Ejournal.unri.ac.id
Jurnal Natur Indonesia 15(1), Februari 2013: 57–62
Synthesis and antimalarial activity 57
Synthesis and Antimalarial Activity
of 2-Phenyl-1,10-Phenanthroline Derivative Compounds
Ruslin Hadanu1*), Mustofa2), and Nazudin1)
1)Department of Chemistry, Faculty of Teachership and Educational Science,
Pattimura University, Poka, Ambon 97233
2)Department of Pharmacology and Toxicology, Faculty of Medicine,
Gadjah Mada University, Sekip Utara, Yogyakarta
Received 13-05-2011 Approved 29-03-2013
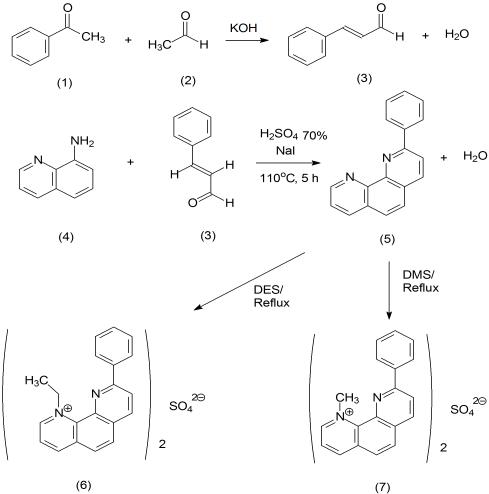
To develop new potential antimalarial drugs of 2-phenyl-1,10-phenanthroline
5 derivatives from 8-aminoquinoline as starting
material were synthesized in good yields. The synthesis of 2-phenyl-1,10-phenanthroline
5 derivatives compounds
with 8-aminoquinoline
4 as starting material through three steps has been carried out. The first step of reactions is aldol
condensation of benzaldehyde
1 with acetaldehyde
2. The result of reactions is cinnamaldehyde
3 (92.14%) in the form of
yellow solid. The second step of reactions was synthesized of 2-phenyl-1,10-phenanthroline
5 (brown solid, 54.63%)
through cyclization of 8-aminoquinoline
4 with cinnamaldehyde
3 compound. The third step of reactions is methylation and
ethylation of 2-phenyl-1,10-phenanthroline using dimethyl sulphate (DMS) and diethyl sulphate (DES) reagents that it was
refluxed for 17 and 19 h, respectively. The results of reactions are (1)-
N-methyl-9-phenyl-1,10-phenanthrolinium sulphate
6
and (1)-
N-ethyl-9-phenyl-1,10-phenanthrolinium sulphate
7 in yield from 90.62% and 89.70%, respectively. The results of
testing
in vitro antiplasmodial activity at chloroquine-resistant
Plasmodium falciparum FCR3 strain to 2-phenyl-1,10-
phenanthroline
5 derivatives obtained that (1)-
N-ethyl-9-phenyl-1,10-phenanthrolinium sulphate
7 compound has higher
antimalarial activity (IC :0.13 ± 0.02 µM) than antimalarial activity of (1)-
N-methyl-9-phenyl-1,10-phenanthrolinium sulphate
6 compound (IC :0.25 ± 0.01 µM) and 2-phenyl-1,10-phenanthroline
5 compound (IC :2.45 ± 0.09 µM). While, the results
of testing
in vitro antiplasmodial activity at chloroquine-resistant
Plasmodium falciparum D10 strain to 2-phenyl-1,10-
phenanthroline
5 derivatives obtained that (1)-
N-methyl-9-phenyl-1,10-phenanthrolinium sulphate
6 compound has higher
antimalarial activity (IC :0.10± 0.04 µM) than antimalarial activity of (1)-
N-ethyl-9-phenyl-1,10-phenanthrolinium sulphate
7 (IC :0.18 ± 0.01 µM) and 2-phenyl-1,10-phenanthroline
5 compound (IC :0.55 ± 0.07 µM).
