (ⱖ200 m apart) to ensure analysis of ⱖ9(but generally ⬃20 –30) distinct islets/animal.
vary from early-onset diabetes without microscopically
Immunohistochemistry. Representative sections were serially incubated
detectable amylin aggregates to late-onset diabetes with
with guinea pig anti-insulin serum, donkey anti-guinea pig IgG–fluorescein
microscopic amyloid deposits. Diabetes pathogenesis and
isothiocyanate, rabbit anti-glucagon, and donkey anti-rabbit IgG–Texas Red
progression in hemizygous animals occurs primarily
then counterstained with Congo red and reimaged to quantitate islet amyloid,
through islet -cell dysfunction with subsequent -cell
morphology, and insulin and glucagon cells. Alternatively, sections wereincubated with combined rabbit anti-glucagon and anti-somatostatin and
loss. Both onset and progression were significantly inhib-
imaged by indirect avidin-biotin-peroxidase.
ited by chronic treatment with tetracycline, an antibiotic
Chronic oral administration of tetracycline. Tetracycline was adminis-
that interacts with aggregates of proteins implicated in
tered orally via the drinking water in light-proof bottles either from the time
amyloid-related diseases (21–23).
of weaning (21 days of age) or from diabetes onset in different studies, at finalconcentrations (0.03 mg/ml or 0.5 mg/ml in water, 18 mol 䡠 l⍀⫺1 䡠 cm⫺1, milliQ;Millipore) and fresh solutions constituted weekly. Polydipsia was defined asfluid intake exceeding twice the average daily intake of the average nondia-
RESEARCH DESIGN AND METHODS
betic adult mouse. Controls comprising hemizygous male mice and nontrans-
Ethics approval. Experimental protocols were approved by the University of
genic littermates received milliQ water alone.
Auckland Animal Ethics Committee and performed in accordance with the
Statistical analysis. Data were analyzed using GraphPad Prism 4 (GraphPad
New Zealand Animal Welfare Act (1999).
Software, San Diego, CA) and diabetes onset and survival using the Mantel-
Materials. Chemicals and kits were from Roche Applied Biosciences, Invitro-
Haenszel log-rank test and the Gehan-Breslow-Wilcoxon test. Correlation
gen, Life Technologies/BRL, or Sigma and were of analytical grade or better,
analyses were performed using Pearson correlation with two-tailed hypothe-
unless stated otherwise.
ses. Descriptive variables were contrasted by one-way ANOVA with Tukey-
Generation of hA/hIAPP transgenic mice. The hA/hIAPP transgene (sup-
Kramer post hoc tests. Blood glucose and fluid intake values were contrasted
plemental Fig. S1A [available at http://diabetes.diabetesjournals.org/cgi/
using mixed models fitted by restricted maximum likelihood (JMP 5.1; SAS
content/full/db09-0548/DC1]) was constructed from PCR-derived fragments
Institute).
P values of ⬍0.05 were considered significant.
using the following primer pairs: RIP5 (GAAAGACTCGAGGATCCCCCAACCAC) and RIP3 (CAGGGCCATGGTGGAACAATGACC), hAMY5 (GAAGC
Human amylin/hIAPP transgenic mice spontaneously
GAAA) and hALB3 (CAACCTCAAGCTTGTCTGGGCAAGGG), hGAPDH5
developed diabetes. Transgenic animals were generated
within a FVB/N background (supplmentary Fig. S1). One
GTCT-AGACTTCCTCCACCTGTCA). It was introduced into the genome by
transgenic line, hereinafter designated as Line 13, had
pronuclear injection (24).
integrated transgene copy numbers of 36 ⫾ 7 and 76 ⫾ 2
Northern analysis. RNA was extracted from liquid nitrogen snap-frozen
tissues using QIAGEN RNeasy Midi Kits according with the rotor-stator tissue
for hemizygous and homozygous animals, respectively
disruption (Ultra-Turrax T8; IKA-Werke, Germany). Total RNA (20 g) was
(data not shown).
denatured and transferred to nylon membranes with 20⫻ SSC (25) and
Diabetes developed spontaneously and reproducibly in
hybridized to 32P-labeled probes (26) at 65°C overnight. Intensities of mRNA
both homozygous and hemizygous Line 13 male and
bands corresponding to hA/hIAPP and mA/mIAPP were not directly compared
female mice, while nontransgenic littermates did not de-
as experimental conditions differed (supplemental Fig. S1
A and
B).
velop hyperglycemia (Fig. 1). All subsequent studies were
Animal studies. Mice were fed ad libitum with Diet 86 (Tegel NRM,
Auckland, Zealand), a dry-pelleted natural-ingredient diet with 3% fat. Blood
performed in male animals (28,29). In homozygous mice,
glucose levels were determined in tail vein blood (Advantage II; Roche) and
diabetes developed in 100% of animals (
n ⫽ 6) by 41 days
diabetes defined when concentrations were ⬎11 mmol/l on two consecutive
and all were dead by 91 days (Fig. 1
A). By comparison,
weekly readings. For survival studies, we applied an agreed euthanasia
median values for time to diabetes onset and lifespan
surrogate end point for death, consistent with current ethical practice.
within the hemizygous group were longer (175 and 272
Euthanasia was performed in the following circumstances: after 20% loss of
days, respectively) compared with homozygous animals
maximum body weight, if significant lethargy developed, following loss ofexploratory behavior with increasing relative immobility, failure to groom,
(35 and 63 days, respectively). In addition, a second,
or any other signs of overt distress. Infection or malignancy were also
independent line (designated Line 20) generated with the
accepted as indications for euthanasia.
same construct and methods also developed spontaneous
Intraperitoneal insulin and glucose tolerance tests. Mice undergoing
diabetes (Fig. 1
A). Thus, the diabetic phenotype was not
intraperitoneal insulin tolerance tests (ITTs) were typically fasted for 6 h.
an artifact arising from insertional mutagenesis.
