Jacemedical.com
Meta-Analysis of Multiple Primary Prevention Trials of
Cardiovascular Events Using Aspirin
Alfred A. Bartolucci, PhD,a,* Michal Tendera, MDb, and George Howard, DrPHa
Several meta-analyses have focused on determination of the effectiveness of aspirin (ace-
tylsalicylic acid) in primary prevention of cardiovascular (CV) events. Despite these data,
the role of aspirin in primary prevention continues to be investigated. Nine randomized
trials have evaluated the benefits of aspirin for the primary prevention of CV events: the
British Doctors' Trial (BMD), the Physicians' Health Study (PHS), the Thrombosis Pre-
vention Trial (TPT), the Hypertension Optimal Treatment (HOT) study, the Primary
Prevention Project (PPP), the Women's Health Study (WHS), the Aspirin for Asymptom-
atic Atherosclerosis Trial (AAAT), the Prevention of Progression of Arterial Disease and
Diabetes (POPADAD) trial, and the Japanese Primary Prevention of Atherosclerosis With
Aspirin for Diabetes (JPAD) trial. The combined sample consists of about 90,000 subjects
divided approximately evenly between those taking aspirin and subjects not taking aspirin
or taking placebo. A meta-analysis of these 9 trials assessed 6 CV end points: total coronary
heart disease, nonfatal myocardial infarction (MI), total CV events, stroke, CV mortality,
and all-cause mortality. No covariate adjustment was performed, and appropriate tests for
treatment effect, heterogeneity, and study size bias were applied. The meta-analysis suggested
superiority of aspirin for total CV events and nonfatal MI, (p <0.05 for each), with nonsignif-
icant results for decreased risk for stroke, CV mortality, and all-cause mortality. There was no
evidence of a statistical bias (p >0.05). In conclusion, aspirin decreased the risk for CV events
and nonfatal MI in this large sample. Thus, primary prevention with aspirin decreased the risk
for total CV events and nonfatal MI, but there were no significant differences in the incidences
of stroke, CV mortality, all-cause mortality and total coronary heart disease.
2011 Elsevier
Inc. All rights reserved. (Am J Cardiol 2011;107:1796 –1801)
The aim of the present analysis was to examine the more
Features of the studies included in the 9 trial
recent trials that have been published since Bartolucci and
meta-analysis are listed in
and add data from those studies to enlarge the
The United States Preventive Services Task de-
sample and thus the power and precision. By adding these
scribed the data collection and analysis from the first 5 primary
studies, it may be increasingly possible to detect moderate,
prevention trials—the British Doctors' Trial (BMD), the Phy-
but potentially meaningful, differences that individual trials
sicians' Health Study (PHS), the Thrombosis Prevention
cannot detect. Added studies to our meta-analysis of the 6
Trial (TPT), the Hypertension Optimal Treatment (HOT)
primary prevention studies include the Aspirin for Asymp-
study, the Primary Prevention Project (PPP)—and the ad-
tomatic Atherosclerosis Trial the Prevention of
dition of the new data from the Women's Health Study
Progression of Arterial Disease and Diabetes
(WHS) was described by Bartolucci and The new
trial, and the Japanese Primary Prevention of Atherosclero-
sources of data are from the AAAT, POPADAD, and JPAD
sis With Aspirin for Diabetes trial.
Because aspirin may have a differential effect on differ-
ent aspects of cardiovascular (CV) disease, outcomes were
In this report, we present a meta-analysis of 9 pri-
classified as follows: (1) total coronary heart disease (CHD)
mary prevention trials with aspirin, including the AAAT,
as nonfatal and fatal myocardial infarction (MI) and death
POPADAD, and JPAD trials, added to the 6 trials in-
due to CHD; (2) nonfatal MI as confirmed MI that did not
cluded in the previous meta-analyses (the Antithrombotic
result in death; (3) total CV events as a composite of CV
Trialists' Collaboration and Bartolucci and
death, MI, or stroke; (4) stroke as ischemic or hemorrhagicstroke that may or may not have resulted in death; (5) CVmortality as death related to CHD or stroke; and (6) all-
aDepartment of Biostatistics, School of Public Health, University of
cause mortality as death related to any cause. Where appli-
Alabama at Birmingham, Birmingham, Alabama; and b3rd Division of
cable (data available), we performed a meta-analysis and
Cardiology Medical University of Silesia, Katowice, Poland. Manuscript
summary overview for each of these end points for the 9
received December 14, 2010; revised manuscript received and accepted
study data sets. All 9 studies were screened for these out-
February 12, 2011.