Keywords: 2-phenyl-1,10-phenanthroline derivatives, antimalarial activity, plasmodium, synthesis
Untuk mengembangkan obat baru antimalaria yang potensial dari senyawa turunan 2-fenil-1,10-fenantrolina
5 telah disintesis
dari 8-aminokuinolina sebagai bahan dasar. Sintesis senyawa turunan 2-fenil-1,10-fenantrolina
5 dari 8-aminokuinolina
4
telah dilakukan melalui tiga tahap reaksi. Langkah pertama adalah reaksi aldol kondensasi benzaldehida
1 dengan asetaldehida
2 menghasilkan senyawa sinnamaldehida
3 (92,14%) dalam bentuk padatan kuning. Langkah kedua adalah sintesis senyawa
2-fenil-1,10-fenantrolina
5 (padatan coklat, 54,63%) melalui siklisasi 8-aminokuinolina
4 dengan senyawa sinnamaldehida
3.
Langkah ketiga adalah reaksi metilasi dan etilasi terhadap senyawa 2-fenil-1,10-fenantrolina
5 menggunakan reagen dimetil
sulfat (DMS) dan dietil sulfat (DES) yang direfluks masing-masing selama 17 dan 19 jam. Hasil reaksi alkilasi tersebut adalah
(1)-
N-metil-9-fenil-1,10-phenanthrolinium sulfat
6 dan (1)-
N-etil-9-fenil-1,10-phenanthrolinium sulfat
7 dengan rendemen
berturut-turut sebesar 90,62% dan 89,70%. Hasil pengujian aktivitas antiplasmodial
in vitro pada
chloroquine-resistant
Plasmodium falciparum strain FCR3 terhadap turunan 2-fenil-1,10-fenantrolin
5 diperoleh bahwa senyawa (1)-
N-etil-9-
fenil-1,10-phenanthrolinium sulfat
7 memiliki aktivitas antimalaria yang lebih tinggi (IC : 0,13 ± 0,02 µM) dibandingkan
dengan aktivitas antimalaria dari senyawa (1)-
N-metil-9-fenil-1,10-phenanthrolinium sulfat
6 (IC : 0,25 ± 0,01 µM) dan
senyawa 2-fenil- 1,10-fenantrolina
5 (IC : 2,45 ± 0,09 µM). Sementara, hasil pengujian aktivitas antiplasmodial
in vitro pada
chloroquine-sensitive P. falciparum strain D10 terhadap senyawa 2-fenil-1,10-fenantrolina
5 diperoleh bahwa senyawa (1)-
N-metil-9-fenil-1,10-fenantrolina sulfat
6 memiliki aktivitas antimalaria yang lebih tinggi (IC : 0,10 ± 0,04 µM) dibandingkan
58 Jurnal Natur Indonesia 15(1): 57–62
Hadanu,
et al.
dengan aktivitas antimalaria dari senyawa (1)-
N-etil-9-fenil-1,10-phenanthrolinium sulfat
7 (IC : 0,18 ± 0,01 M) dan senyawa
2-fenil-1,10-fenantrolina
5 (IC : 0,55 ± 0,07 pM).
Kata Kunci: aktivitas antimalaria, plasmodium, sintesis, turunan 2-fenil-1,10-fenantrolina
The halofantrine as new anti malaria has good
Malaria is the most important parasitic disease in the
therapeutic effects (Basco
et al. 1994). Halofantrine as more
world. Its etiological agents are protozoa of the genus
active against strains of
P. falciparum that are resistant to
Plasmodium. Plasmodium falciparum is the most virulent
chloroquine, pyrimethamine, and quine (Rang
et al. 2003).
among the four species infecting humans and is responsible
However, halofantrine is known to have some unwanted
for most of mortality. In 2008, among 3.3 billion people at
side effects, such as abdominal pain, nausea, vomiting,
risk, there were 243 million malaria cases, causing an
diarrhea, orthostatic, hypertension, prolongation of QTc
estimated 863,000 deaths, mostly of children under five years.
intervals, pruritus, rash, and hepatotoxic (Karbwang
et al.