Thereafter, actrapid (0.5 mIU/g body wt; Novo Nordisk) was injected intra-
Intraperitoneal ITTs performed at 85 days showed that
peritoneally in conscious animals (29 G needle). Glucose levels were mea-sured
hemizygous mice developed spontaneous diabetes within
intraperitoneal glucose tolerance tests (GTTs), mice were overnight fasted
a background of normal insulin sensitivity (Fig. 1
B). Time
DIABETES, VOL. 59, JANUARY 2010
J.F. AITKEN AND ASSOCIATES
100 200 300 400 500 600
Diabetes to death (days)
Percent amyloid/islet area
Diabetes onset (days)
FIG. 1. Characterization of spontaneous diabetes in homozygous and hemizygous hA/hIAPP transgenic mice. A: Survival curves for two distinct
lines of hA/hIAPP transgenic mice that were generated by separate injections of the same construct in the FVB/N background: homozygous line
13 (‚
, n ⴝ
6); hemizygous line 13 (E
, n ⴝ
14); and hemizygous line 20 (ƒ
, n ⴝ
7). Closed symbols represent corresponding nontransgenic
littermates. B: Intraperitoneal insulin tolerance tests in hemizygous line 13 mice (E
, n ⴝ
11) versus nontransgenic littermates (F
, n ⴝ
6) at 85
days of age. Animals were fasted overnight (17 h) then injected with Actrapid (0.75 mIU/g body wt). Data are means ⴞ
SE. C: Correlational
analysis between time to diabetes onset and time of diabetes onset to death in hemizygous line 13 mice (P ⴝ
NS, n ⴝ
19). D: Amyloid areas were
positively correlated with lifespan in diabetic water-treated (E
, dashed line; n ⴝ
14, R2 ⴝ
0.78, P < 0.001) and nondiabetic hemizygous animals
(half-closed circles, n ⴝ
4, R2 ⴝ
0.95, P < 0.05). Arrows indicate animals with representative islets shown in Fig. 2.
to diabetes onset was not correlated with the period
These data indicated that an ⬃2- to 2.5-fold increase in
between diabetes onset and death, showing that age did
expression of hA/hIAPP above endogenous and nonamy-
not influence the rate of progression of diabetes once it
loidogenic mA/mIAPP in hemizygous animals was suffi-
had begun (Fig. 1
C).
cient to evoke diabetes.
Severity of diabetes progression was directly related
Amyloid deposition was dissociated from diabetes
to pancreatic expression of amyloidogenic human
and positively correlated with lifespan. To investigate
amylin. In homozygous mice, at an age where they were
the relationship between amyloid deposition and diabetic
glucose intolerant (30 days; intraperitoneal GTTs not
phenotype, islet amyloid area was quantitated microscopi-
shown), plasma hA/hIAPP concentrations were 1.8-fold
cally. Of the diabetic hemizygous animal cohort examined,
higher than in hemizygous animals and ⬃3.7-fold above
there was a positive correlation between amyloid content
nontransgenic control mA/mIAPP concentrations (Table
and lifespan in animals that had survived ⬎220 days (44%
1). Normal plasma insulin was maintained in homozygous
frequency,
P ⬍ 0.001) (Fig. 1
D and Fig. 2,
top two panels).
mice at 30 days despite a 72% reduction in pancreatic
However, amyloid was rarely observed in terminally diabetic
insulin content. Thus, at a time point similar to the median
animals killed at lifespans ⬍220 days (49% frequency). As
time to diabetes (35 days), substantial pancreatic insulin
expected, amyloid deposition was never observed in the
depletion had occurred in homozygous transgenic mice
islets of nontransgenic animals (Fig. 2,
middle panel). Inter-
before a reduction in circulating insulin concentrations
estingly, some hemizygous animals remained nondiabetic
was detectable. Interestingly, and by contrast, pancreatic
(7% frequency) but showed the presence of islet amyloid
insulin concentrations in hemizygous animals were rela-
(Fig. 1
D and Fig. 2,
second to bottom panel) with a similar
tively unchanged at a time point (183 days) similar to the
relationship between amyloid content and lifespan as ob-
respective median onset time to diabetes (175 days).
served for diabetic hemizygous animals (
P ⬍ 0.05). Amyloid
DIABETES, VOL. 59, JANUARY 2010
TETRACYCLINE CURBS DIABETES IN hA/hIAPP MICE
TABLE 1Hormone concentrations in plasma and pancreatic tissue from fed wild-type (⫺/⫺) and hemizygous (⫹/⫺) and homozygous (⫹/⫹)hA/hIAPP transgenic mice at 30 days and 4 months of age
Pancreas (pmol/mg)
221 ⫾ 55‡ (5)
1,261 ⫾ 142 (8)
1,028 ⫾ 121(16)
Data are means ⫾ SE. The ELISA assay used to measure hA/hIAPP has a reported ⱕ1% cross-reactivity with human insulin, glucagon,glucagon-like peptide-1, pancreatic polypeptide, calcitonin, calcitonin gene–related peptide, and adrenomedullin and does not detectablycross-react with mA/mIAPP (39). The in-house radioimmunoassay used to measure mA/mIAPP had 7% cross-reactivity with hA/hIAPP andthus was used only in nontransgenic littermates. *Two pooled analyses representing a total of five animals. †
P ⬍ 0.05, two-tailed
t test. ‡
P ⬍0.001 vs. both groups, one-way ANOVA with Tukey's post hoc test. §
P ⬍ 0.01, two-tailed
t test. —, not determined; ND, not detected.
was also absent from all terminally diabetic homozygous
not significant by the Mantel-Haenszel log-rank test. As
Line 13 mice analyzed (Fig. 2,
bottom panel).
proportional hazards might not apply in this case, however,
End-stage diabetes was characterized by selective
the data were reanalyzed using the Gehan-Breslow-Wilcoxon
loss of 
-cells from the islets. Immunohistochemistry of
test, which does not rely on this assumption. The latter
pancreatic islet sections using antisera to stain both ␣- and
analysis did reveal a significant difference in median time to
␦-cells showed that almost all the islet cells in terminally diabetes onset (
P ⬍ 0.05). Following diabetes onset, progres-diabetic homozygous and hemizygous mice were non–-
sive hyperglycemia resulted in the development of polydipsia
cells (Fig. 2). Glucagon staining also revealed a change in
at blood glucose concentrations of ⬃25 mmol/l (Fig. 3
A and
␣-cell distribution from the physiological location at the
B). Since the upper limit of blood glucose measurement hereislet periphery to a more dispersed distribution throughout
was 33 mmol/l, the maximum reported values are lower-limit
diabetic islets, a substantive change in histomorphology
estimates of actual glucose concentrations in advanced
(Fig. 2). Taken together, these findings show that end-
stage diabetes in both homozygous and hemizygous ani-
Most importantly, in tetracycline-treated hemizygous
mals was associated with islet -cell loss and altered islet
animals, there was a significant delay in disease progres-
architecture. Consistent with these observations, plasma
sion following onset of hyperglycemia, as measured by
insulin and amylin concentrations in terminally killed
retardation in the rates of both blood glucose elevation
homozygous and hemizygous animals were below the
(Fig. 3
A,
P ⬍ 0.01) and fluid intake (Fig. 3
B,
P ⬍ 0.01)
limits of detection (data not shown). We also performed
compared with water-treated transgenic animals. Tetracy-
studies for terminal deoxynucleotidyl transferase-medi-
cline had no effects on either blood glucose concentrations
ated dUTP nick-end labeling and caspase-3 activation in
or fluid intake in nontransgenic littermates over the same
islets from animals at different stages of diabetes, but rates
time period. We did not quantitate blood tetracycline
were not significantly different between those from dia-
levels; however, assuming a similar bioavailability to oral
betic and control (nondiabetic) animals (results not
doxycycline or minocycline administered to mice (32), we
shown). These findings are consistent with the observa-
estimate that plasma tetracycline concentrations during
tion that although apoptosis is widespread in biology,
the polydipsic phase could reach low micromolar levels.
dying cells are seldom seen in situ because of their rapid
Survival analysis (Fig. 3
C) showed that tetracycline-
clearance by phagocytosis (30). They do not, however,
treated transgenic animals had a significantly increased
exclude the possibility that the sensitivity of the assays
median survival (34%,
P ⬍ 0.05) for the period from
used may not have been sufficient to detect any differences
diabetes onset to death (155 days,
n ⫽ 17) compared with
in numbers of rare apoptotic cells.
the water-treated control group (116 days,
n ⫽ 19).