This study was supported by an unrestricted research grant from Bayer
Data from the United States Preventive Services Task
HealthCare AG (Leverkusen, Germany).
*Corresponding author: Tel: 205-934-4906; fax: 205-975-2540.
Force for each patient trial and data from the WHS, AAAT,
E-mail address: (A.A. Bartolucci).
JPAD, and POPADAD were combined for analysis. For
0002-9149/11/$ – see front matter 2011 Elsevier Inc. All rights reserved.
Preventive Cardiology/Meta-Analysis of Aspirin in Primary Prevention
Table 1Features of the trials included in the 9 study meta-analyses
6-Study* Meta-Analysis
Aspirin dose (mg/dl)
75–300; 100–325
Healthy men/women, DBP
Men/women, low brachial index
Type 1 and 2 diabetes
* Details of the 6 individual primary prevention trials (WHS, BMD, PHS, HOT, PPP, and TPT) are given in Bartolucci and DBP ⫽ diastolic blood pressure.
Statistical significance of cardiovascular end points in the primary
Meta-analysis of predefined end points for the 9 study meta-analysis
prevention trials
All-cause mortality
* Coronary and cerebrovascular death only.
neity across the studies, between-study variation, and
X ⫽ statistically significant advantage of aspirin versus placebo
within-study variation or patient selection. However, given
(p ⬍0.05).
the summary data, within-study variation is not easily as-sessed. The standard procedure for the assessment of small
each previously described end point, a meta-analysis was
study effects (i.e., a trend for relatively smaller studies to
performed for the comparison of aspirin with placebo or
show larger treatment effects) has been the use of funnel
control. A summary odds ratio with 95% confidence inter-
plots using Egger's There has been considerable
val was calculated. The odds ratio is the appropriate effect
discussion regarding the properties of this The
size statistic for our 5 risk ratio outcomes noted in the