From 109 countries endemic for malaria, 45 were within the
1991; Bassi
et al. 2006). The 1,10-phenanthroline derivatives
World Health Organization (WHO 2009) Especially in African
are similar to halofantrine as antimalarial drug which its added
Region (Fernández
et al. 2011; Fidock
et al. 2004; Olumese
at heterocyclic with two nitrogen atoms. (In 2000, Yapi
2005; Kayembe
et al. 2010). Malaria remains one of the most
reported) that the 1,10-phenanthroline ring system appeared
important diseases of the developing world, killing 1–3
as new class of potential antimalarial compound (Yapi
et al.
million people and causing disease in 300–500 million people
annually (Fidock
et al. 2004; Olumese 2005, Kayembe
et al.
Now, part of our research was concerning the synthesis
2010)
. Malaria endemic areas include Africa, South East Asia,
and biological activity of 1,10-phenanthroline derivatives.
India and South America; however, the disease is spreading
In this program continuation of these studies, we report in
to new areas, such as Central Asia, and Eastern Europe.
this paper our results concerning the synthesis and the
Local transmission of malaria in the United States, unheard
determination of the biological activity of compound type
of in the era between World War II and 1980, now accounts
for an increasing number of cases (Molyneux
et al. 1989).
(Widjayanti
et al. 2006). Yapi
et al. (2006) have synthesized
Clinical cases in the US now average 1,300 per year
diaza-analogs of phenanthrene by substituting the two
(Wernsdorfer 1991). Worldwide, the majority of deaths occur
nitrogen atoms in the phenanthrene skeleton. Antiplasmodial
in children; other high risk groups include pregnant women,
activity of series of diaza-analogs of phenanthrene derived
refugees, migrant workers, and non immune travelers-over
from 3-amino-, 5-amino-, 6-amino-, 8-aminoquinoline and
20 million Western tourists at risk annually (fact sheets from
5-isoquinoline showed that among the molecules evaluated
Malaria Foundation International). Although four species
the 1,10-phenanthroline skeleton was the most active
of the genus
Plasmodium cause human malaria,
compound
in vitro on both chloroquine-resistant (FcB1)
P. falciparum is the deadliest and will be the subject of this
and chloroquine-sensitive (Nigerian) strain with an IC of
about 0.13 μM. Based on the skeleton, (Mustofa
et al. 2003)
The traditional remedies are no longer effective and
have also synthesized thirteen derivatives of 1,10-
the incidence of malarial by
P. falciparum, the most
phenanthroline and evaluated the
in vitro antiplasmodial
dangerous species of parasite, continues to grow, while some
activity (Yapi
et al. 2000) and their Quantitative Structure
traditional drugs such as chloroquine and its congeners are
Activity Relationship (QSAR) Mustofa
et al. 2003. The
losing their activity due to the increasing multi drug
resulting of the QSAR analysis found the best theoretical
resistance (Yapi
et al. 2000; Yapi
et al. 2006). Therefore, it is
activity of six new compounds and its was synthesized and
essential to find new drugs of anti malaria having a
evaluated their
in vitro antiplasmodial activity through
pharmacological activity higher than of currently available
experiment in laboratory.
drugs of anti malaria. In this connection, quantitative
This study were synthesized of 2-phenyl-1,10-
structure-activity relationship (QSAR) analysis plays an
phenanthroline
5 derivatives from 8-aminoquinoline
4 as
important role to minimize trial and error in designing new
starting material were obtained two compounds of 2-phenyl-
antimalarial drugs.
1,10-phenanthroline
5 derivatives i.e. (1)-
N-methyl-9-phenyl-
Synthesis and antimalarial activity 59
1,10-phenanthrolinium sulphate 6 and (1)-N-ethyl-9-phenyl-
500 MHz. MS spectrum were recorded on GC-MS Shimadzu
1,10-phenanthrolinium sulphate 7 which were synthesized
through 3 stages reaction. The reactions condition and the
Procedure. Synthesis of Cinnamaldehyde (3). Ethanol
results of synthesis of 2-phenyl-1,10-phenanthroline 5
(15 mL) was transferred into a 125-mL Erlenmeyer flask, and
derivative compounds were described in Figure 1.