Chronic oral administration of tetracycline (0.03
Examination of pancreata from terminally killed tetra-
mg/ml drinking water) from weaning improved glyce-
cycline-treated animals also showed a positive correlation
mic control and lifespan in hemizygous mice (study
between islet amyloid content and lifespan (Fig. 3
D,
P ⬍
1). We previously reported that tetracycline interacts with
0.001,
n ⫽ 12,
R2 ⫽ 0.91). This finding was similar to that
amylin fibrils based on evidence from thioflavin-T fluores-
observed for water-treated terminally diabetic hemizygous
cence and radioprecipitation assays (31). Here, we provide
mice wherein the corresponding regression slope was not
additional in vitro evidence for specific interactions between
significantly different (Fig.
1D). Interestingly, of all the
tetracycline and hA/hIAPP by circular dichroism spectros-
pancreata examined in this study the highest amyloid
copy (supplementary Fig. S2). In addition, to investigate
content occurred in a tetracycline-treated mouse, a finding
whether tetracycline might modulate indexes of aggregation-
consistent with the correspondingly increased lifespan
evoked diabetes in vivo, hemizygous mice were randomly
observed in this group.
assigned to groups that were administered either water alone
Tetracycline (0.5 mg/ml drinking water) improved
(control) or water containing 0.03 mg/ml tetracycline. Trans-
glucose tolerance and delayed diabetes onset in
genic animals treated with tetracycline (0.03 mg/ml) devel-
hemizygous animals (study 2). To investigate whether
oped diabetes with an apparently earlier median time to
the observed antidiabetes effects of tetracycline might be
onset (85 days,
n ⫽ 17) compared with those administered
attributable to potential indirect insulin-sensitizing effects,
water only (121 days,
n ⫽ 20), which is a difference that was
parallel GTTs and ITTs were performed in independent
DIABETES, VOL. 59, JANUARY 2010
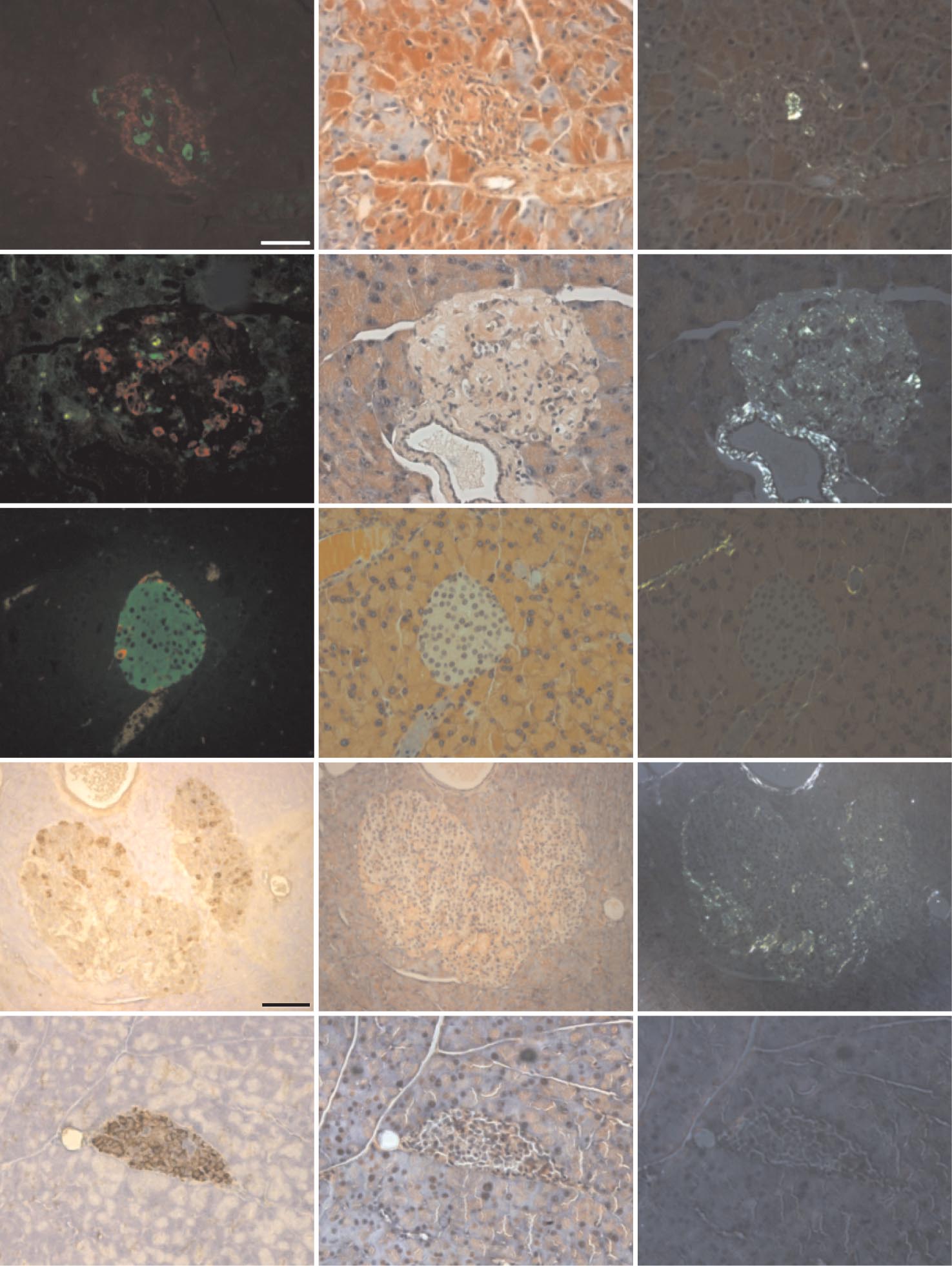
J.F. AITKEN AND ASSOCIATES
FIG. 2. Amyloid visualized by light microscopy was dissociable from occurrence of diabetes in hemizygous hA/hIAPP transgenic mice.