technique of Macaskill et was used to adjust for this
previous paragraph. The odds ratio is the ratio of the odds of
shortcoming (also see Harbord et
an event occurring in 1 group to the odds of it occurring inanother group. The term is also used to refer to sample-based estimates of this ratio. Obviously, an odds ratio of 1
would indicate even odds or no difference between the 2
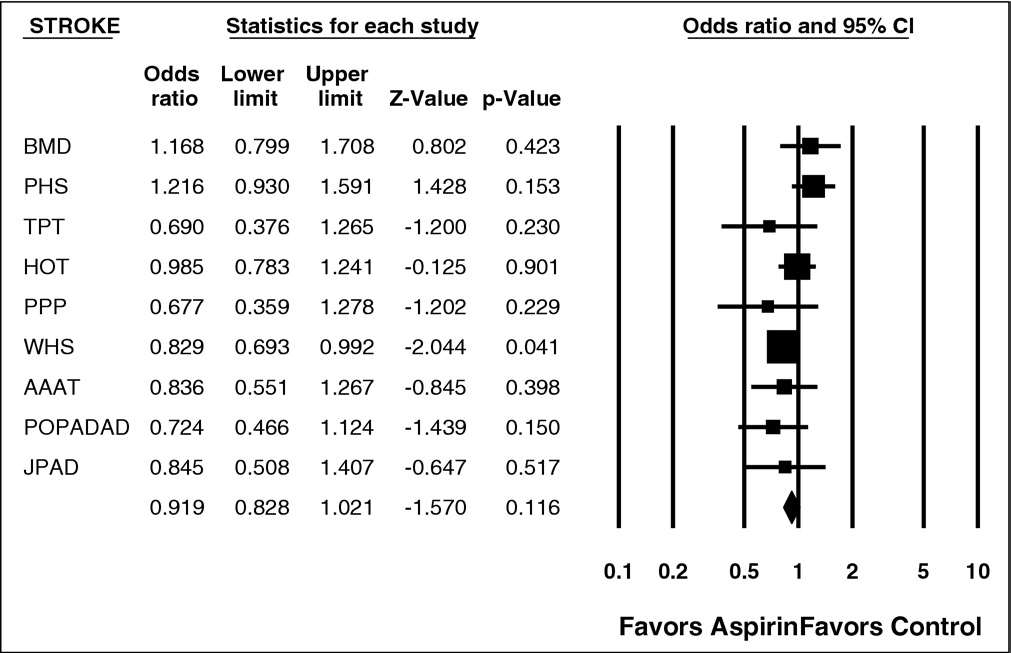
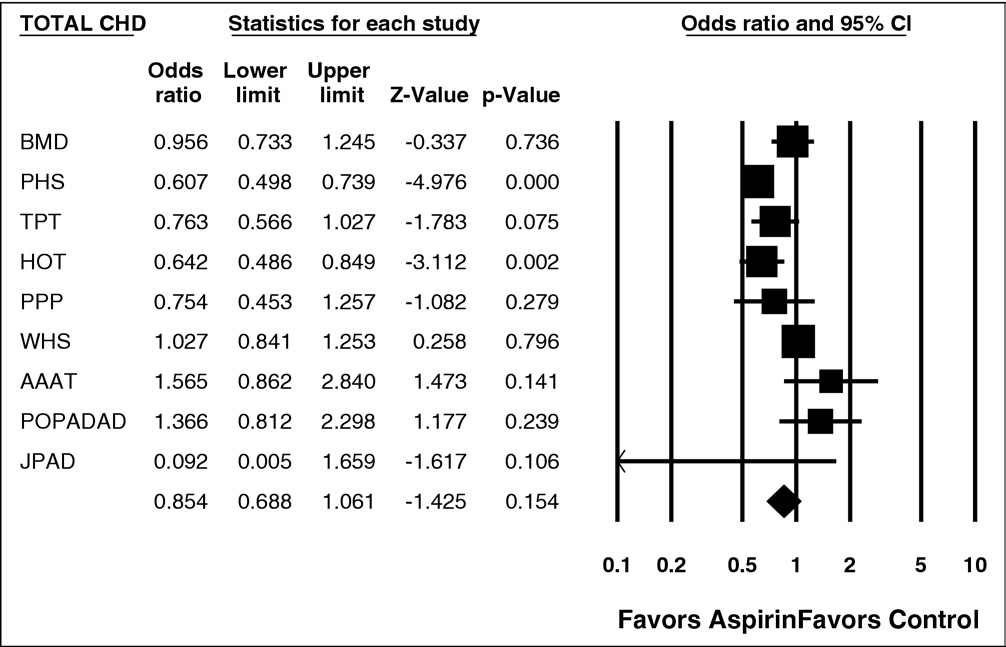
Among the 9 trials in the analysis, 50,868 subjects were
groups of aspirin and control with respect to the odds of anevent such as MI. Calculation of the overall effect combin-
treated with aspirin and 49,170 received placebo or control.
ing the 9 studies used the Mantel-Haenszel chi-square sta-
lists each study and indicates if statistical signifi-
tistic with 1 degree of freedom. This test does not assume
cance of aspirin versus placebo was reached when using the
that patients in 1 study can be directly compared with those
odds ratio for any of the end points or groups of end points.
in another study, and it does not assume that any treatment
The combined effects of aspirin on these end points are
effects are similar in different studies. It does not assume
listed in In subjects treated with aspirin, there was
homogeneity but does take into account heterogeneity. Het-
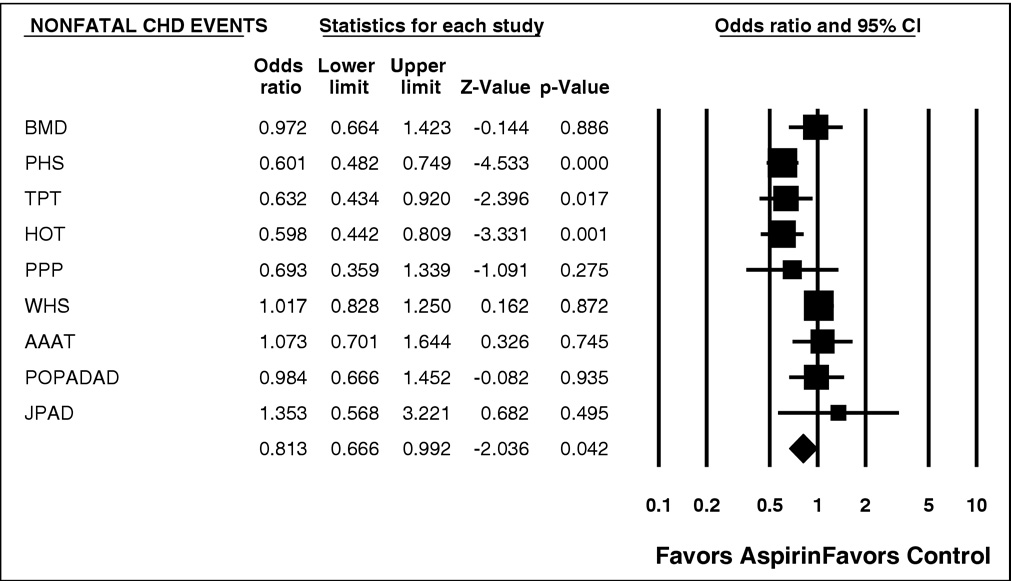
significantly decreased risk for nonfatal MI (p ⫽ 0.042) and
erogeneity was calculated using the chi-square test with n ⫺
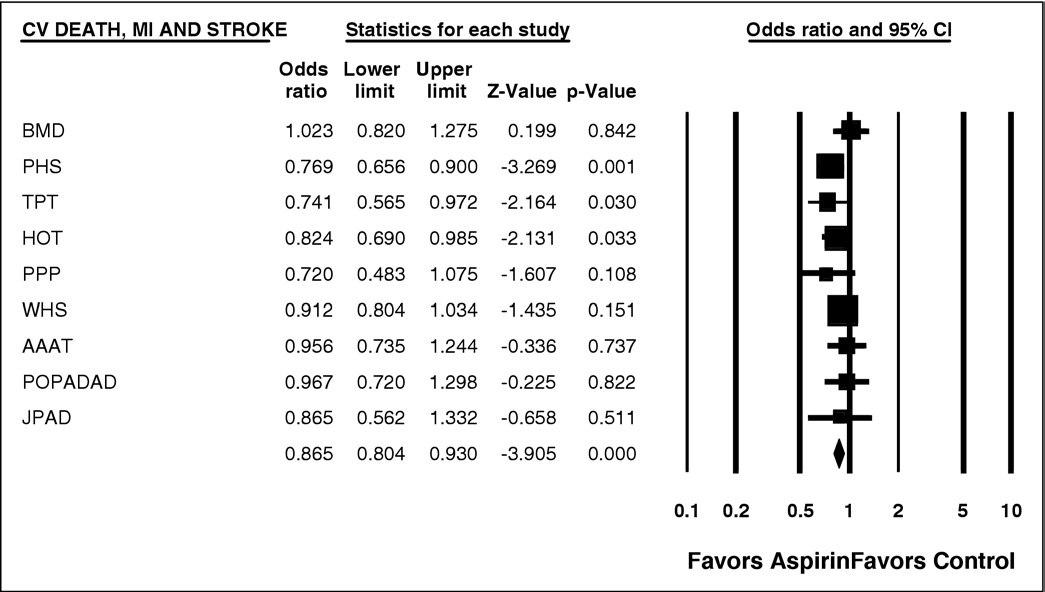
total CV events (p ⫽ 0.001). There was significant hetero-
1 degrees of freedom, where n represents the number of
geneity (p ⱕ0.01) for several of the end points listed in
studies contributing to the meta-analysis. Forest plots were
(e.g., for total CHD events and nonfatal MI). The
used to assess if there was significant heterogeneity (defined