20 mL of 10% NaOH solution. Using a thermometer, cool the
solution to 20ºC. In a medium size tube, mix 2 mL of
MATERIALS AND METHODS
benzaldehyde with 15 drops of acetaldehyde, and leave it at
Materials. The 8-aminoquinoline p.a. (Merck),
room temperature for 5 minutes. Then, add the mixture to the
dymethyl sulphate (DMS) p.a. (Merck), dymethyl sulphate
ethanol-NaOH solution in small portions and stir with
(DES) p.a. (Merck), H SO 70% p.a. (Merck), acetaldehyde
magnetic stirrer for 30 minutes. Cool the mixture using the
p.a. (Merck), benzaldehyde p.a. (Merck), HCl p.a. (Merck),
ice-water bath. The product was filtrated and hand-dried to
NaOH p.a. (Merck), NaI p.a. (Merck), KOH p.a. (Merck),
collect the yellow oils to give of the cinnamaldehyde 3
Na SO anhydrous p.a. (Merck), HBr p.a. (Merck), NaHCO
product (6.09 g; 92.04%). The product was characterized by
p.a. (Merck), acetone p.a. (Merck), CH Cl p.a. (Merck), CHCl
means of spectrum. IR spectrum (KBr) ύ (cm-1): 3062.7-3031.9
p.a. (Merck), CCl p.a. (Merck), dimethyl sulfoxide (DMSO)
(HC=), 2927.7 (-C-H), 1685.7 (C=O), 1600.8 and 1462.8 (C=C
p.a. (Merck), gas N , Na SO p.a. (Merck), TLC plat, silica
aromatic); NMR spectrum (60 MHz, DMSO-d , TMS)
gel, hexane p.a. (Merck), benzene p.a. (Merck).
δ (ppm): 10.1 (1H, s, CHO), 8.2-7.9 (3H, m, H
Instruments. The melting point of compound were
7.3 (4H, m, H
). MS spectrum (EI) m/z: 132 (M), 131 (M-
determined with melting point electro thermal 9100. The
.H), 103 (131-C=O), 77 (103-C H ), and 51 (77- C H ).
spectrum of structures compound measurements were taken
Synthesis of 2-phenyl-1,10-phenanthroline (5). The
using the instruments: Shimadzu FTIR-8201 PC; 1H-NMR
cinnamaldehyde 3 compound (2.64 g; 20 mmol) was added
JEOL 60 MHz, JEOL 500 MHz and GC-MS Shimadzu QP
over 5 h to a stirred solution of the 8-aminoquinoline 4
5000. In general, the melting point of compounds were
(1.73 g; 10 mmol) and NaI (12 mmol) in H SO 70% (5 mL) at
determined on melting point electro thermal 9100 and are
110oC. After 1 h at 110oC the dark brown reaction mixture was
not corrected. The spectrum of structures compound
cooled to room temperature, poured into 1 M Na CO
measurements were taken using the following instruments:
(50 mL) and extracted with CH Cl (3 x 50 mL). The
FTIR spectrum were taken on Shimadzu FTIR-8201 PC; 1H-
combination of organic layers were extracted with CH Cl .
NMR spectrum were obtained on JEOL 60 MHz and JEOL
Removal of the solvent in vacuo afforded the appropriate
1,10-phenanthroline skeleton. The products were purified
by filtration through silica gel using CH Cl as solvent to
give brown solid compound of 2-phenyl-1,10-phenanthroline
5 (2.80 g, 54.63%, m.p.: 145-148oC. The product was
characterized by means of spectrum. IR spectrum (KBr) ύ
(cm-1): 3409.9 (O-H hydrogen bonding), 3028.0 (HC=), 2900.0-
2854.5 (-C-H), 1596.9 and 1462.8 (C=C aromatic); NMR
spectrum (500 MHz, DMSO-d6, TMS) δ (ppm): 8.30-6.43
(12H, m, Ph); MS spectrum (EI) m/z: 256 (M), 230 (M-C H ),
204 (230-C H ), 179 (204-C H ), 127 (179-NC H ), 101 (127-
.C H) and 77 (102-.C H).
rolinium sulphate (6). The 2-phenyl-1,10-phenanthroline 5
(0.51 g; 2 mmol) and DMS (1.26 g, 20 mmol) in acetone (20
mL) was refluxed for 17 h. The resulting mixture was then
cooled. The precipitate which formed was filtered, and
Recrystalization
Figure 1 Synthesis of 2-phenyl-1,10-phenanthroline 5 derivatives
dichloromethane:diethyl ether (1:1). The precipitate which
60 Jurnal Natur Indonesia 15(1): 57–62
Hadanu, et al.
formed was filtered and washed with acetone to give brown
The parasite control in the presence without chemicals
solid compound (0.58 g; 90.62%) of (1)-N-methyl-6-nitro-
(mean of the corresponding wells was referred to as 100%).