Photomicrographs show serial pancreatic islet sections from nontransgenic and human amylin transgenic animals. Left photomicrographs from
top three panels show insulin (green) and glucagon (red) immunoreactivity. Bottom two left photomicrographs show islet sections incubated
with antisera to somatostatin and glucagon revealing brown cytoplasmic staining. Middle and right panels show corresponding light- and
polarized-microscopic field views of adjacent islet sections stained with Congo red. Amyloid birefringence is apple green, whereas that
corresponding to collagen is silvery. The scale bar (50 m) shown in top left photomicrograph applies to all images except for those
corresponding to the 600-day nondiabetic hemizygous mouse (second to bottom row), which represents 100 m. (A high-quality color digital
representation of this figure is available in the online issue.)
cohorts of animals with or without equivalent tetracycline
and 60 days treatment (Fig. 4C). Survival analysis indicated a
treatment (Fig. 4). Hemizygous mice were administered
trend toward delayed diabetes onset in the tetracycline-
tetracycline in their drinking water from 30 days post-
treated group (P ⫽ 0.06, data not shown).
weaning at 0.5 mg/ml to approximate the increased drug
In a parallel study, a second independent hemizygous
intake observed during polydipsia in the previous study.
cohort was treated with or without tetracycline immediately
GTTs performed on a hemizygous cohort revealed no base-
postweaning. ITTs performed after 60 and 90 days treatment
line differences (0 days treatment; Fig. 4A) but significantly
showed that improved glucose tolerance was not due to any
improved glucose tolerance in the tetracycline-treated mice
insulin-sensitizing effect of tetracycline (Fig. 4D and E).
compared with water-treated controls at 30 days (Fig. 4B)
Tetracycline treatment also significantly delayed the median
DIABETES, VOL. 59, JANUARY 2010
TETRACYCLINE CURBS DIABETES IN hA/hIAPP MICE
Blood glucose (mM)
Fluid intakee (m/day)
Weeks from diabetes onset
Weeks from diabetes onset
Percent amyloid/islet area
0 200 400 600 800
Weeks from diabetes onset
FIG. 3. Chronic administration of tetracycline (0.03 mg/ml of drinking water) in hemizygous mice from the time of weaning ameliorated diabetes
and prolonged survival. Weekly blood glucose values (A) and fluid intake (B) in tetracycline-treated (䡺) versus water-treated (control) (E)
animals. Each point represents the mean ⴞ SE of values derived from n ⴝ 7–20 animals; (F, f): values for corresponding nontransgenic
littermates. Mean drug intake (mg 䡠 kgⴚ1 䡠 dayⴚ1, mean ⴞ SD) per animal was calculated from weekly fluid intake and weight measurements over
the phases of pre-diabetes (5.4 ⴞ 1, n ⴝ 13), diabetes to polydipsia (4.4 ⴞ 0.7, n ⴝ 16), and polydipsia to death (44 ⴞ 13, n ⴝ 15). Body weights
at diabetes onset were also not significantly different between the control and drug-treated groups. Following onset of polydipsia, the
tetracycline concentrations in the water of matched nontransgenic littermates were increased proportionately to maintain matched drug intake,
and no adverse effects on fluid palatability were observed. Within the transgenic control group, 2 of 22 mice that spontaneously developed
diabetes were omitted from the final analysis due to development of an eye infection and a tumor, respectively, whereas no animals were excluded
from the tetracycline-treated transgenic group. C: Percent survival from diabetes onset (䡺, n ⴝ 17; E, n ⴝ 19). **P < 0.01; *P < 0.05. D:
Relationship between total area of amyloid deposits and lifespan in tetracycline-treated mice (P < 0.001, n ⴝ 12, R2 ⴝ 0.91).
time to diabetes onset in this cohort of mice as compared
cycline-treated groups was dosage dependent with signif-
with the corresponding water-treated control group (Fig. 4F,
icant alleviation of hyperglycemia (P ⬍ 0.01; Fig. 5A) and
98 vs. 66 days, P ⬍ 0.05). These studies showed that
corresponding fluid intake (P ⬍ 0.05; Fig. 5B) in the 0.5
improved glucose tolerance and delayed onset of diabetes in
mg/ml tetracycline–treated group compared with the wa-
tetracycline-treated hemizygous mice were not due to ex-
ter-treated controls. The 0.5 mg/ml tetracycline–treated
trapancreatic insulin-sensitizing actions or other putative
group had a 254% increase in median survival from diabe-
systemic effects of tetracycline on glucose homeostasis.
tes onset to death compared with the water-treated con-
Amelioration of diabetes and increased lifespan by
trol group (208 vs. 82 days, respectively, P ⬍ 0.05; Fig. 5C),
tetracycline were dosage dependent (study 3). To
and there was a significant trend to dosage dependency
replicate and extend our previous findings, in particular
among the three groups (P ⬍ 0.05).
with regard to dosage-related effects, further groups of
Diabetes pathogenesis in hemizygous mice occurred
hemizygous animals that had been administered water
through islet -cell dysfunction followed by -cell
from the time of weaning were randomly assigned at the
loss. To examine more precisely the effects of tetracycline
time of disease onset to one of the following groups:
treatment on islet -cell mass, pancreatic islets were
water-only control (n ⫽ 12), water containing 0.03 mg/ml
examined in a further independent cohort of diabetic mice
tetracycline (n ⫽ 12) (study 3a, Fig. 5), or water containing
studied at 6 weeks after disease onset. Hemizygous ani-
0.5 mg/ml tetracycline (n ⫽ 10) (study 3b, Fig. 5). The
mals were randomly assigned at disease onset to one of
extent of suppression of disease progression in the tetra-
two groups: either water-treated control or treatment with
DIABETES, VOL. 59, JANUARY 2010
J.F. AITKEN AND ASSOCIATES
Blood Glucose (mM)
Starting Blood Glucose (%)
Blood Glucose (mM)
Starting Blood Glucose (%)
Percent Diabetic 20
Blood Glucose (mM 2
Time to diabetes onset (days)
FIG. 4. Tetracycline (0.5 mg/ml of drinking water) improved glucose tolerance and delayed diabetes onset. Glucose tolerance tests were compared
between tetracycline-treated (〫) and control water-treated (E) hemizygous mice at baseline (time ⴝ 0) (A) and after 30 (B) and 60 (C) days
treatment. Statistical analysis by two-way ANOVA showed that curves corresponding to tetracycline-treated and control animals differed
significantly after both 30 (P < 0.01) and 60 (P < 0.001) days treatment. Insulin tolerance tests were carried out in a second cohort of animals
after 60 (D) and 90 (E) days equivalent tetracycline treatment. Each point represents the means ⴞ SE of values derived from n ⴝ 12–25 animals.
F: Survival analysis for the second cohort showed significantly delayed diabetes onset in tetracycline-treated mice (n ⴝ 18) versus the
water-treated control group (n ⴝ 20). ***P < 0.01; *P < 0.05. In other experiments with nontransgenic littermates, glucose tolerance and insulin
tolerance tests performed at 0, 60, and 90 days treatment showed that tetracycline administration did not intrinsically affect glucose tolerance
or insulin sensitivity (not shown).
DIABETES, VOL. 59, JANUARY 2010
TETRACYCLINE CURBS DIABETES IN hA/hIAPP MICE
Blood Glucose (mM)
Fluid Intake (ml/day) 10
Weeks from diabetes onset
FIG. 5. Suppression of diabetes progression by tetracycline treatment was dosage dependent. Tetracycline was administered to hemizygous
animals from the time of diabetes onset at concentrations of either 0.03 mg/ml (䡺) or 0.5 mg/ml (〫) of drinking water and results compared with
those in water-treated control animals (E). Weekly blood glucose values (A) and fluid intakes (B) are shown. Data are means ⴞ SE of values
derived from 6 to 12 animals per point. **P < 0.01; *P < 0.05 for the 0.5 mg/ml tetracycline-treated compared with water-treated control
hemizygous animals. C: Percent survival shown is in animals treated with 0.03 mg/ml tetracycline (n ⴝ 12, P ⴝ 0.08) or 0.5 mg/ml tetracycline (n ⴝ
10, P < 0.05) compared with those receiving water only (n ⴝ 12). There was no significant difference in bodyweight at diabetes onset or in the
median time to diabetes onset across all three diabetic groups, nor did tetracycline exert effects on bodyweight as indicated by similar growth
rates of tetracycline-treated and nontransgenic littermates. No animals were excluded from these analyses.
0.5 mg/ml tetracycline, as was described above for study
6I). These findings showed that insulin area was main-
3b. Interestingly, visual inspection of islets from hemizy-
tained within the islets during early- and mid-stage diabe-
gous mice with early- to mid-stage diabetes (Fig. 6A)
tes in hemizygous transgenic mice with no substantial
showed they were structurally well preserved, with insulin
reductions in pancreatic insulin content or serum insulin
areas similar to those of matched nontransgenic litter-
concentrations. They are consistent with islet -cell loss
mates (Fig. 6B). Also, quantitative analyses of islet areas
as a correlate of later-stage, more severe diabetes (blood
revealed no significant between-group difference at this
glucose ⬎15 mmol/l).
time-point (Fig. 6C). By contrast, animals with advanceddiabetes had comparatively elevated blood glucose con-centrations and reduced insulin areas, consistent with islet
-cell loss (Fig. 6D).
Our findings demonstrate that the amyloid deposits found
These qualitative observations were confirmed in an
in the pancreatic islets of hA/hIAPP transgenic mice are
exhaustive, quantitative blinded comparison of insulin
not intrinsically cytotoxic, an observation consistent with
areas of 9 –30 islets from individual tetracycline-treated
the reported occurrence of islet amyloid in nondiabetic
(Fig. 6E) and water-treated (Fig. 6F) hemizygous mice.
humans (2). Perhaps most notably, amyloid deposition
Although there was no significant difference in mean
was not observed in homozygous animals with severe,
insulin area–to–islet area ratios between the two groups
early-onset diabetes but was present in both diabetic and
(75 ⫾ 4%, n ⫽ 8 vs. 71 ⫾ 7%, n ⫽ 12, respectively), there
nondiabetic hemizygous animals, where it was positively
was a significant difference in their variance (F test, P ⫽
rather than negatively correlated with lifespan.
0.029), pointing to a between-group difference. This finding
In homozygous transgenic animals, the lack of visible
was extended in further correlational analyses, which
amyloid deposition coupled with the higher intrinsic hA/
showed that there were significant inverse relationships
hIAPP expression and significantly depleted pancreatic
between blood glucose concentrations and mean islet
insulin concentrations at the median time to diabetes
insulin area in each group (Fig. 6G). Here, there was a
onset were all consistent with islet -cell loss as the major
significant difference in the regression slopes between
diabetogenic mechanism. Evidence from other hA/hIAPP
tetracycline- and water-treated mice (Fig. 6G). In particu-
transgenic murine lines has pointed to a role for soluble
lar, insulin area–to–islet area ratios in diabetic hemizygous
oligomers in the increased frequency of -cell apoptosis in
mice, wherein blood glucose concentrations were ⬍15
late-stage diabetes (15,18,19). The corresponding lack of
mmol/l, were comparable not only between tetracycline
amyloid in our homozygous mice is consistent with this
and water-treated mice but also with values in nontrans-
pathogenic mechanism and supports a growing body of
genic nondiabetic mice, for example as represented by
experimental data consistent with the hypothesis that
mouse no. 352 (Fig. 6G, ⽧).
cytotoxic effects of prefibrillar aggregates of other amyloi-
We quantified pancreatic and serum insulin concentra-
dogenic proteins, such as -amyloid and ␣-synuclein, can
tions by ELISA in further separate groups of hemizygous
elicit cell death (33–37).
mice 6 weeks after the onset of diabetes. Interestingly,
Our findings also show that tetracycline can partially
although some reduction in total pancreatic insulin con-
suppress the progression of diabetes in hemizygous ani-
tent was evident in some animals, there was no apparent
mals. In study 1, it slowed the rate of deterioration in
relationship between insulin content and blood glucose
blood glucose and polydipsia after diabetes onset, com-
concentrations (Fig. 6H). Similarly, there was no evident
pared with matched control animals, which translated into
reduction in serum insulin concentrations, and indeed
a 34% increase in median survival. By contrast, tetracy-
some hemizygous animals displayed comparatively high
cline did not delay diabetes onset in this study, although
serum insulin concentrations during early diabetes (Fig.