1,10-phenanthrolinium sulphate 6; m.p.: 188-190oC. The
Concentrations inhibiting 50% of the parasite (IC ) were
product was characterized by spectroscopy method. IR
determined by SPPS 13.0 software. The IC that indicated
spectrum (KBr) ύ (cm-1): 3429.2 (O-H hydrogen bonding),
antiplasmodial activity of chemicals compound to determine
2950.9; 2923.9; and 2866.0 (-C-H), 1600.8 and 1500.0 (C=C
by probit analysis method with percentage of concentration
aromatic); 1365.5 (CH ); NMR spectrum (500 MHz, DMSO-
inhibition versus chemical doses.
d , TMS) δ (ppm): 9.37 (1H, d, H ), 9.10 (1H, d, H ), 8.75-8.74
(1H, t, H ), 8.58-8.54 (1H, d, H ), 7.94-7.93 (1H, d, H ), 7.83-
RESULTS AND DISCUSSION
7.82 (1H, d, H ), 7.76-7.72 (1H, d, H ), 7.68-7.64 (1H, s, H ),
The synthesis of 2-phenyl-1,10-phenanthroline 5
7.50-7.30 (1H, t, H ), 7.21-7.13 (1H, t, H ), 6.94-6.92 (1H, t,
derivate was carried out through three steps (Figure 1). The
H ), 6.80-6.75 (1H, t, H ), 4.78 (3H, s, CH ), and 3.57-3.49
first step is synthesis of cinnamaldehyde 3 compound by
(H O, s; hydrogen bonding).
aldol condensation reaction. This reaction used sodium
hydroxide (NaOH) as base catalyst. The condensation of
linium sulphate (7). The 2-phenyl-1,10-phenanthroline 5
acetaldehyde with benzaldehyde to give cinnamaldehyde 3
(0.51 g; 2 mmol) and DES (0.51 g; 20 mmol) in acetone
compound as product of reaction (6.09 g; 92.04%). The
(25 mL) was refluxed for 19 h. The resulting mixture was then
second step is synthesis of 2-phenyl-1,10-phenanthroline 5
cooled. The precipitate which formed was filtered, and
from 8-aminoquinoline 4 and cinnamaldehyde 3 through
washed with acetone. Recrystalization with dichloromethane:
cyclization reaction. The third step is synthesis of the (1)-N-
diethyl ether (1:1). The precipitate which formed was filtered
alkyl-9-phenyl-1,10-phenanthrolinium salts compound from
and washed with acetone to give brown solid compound
2-phenyl-1,10-phenanthroline 5 using DMS and DES reagent
(0.61 g; 89.70%) of (1)-N-ethyl-6-nitro-1,10-phenan-
as donor of (1)-N-methyl-9-phenyl-1,10-phenanthrolinium
throlinium sulphate 7; m.p.: 188-190oC. The product was
characterized by means of spectroscopic. IR spectrum (KBr)
sulphate 7 compounds (Figure 1).