the lack of an effect may simply have reflected the
DIABETES, VOL. 59, JANUARY 2010
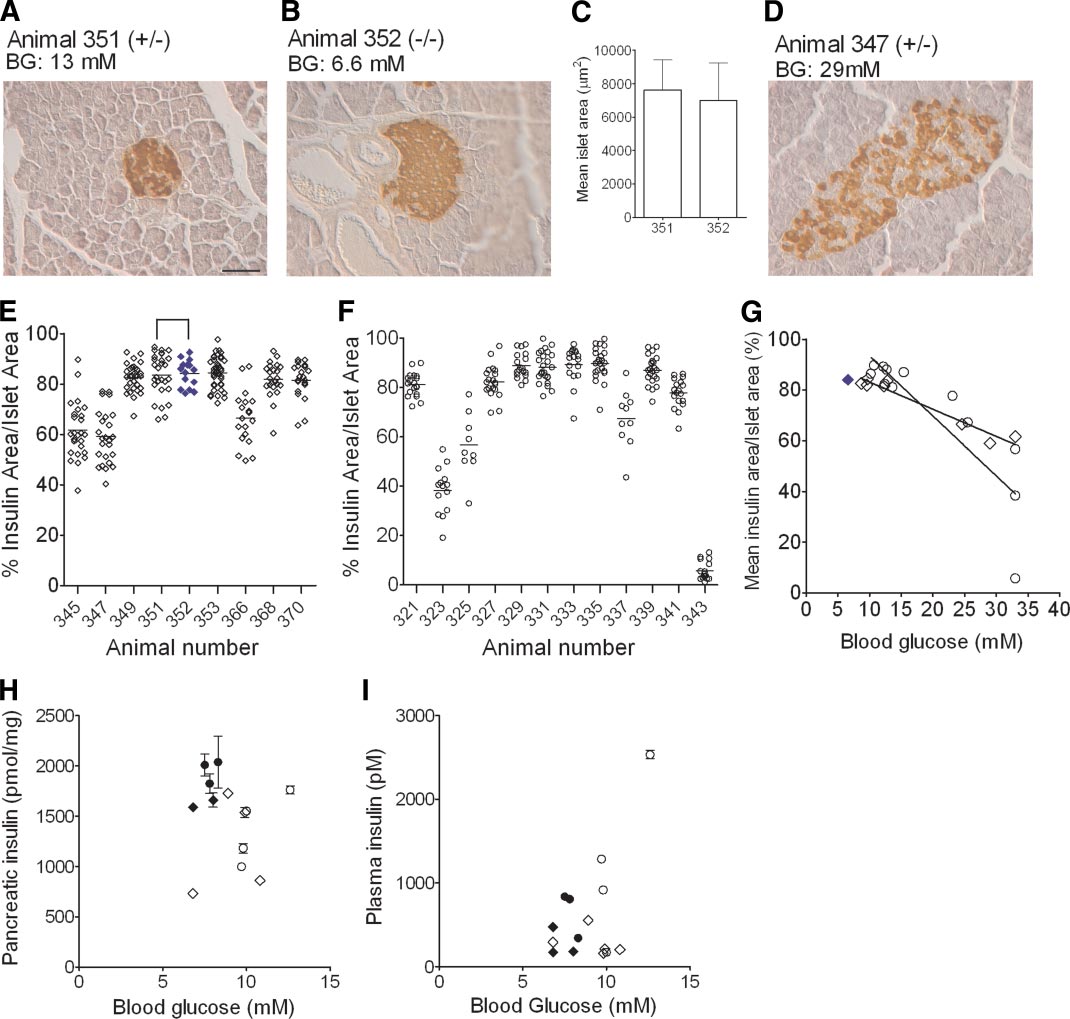
J.F. AITKEN AND ASSOCIATES
FIG. 6. Quantitative islet immunohistochemistry indicates that -cell dysfunction preceded -cell loss in diabetic hemizygous mice. Pancreatic
islets were analyzed by blinded insulin histochemistry in hemizygous and matched nondiabetic control mice at time points that corresponded to
6 weeks after diabetes onset in each transgenic mouse. A: Insulin staining in a representative islet from a tetracycline-treated (0.5 mg/ml)
transgenic mouse (animal no. 351, ⴙ/ⴚ). B: An islet from its corresponding matched nontransgenic control (animal no. 352, ⴚ/ⴚ). C: Islet areas
did not differ significantly between these two animals. By contrast, shown in D, is an islet from a hemizygous animal with markedly elevated blood
glucose accompanied by a decreased area of islet insulin staining. Scale bar represents 50 m. Quantitative analyses of insulin area–to–islet area
ratios from 9 to 30 islets per animal are shown in a series of individual tetracycline-treated (n ⴝ 9) (E) or water-treated (n ⴝ 12) (F) diabetic
hemizygous transgenic mice; each datum point represents the ratio from a single islet and horizontal lines are arithmetic means. For comparison,
islet analyses for animal no. 352, which is the tetracycline-treated nontransgenic and nondiabetic age-matched control for hemizygous animal no.
351, are shown in E as closed blue diamonds. G: Linear correlation analysis of the relationships between blood glucose values and insulin
area–to–islet area ratios were demonstrated in both groups (both P < 0.0001); there was also a significant difference between the slopes of the
curves in the two groups (P < 0.0001); the solid blue diamond corresponds to the nontransgenic animal whose islet was shown in B. Blood glucose
and pancreatic insulin content (H) and serum insulin concentrations (I) in animals (n ⴝ 3 replicates/mouse); these latter analyses were
performed in separate cohorts of water-treated (E) and tetracycline-treated (0.5 mg/ml, 〫) hemizygous mice studied at the time point 6 weeks
after diabetes onset. Data from matched nontransgenic and nondiabetic mice studied at the corresponding time point are indicated by closed
black symbols. Data in C, H, and I are means ⴞ SE. A, B, and D: BG, blood glucose. (A high-quality color digital representation of this figure is
available in the online issue.)
relatively low dosage administered during the pre-diabetic
weaning, significantly delayed disease onset and progres-
phase. The likelihood that this explanation is correct was
sion. The observed improvement in glucose tolerance in
confirmed in study 2, wherein tetracycline administration
tetracycline-treated hemizygous mice after 30 and 60 days
at the higher dosage of 0.5 mg/ml from 30 days after
of treatment is also consistent with observations from
DIABETES, VOL. 59, JANUARY 2010
TETRACYCLINE CURBS DIABETES IN hA/hIAPP MICE
study 3, where we again used the higher drug dosage (0.5
mechanism occurs by default in homozygous mice which
mg/ml) but with administration from the time of disease
possess higher intrinsic hA/hIAPP expression.
onset. In this study, tetracycline exerted a clear, dosage-
In summary, our findings show that islet -cell dysfunc-
dependent effect to delay the deterioration in blood glu-
tion and not mature extracellular amyloid is the underpin-
cose regulation and polydipsia, with the higher dosage
ning cause for diabetes pathogenesis in hemizygous hA/
causing an increase in median survival of 254% in the
hIAPP transgenic mice. Moreover, deposition of micro-
period following diabetes onset.
scopically visible amyloid was positively correlated with
We found no evidence that the antidiabetes effects
lifespan, showing that tissue hA/hIAPP deposits are not
evoked by tetracycline in the hemizygous mice were due
intrinsically cytotoxic. Treatment with an effective dosage
to any putative extrapancreatic effects, since ITTs re-
of tetracycline delayed the onset and impeded the progres-
vealed that it exerted no detectable systemic insulin-
sion of diabetes in hemizygous mice. Consequently, any
sensitizing effects in either hemizygous mice or their
intervention that allows progressive deposition of (appar-
nontransgenic littermates. Furthermore, tetracycline had
ently benign) islet amyloid through mechanisms that re-
no measurable effects on glucose regulation in nontrans-
duce the cytotoxicity of prefibrillar aggregates might be
genic littermates. When taken together with the histolog-
expected to prevent islet -cell degeneration.