ύ (cm-1): 3438.8 (O-H hydrogen bonding), 3058.9 (C -H),
Synthesis of (1)-N-methyl-9-phenyl-1,10 phenanthro-
2989.5 and 2879.5 (-C-H), 1625.9 and 1525.6 (C=C aromatic);
linium sulphate 6 was conducted from 2-phenyl-1,10-
1438.8 (CH ), and 1357.8 (CH ); NMR spectrum (500 MHz,
phenanthroline 5 by DMS reagent in acetone which refluxing
DMSO-d , TMS) δ (ppm): 9.20-8.20 (12H, H ), 2.49 (3H, m,
17 hours. The structure of (1)-N-methyl-9-phenyl-1,10-
) and 1.1 (3H, t, H
phenanthrolinium sulphate 6 was determined by FTIR and
Biological Activity. Parasites were cultured according
1H-NMR spectrum. The FTIR spectrum showed typical
to method described by Trager and Jensen (1976) with
spectra at 1357.8 cm-1 that to indicate the presence of methyl
modification. FCBr3 was considered as a chloroquine
group, while the 1H-NMR spectrum showed one singlet at
resistant strain and D10 were considered as a chloroquine
4.78 (3H), assigned to the methyl group. Treatment of (1)-N-
sensitive strain. Culture medium was replaced daily and the
ethyl-9-phenyl-1,10-phenanthrolinium sulphate 7 compound
cultures were synchronized by 5% D-sorbitol lysis (Merk,
with DES in acetone which refluxing for 19 hours gave the
Darmstadt, Germany). The method used for in vitro
salt compound. The product of ethylation of reaction
antimalarial activity testing was adapted from visual method.
washing with acetone and the structure was determined by
The molecules were tested 3 times in triplicate in 96-well
FTIR and 1H-NMR spectrum. Similarly, the FTIR spectrum
plates (TPP, Switzerland) with cultures at ring stage at
0.5-1.0% parasitemia (hematocrit 1%). For each test, the
showed typical spectrum at 1438.8 and 1357.8 cm-1,
parasite cultures were incubated with the chemicals
respectively, assigned to the methyl and methylene groups,
at decreasing concentrations for 24 and 72 h. The first
while the 1H-NMR spectrum of (1)-N-ethyl-9-phenyl-1,10-
dilution of the product (10 mg/mL) was perfomed with
phenanthrolinium sulphate 7 compounds showed one triplet
dimethylsulfoxide (DMSO, Merck), and the following with
at 2.49 (3H, m, H ) and 1.1 (3H, t, H ), respectively,
RPMI 1640. Parasites growth was estimated by coloring with
that indicated the presence of methyl and methylene groups.
giemsa (10%) for 30 second and calculated by β-caunter.
Widjayanti et al. (2006) reported the activities of 8 new
Synthesis and antimalarial activity 61
Table 1 Parasite growth inhibition and IC
of 2-phenyl-1,10-phenanthroline on FCR-3 strain
% Inhibition (mean ± SD)
Compound of 5
Compound of 6
Compound of 7
Table 2 Parasite growth inhibition and IC
of 2-phenyl-1,10-phenanthroline on D10 strain
Concentration (ng/mL)
% Inhibition (mean ± SD)
Compound of 5
Compound of 6
Compound of 7
ND : Not Determined
compounds of N-alkyl- and N-benzyl-1,10-phenanthrolinium
tively. The result of antiplasmodium evaluation to all 2-
derivatives: 1) (1)-N-methyl-1,10-phenanthrolinium sulphate,
phenyl-1,10-phenanthroline derivatives toward FCR 3 and
2) (1)-N-ethyl-1,10-phenanthrolinium sulphate, 3) (1)-
D10 strain of P. falciparum were presented in Table 1 and 2,
N-t-buthyl-1,10-phenanthrolinium chloride, 4) (1)-N-
completely. Based on the Table 1 and 2 showed having the
benzyl-1, 10-phenanthrolinium chloride, 5) (1)-N-benzyl-1,
highest antiplasmodial activity in FCR3 strain is (1)-N-
10-phenanthrolinium bromide, 6) (1)-N-benzyl-1,10-
ethyl-9-phenyl-1,10-phenanthrolinium sulphate 7 to equal
phenanthrolinium iodide, 7) (1)-N-(4-methoxybenzyl-1,10-
0.13 ± 0.02 µM, while in the D10 strain having the highest
phenanthrolinium chloride, and 8) (1)-N-(4-benzyloxy-3-
antiplasmodial activity is (1)-N-methyl-9-phenyl-1,10-
phenanthrolinium sulfate 6 to equal 0.10 ± 0.04 µ M.