ical analysis of islets 6 weeks after diabetes onset, thesedata point toward preservation of islet -cell function as
the mechanism underlying tetracycline's actions to delay
These studies were supported by the Endocore Research
onset and progression of diabetes, possibly through inter-
Trust, the University of Auckland Research Committee,
actions with soluble nascent prefibrillar aggregates.
the Maurice & Phyllis Paykel Trust, the Auckland Medical
Finally, our findings show that -cell dysfunction and
Research Foundation, and Lottery Health (New Zealand).
not -cell loss was responsible for the initial development
G.C. acknowledges support by program grants from the
of diabetes in these hemizygous mice. Compared with the
Foundation for Research, Science, and Technology and by
rapid onset and development of diabetes in homozygous
the Health Research Council of New Zealand.
animals, diabetes onset and progression were significantly
No potential conflicts of interest relevant to this article
more gradual in the hemizygous group. Strikingly, non-
were reported.
transgenic controls and hemizygous animals had similar
We thank John Todd, Cynthia Tse, and John Scott for
total pancreatic insulin content at the median time of
helpful discussions and gratefully acknowledge Xiaoling
diabetes onset. Significantly, their insulin areas were also
Li, Vita Chien, Rosemary Smith, Nataliya Olerskeya, Beryl
comparable in the early- and mid-stages of diabetes,
Davy, and Vernon Tintinger for technical assistance and
indicating that no significant loss of -cells had ensued to
Vivian Ward for excellent graphics support.
this point in disease progression. Direct measurements ofpancreatic and serum insulin concentrations 6 weeks
following diabetes onset, although variable, were also
1. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. -Cell
consistent with the above finding, in that there were no
deficit and increased -cell apoptosis in humans with type 2 diabetes.
substantive reductions in insulin content and no clear
associations with blood glucose concentrations. This ef-
2. Zhao HL, Lai FM, Tong PC, Zhong DR, Yang D, Tomlinson B, Chan JC.
fect is consistent with the development of glucose blind-
Prevalence and clinicopathological characteristics of islet amyloid in
ness in the islets of at least some hemizygous mice (38).
Chinese patients with type 2 diabetes. Diabetes 2003;52:2759 –2766
3. Opie EL. The relation of diabetes mellitus to lesions of the pancreas:
Our experimental analyses were clearly able to discrim-
hyaline degeneration of the islands of Langerhans. J Exp Med 1901;5:527–
inate a 20% loss in insulin staining area or in islet -cell
mass, but such absolute reductions only occurred in
4. Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purifica-
association with advanced diabetes. That no significant
tion and characterization of a peptide from amyloid-rich pancreases of
reduction in insulin staining area occurred in early- to
type 2 diabetic patients. Proc Natl Acad Sci U S A 1987;84:8628 – 8632
5. Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson
mid-stage diabetes clearly demonstrates that hA/hIAPP
KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the
expression does not initially evoke diabetes in hemizygous
diabetic cat are derived from a neuropeptide-like protein also present in
mice by eliciting overt islet -cell loss. In support of this
normal islet cells. Proc Natl Acad Sci U S A 1987;84:3881–3885
conclusion, extensive immunohistochemical analyses re-
6. Goldsbury C, Goldie K, Pellaud J, Seelig J, Frey P, Muller SA, Kistler J,
vealed no evidence for -cell apoptosis in pancreatic
Cooper GJ, Aebi U. Amyloid fibril formation from full-length and fragments
sections from hemizygous mice 6 weeks following diabe-
of amylin. J Struct Biol 2000;130:352–362
7. Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. The aggregation
tes onset, as measured by caspase-3 or terminal deoxynu-
potential of human amylin determines its cytotoxicity towards islet
cleotidyl transferase-mediated dUTP nick-end labeling
beta-cells. FEBS J 2006;273:3614 –3624
staining (data not shown), although the assays we used
8. Janciauskiene S, Ahren B. Different sensitivity to the cytotoxic action of
may have been insufficiently sensitive to detect rare apo-
IAPP fibrils in two insulin-producing cell lines, HIT-T15 and RINm5F cells.
ptotic cells in affected islets.
Biochem Biophys Res Commun 1998;251:888 – 893
Our findings indicate that below a certain threshold,
9. Zhang S, Liu J, Saafi EL, Cooper GJ. Induction of apoptosis by human
amylin in RINm5F islet -cells is associated with enhanced expression of
hA/hIAPP expression causes islet -cell dysfunction lead-
p53 and p21WAF1/CIP1. FEBS Lett 1999;455:315–320
ing to impaired insulin production, processing, and/or
10. Zhang S, Liu H, Yu H, Cooper GJ. Fas-associated death receptor signaling
secretion at the outset of diabetes (14). Here, we could not
evoked by human amylin in islet -cells. Diabetes 2008;57:348 –356
investigate insulin processing due to the unavailability of
11. Zhang S, Liu J, MacGibbon G, Dragunow M, Cooper GJ. Increased
an appropriate mouse-specific proinsulin ELISA. As amy-
expression and activation of c-Jun contributes to human amylin-induced
lin expression in our mice is under control of the rat
apoptosis in pancreatic islet -cells. J Mol Biol 2002;324:271–285
12. Zhang S, Liu H, Liu J, Tse CA, Dragunow M, Cooper GJ. Activation of
insulin 2 promoter, we expect that rising blood glucose
activating transcription factor 2 by p38 MAP kinase during apoptosis
would establish a positive feedback cycle that progres-
induced by human amylin in cultured pancreatic beta-cells. FEBS J
sively facilitates islet -cell loss. Presumably, this latter
2006;273:3779 –3791
DIABETES, VOL. 59, JANUARY 2010
J.F. AITKEN AND ASSOCIATES
13. Zhang S, Liu J, Dragunow M, Cooper GJ. Fibrillogenic amylin evokes islet
26. Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A
-cell apoptosis through linked activation of a caspase cascade and JNK1.
J Biol Chem 2003;278:52810 –52819
27. van Hulst KL, Born W, Muff R, Oosterwijk C, Blankenstein MA, Lips CJ,
14. Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes,
Fischer JA, Ho¨ppener JW. Biologically active human islet amyloid polypep-
and the toxic oligomer hypothesis. Endocr Rev 2008;29:303–316
tide/amylin in transgenic mice. Eur J Endocrinol 1997;136:107–113
15. Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK,
28. Kahn SE, Andrikopoulos S, Verchere CB, Wang F, Hull RL, Vidal J.
Butler PC. Spontaneous diabetes mellitus in transgenic mice expressing
Oophorectomy promotes islet amyloid formation in a transgenic mouse
human islet amyloid polypeptide. Proc Natl Acad Sci U S A 1996;93:7283–
model of type II diabetes. Diabetologia 2000;43:1309 –1312
29. Geisler JG, Zawalich W, Zawalich K, Lakey JR, Stukenbrok H, Milici AJ,
16. Soeller WC, Janson J, Hart SE, Parker JC, Carty MD, Stevenson RW,
Soeller WC. Estrogen can prevent or reverse obesity and diabetes in mice
Kreutter DK, Butler PC. Islet amyloid-associated diabetes in obese A(vy)/a
expressing human islet amyloid polypeptide. Diabetes 2002;51:2158 –2169
mice expressing human islet amyloid polypeptide. Diabetes 1998;47:743–
30. Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of
apoptotic cells. Trends Cell Biol 1998;8:365–372
17. Ho¨ppener JW, Oosterwijk C, Nieuwenhuis MG, Posthuma G, Thijssen JH,
31. Aitken JF, Loomes KM, Konarkowska B, Cooper GJ. Suppression by
Vroom TM, Ahren B, Lips CJ. Extensive islet amyloid formation is induced
polycyclic compounds of the conversion of human amylin into insoluble
by development of type II diabetes mellitus and contributes to its progres-
amyloid. Biochem J 2003;374:779 –784
sion: pathogenesis of diabetes in a mouse model. Diabetologia 1999;42:
32. Smith DL, Woodman B, Mahal A, Sathasivam K, Ghazi-Noori S, Lowden PA,
Bates GP, Hockly E. Minocycline and doxycycline are not beneficial in a
18. Butler AE, Janson J, Soeller WC, Butler PC. Increased -cell apoptosis
model of Huntington's disease. Ann Neurol 2003;54:186 –196
prevents adaptive increase in -cell mass in mouse model of type 2
33. Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of
diabetes: evidence for role of islet amyloid formation rather than direct
islet amyloid polypeptide toxicity is membrane disruption by intermediate-
action of amyloid. Diabetes 2003;52:2304 –2314
sized toxic amyloid particles. Diabetes 1999;48:491– 498
19. Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes due
34. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease:
to a progressive defect in -cell mass in rats transgenic for human isletamyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes
progress and problems on the road to therapeutics. Science 2002;297:353–
2004;53:1509 –1516
20. Scrocchi LA, Chen Y, Waschuk S, Wang F, Cheung S, Darabie AA,
35. Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M,
McLaurin J, Fraser PE. Design of peptide-based inhibitors of human islet
Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE,
amyloid polypeptide fibrillogenesis. J Mol Biol 2002;318:697–706
Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from A1– 42
21. Forloni G, Colombo L, Girola L, Tagliavini F, Salmona M. Anti-amyloido-
are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A
genic activity of tetracyclines: studies in vitro. FEBS Lett 2001;487:404 – 407
1998;95:6448 – 6453
22. Cardoso I, Merlini G, Saraiva MJ. 4⬘-iodo-4⬘-deoxydoxorubicin and tetra-
36. Goldberg MS, Lansbury PT Jr: Is there a cause-and-effect relationship
cyclines disrupt transthyretin amyloid fibrils in vitro producing noncyto-
between alpha-synuclein fibrillization and Parkinson's disease? Nat Cell
toxic species: screening for TTR fibril disrupters. FASEB J 2003;17:803–
Biol 2000;2:E115–119
37. Sousa MM, Cardoso I, Fernandes R, Guimaraes A, Saraiva MJ. Deposition
23. Tagliavini F, Forloni G, Colombo L, Rossi G, Girola L, Canciani B, Angeretti
of transthyretin in early stages of familial amyloidotic polyneuropathy:
N, Giampaolo L, Peressini E, Awan T, De Gioia L, Ragg E, Bugiani O,
evidence for toxicity of nonfibrillar aggregates. Am J Pathol 2001;159:1993–
Salmona M. Tetracycline affects abnormal properties of synthetic PrP
peptides and PrP(Sc) in vitro. J Mol Biol 2000;300:1309 –1322
38. Andrikopoulos S, Verchere CB, Terauchi Y, Kadowaki T, Kahn SE. -Cell
24. Hogan BL, Costantini F, Lacy E: Manipulating the Mouse Embryo: A
glucokinase deficiency and hyperglycemia are associated with reduced
Laboratory Manual. Cold Spring Harbor, NY, Cold Spring Harbor Labora-
islet amyloid deposition in a mouse model of type 2 diabetes. Diabetes
2000;49:2056 –2062
25. Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory
39. Percy AJ, Trainor DA, Rittenhouse J, Phelps J, Koda JE. Development of
Manual. 2nd ed. Cold Spring Harbor, NY, Cold Spring Harbour Laboratory,
sensitive immunoassays to detect amylin and amylin-like peptides in
unextracted plasma. Clin Chem 1996;42:576 –585
DIABETES, VOL. 59, JANUARY 2010
Source: http://diabetes.diabetesjournals.org/content/diabetes/59/1/161.full.pdf
SOMMAIRE : 1. A l'honneur, page 1 2. Le mot du président, page 2 3. En souvenir de Pierrot COMBESSIS, Roger FIORIO, page 3 4. Devoir de mémoire, Pierre SONNIER, page 4 5. Notre assemblée générale 2009 (extraits du CR), pages 5 et 6 6. La construction européenne, Paul BLANC, page 7 7. Activités de la section, page 8 8. Combat de BRIOUDE, René VITTOZ, page 9 et 10 9. Algérie 1957, le ralliement de Si Chérif, Stéphane FRACHET, pages 11 et 12 10. Le centre d'information de Kabylie, Robert BAYLE, pages 13 et 14 11. Laos, l'ethnie hmong dispersée, Michel BAIN, pages 15 et 16 12. Le beurre de qualité, « pour rire », Albert GRAS, page 17 13. Info « Dernières », page 18 et 19 14. Quelques photos AG de section 2009, page 20
n-3 Fatty acid derived endocannabinoids: a new link between fish oil and inflammation Michiel G.J. Balvers Thesis committee Thesis supervisor Prof. dr. R.F. Witkamp Professor of Nutrition and Pharmacology, Wageningen University Thesis co-supervisors Dr. ing. K.C.M. Verhoeckx Medior scientist, TNO, Zeist Dr. H.M. Wortelboer Senior scientist, TNO, Zeist Other members Prof. dr. ir. A.H. Kersten, Wageningen University Dr. R.H.H. Pieters, Utrecht University & University of Applied Sciences Utrecht Prof. dr. J. van der Greef, Leiden University & TNO, Zeist Prof. dr. J. Garssen, Utrecht University & Danone Research, Wageningen This research was conducted under the auspices of the Graduate School VLAG