In another compound, Hadanu et al. (2007) reported the
This treatment with 2-phenyl-1,10-phenanthroline
activities of 1 new compound of (1)-N-(4-methoxybenzyl-
derivative compounds significantly inhibited parasitemia of
1,10-phenanthrolinium bromide. All compounds tested
P. falciparum FCR3 strain D10 strain (Table 1 and 2).
antiplasmodial activities, the compound of (1)-N-benzyl-1,10-
Although the suppression of parasitemia was never
phenanthrolinium bromide had the highest activities (0.10 ±
complete (100% inhibition of parasite growth), the results
0.13 µM) against P. falciparum strain FCR3 and the (1)-N-
indicated antiplasmodial potency. In the P. falciparum FCR3
benzyl-1,10-phenanthrolinium bromide had highest activity
strain, the (1)-N-ethyl-9-phenyl-1,10-phenantrolinium sulfate
(IC : 0.33 ± 0.34 µM) on P. falciparum strain D10.
7 compound have higher activity than (1)-N-methyl-9-
In this research, the result of evaluation antiplasmodial
phenyl-1,10-phenanthrolinium sulphate 6 and 2-phenyl-1,10-
activities using chloroquine-resistant FCR3 strain is
phenanthroline 5 compound, but in the P. falciparum D10
summarized in Table 1. While, the result of investigation
antiplasmodial activities using chloroquine sensitive D10
sulphate 6 compound have higher activity than (1)-N-ethyl-
strains is summarized in Table 2. In this study, the
9-phenyl-1,10-phenanthrolinium sulphate 7 and 2-phenyl-
antiplasmodial activity of 1,10-phenanthroline derivatives
1,10-phenanthroline 5 compound.
showed that 2-phenyl-1,10-phenanthroline 5, (1)-N-methyl-
9-phenyl-1,10-phenantrolinium sulphate 6, and (1)-N-ethyl-
9-phenyl-1,10-phenantrolinium sulphate 7 were active
The 1,10-phenanthroline derivative compounds i.e. 2-
against P. falciparum FCR3 with an IC 2.45 ± 0.09, 0.25 ±
0.01 and 0.13 ± 0.02 µM, respectively, and D10 strains with
phenanthrolinium sulphate 6 and (1)-N-ethyl-9-phenyl-1,10-
an IC 0.55 ± 0.07, 0.10 ± 0.04, and 0.18 ± 0.01 µM, respec-
phenanthrolinium sulphate 7 were synthesized,
62 Jurnal Natur Indonesia 15(1): 57–62
Hadanu, et al.
characterized, and evaluated of in vitro antiplasmodial
quinones isolated from four plants used by traditionalhealers in the Democratic Republic of Congo. J Med
activity. Results of in vitro antiplasmodial activity on
Plant Res 4(11): 991–994.
chloroquine-resistant P. falciparum FCR3 strain were
Molyneux, M.E., Taylor, T.E., Wirima, J.J & Borgstein, A.
determined by microscopic method after 72 h incubation
1989. Clinical features and prognostic indicators in
showing the highest antiplasmodial activity in FCR3 strain
paediatric cerebral malaria: a study of 131 comatose
Malawian children. Q J Med 71(265): 441–459.
is (1)-N-ethyl-9-phenyl-1,10-phenanthrolinium sulphate 7 to
Mustofa., Yapi, A.D., Valentin, A & Tahir, I. 2003. In Vitro
equal 0.13 ± 0.02 µM, while in the D10 strain having the
antiplasmodial activity of 1,10-phenanthroline
highest antiplasmodial activity is (1)-N-methyl-9-phenyl-
derivatives and its quantitative structure-activity
1,10-phenanthrolinium sulphate 6 to equal 0.10 ± 0.03 µM.
relationship. BIKed 35(2): 67–64.
Olumese, P. 2005. Epidemiology and surveillance: Changing
the global picture of malaria myth or reality?. AcTrop
The study was funded by Research Grant Hibah
Rang, H.P., Dale, M.M., Ritter, J.M & Moore, P.K. 2003.
Bersaing 2009-2010 from Minister of National Education,
Pharmacology, 5th ed, Edinburg: Churchill Livingstone.
Indonesian Government with number of contract project:
Solikhah, E.N., Supargiono., Jumina., Wijayanti, M.A.,
Tahir, I., Hadanu, R & Mustofa. 2006. In vitro
06/H13/SPPP-HBPF/2010, date 27th May 2010. We are also
antiplasmodial activity and cytotoxicity of new
grateful to PT. Konimex Indonesia for providing the
(1)-N-alkyl and (1)-N-benzyl-1,10-phenantrolinium
chloroquine diposphate used in this research.
derivatives. Trop Med Public Health 36(6): 1072–1077.
Trager, W & Jensen, J.B. 1976. Human malaria parasites in
continuous culture. Science 193(4254): 673–675.
Wensdorfer, W.H & Payne, D. 1991. The dynamics of drugs
Basco, L.K., Ruggeri, C & Le Bras, J. 1994. Molecules
resistance in P. falciparum. Pharmac Ther 50: 95-121.
Antipaladiques: Relations Structure-Activity. Edition
WHO. 2009. World Health Organization, Malaria Unit, Global
malaria control. Bull. WHO 71(34): 281–284.
Bassi, P.U., Buratai, B.I & Kuchali, W. 2006. Effect of
Widjayanti, M.A., Solikhah, E.N., Tahir, I., Hadanu, R.,
halofantrine administration on some liver and heart
Jumina., Supargiono & Mustofa. 2006. Antiplasmodial
enzymes in healthy human volunteers. Afr J Biomed
activity and acute toxicity of N-alkyl- and N-benzyl-1,10-
Res 9(1): 31–35.
phenanthroline derivatives in mousa malaria model. J
Fidock, D.A., Rosenthal, P.J., Croft, S.L., Brun, R & Nwaka,
Health Sci 52(6): 794–799.
S. 2004. Antimalarial drug discovery: efficacy models
Yapi, A.D., Mustofa, M., Valantin, A., Chavignon, O., Teulade,
for compound screening. Nature Rev 3(6): 509–520.
J., Mallie, M., Chappat, J & Blace, Y. 2000. New potensial
Fernández, A & Valdés, C. 2011. Acridine and acridinones:
antimalarial agents: synthesis and biological activities
old and new structures with antimalarial activity. OMed
of original diaza-analogs of phenanthrene. J Chem Pham
Chem J 5: 11–20.
Bull 48(12): 1886–1889.
Hadanu, R., Mastjeh, S., Jumina., Mustofa., Wijayanti, M.
Yapi, A.D., Valentin, A., Chezal, J.M., Chavignon, O.,
A & Sholikhah, E.N. 2007. Synthesis and antiplasmodial
Chaillot, B., Gerhardt, R., Teulade, J.C & Blace, Y.
activity testing of (1)-N-(4-methoxybenzyl)-1,10-
2006. In vitro and in vivo antimalarial activity of
phenanthrolinium bromide. Indo JChem 7(2): 197-201.
derivatives of 1,10-phenanthroline framework, Arch.
Karbwang, J. & Banchang, K.N. 1994.Clinical pharma-
Pharm. Chem Life Sci 339(4): 201–206.
cokinetics of halofantrine. Clin Pharmacokinet 27: 104–
119.
Kayembe, J.S., Taba, K.M., Ntumba, K., Tshiongo, M.T.C &
Kazadi, T.K. 2010. In vitro anti-malarial activity of 20
Source: http://ejournal.unri.ac.id/index.php/JN/article/download/2120/2086
C H A P T E R 3 3-D US Imaging of the Carotid Arteries Aaron Fenster, Grace Parraga, Anthony Landry, Bernard Chiu, Michaela Egger,and J. David Spence Determining the severity of carotid atherosclerotic stenosis has been an importantstep in establishing patient management pathways and identifying patients who canbenefit from carotid endarterectomy versus those who should be treated using life-style and pharmaceutical interventions. Recently a number of research groups havedeveloped phenotypes other than carotid stenosis using noninvasive imaging. Mon-itoring carotid plaque progression/regression and identifying vulnerable orhigh-risk plaques that can lead to thrombogenic events using noninvasive imagingtools now involve multiple disciplines and multiple modalities, including imageprocessing.
Technology Innovation Management Review TIM Lecture Series The Expanding Cybersecurity Threat It used to be that not a month would go by without some new data breach being reported. Then it seemed not a week would go by. Today, we see daily reports about some new attack vector, some new cyber- espionage group, some new kind of cyber-attack occurring against our