data for nonfatal MI and total CV events are shown in
as p ⬍0.01) and allowed assessment by considering the
and respectively.
direction of the results. A weighting factor was also used
to present different outcomes in the studies
that depended in part on the size of the study, which in turn
included in the current meta-analysis. They show that most
affected the inverse variance formula that the Mantel-Haen-
studies have odds ratios ⬍1, with an overall advantage of
szel procedure uses to calculate heterogeneity. The random-
aspirin over placebo. We had the ability to reexamine our
effects model also helps further account for the heteroge-
results with respect to previous risk for CHD in studies that
The American Journal of Cardiology (www.ajconline.org)
Figure 1. Forest plot of nonfatal CHD events. CI ⫽ confidence interval.
Figure 2. Forest plot of CV deaths, MI, and stroke. CI ⫽ confidence interval.
included TPT, PPP, AAAT, POPADAD, and JPAD, and we
We included the 2 trials conducted in patients with diabetes
found no change in our results. The remainder of the studies
mellitus and no symptoms of CV disease (POPADAD and
showed no previous risk for CHD. The results were consis-
JPAD). Although patients with diabetes might represent a
tent with those listed in all in favor of aspirin.
population different from those with high CV risk on the basisof the presence of classic risk factors alone, the studies clearly
represent the primary prevention setting.
It is evident that aspirin is beneficial for patients who
Systematic analysis of the outcomes from the 9 trials con-
have previously been diagnosed with CHD and is probably
firmed that aspirin decreases the incidence of nonfatal MI and
beneficial to all patients at high risk for developing CHD, on
CV events. However, aspirin had no statistically significant
the basis of an appropriate assessment of known risk factors.
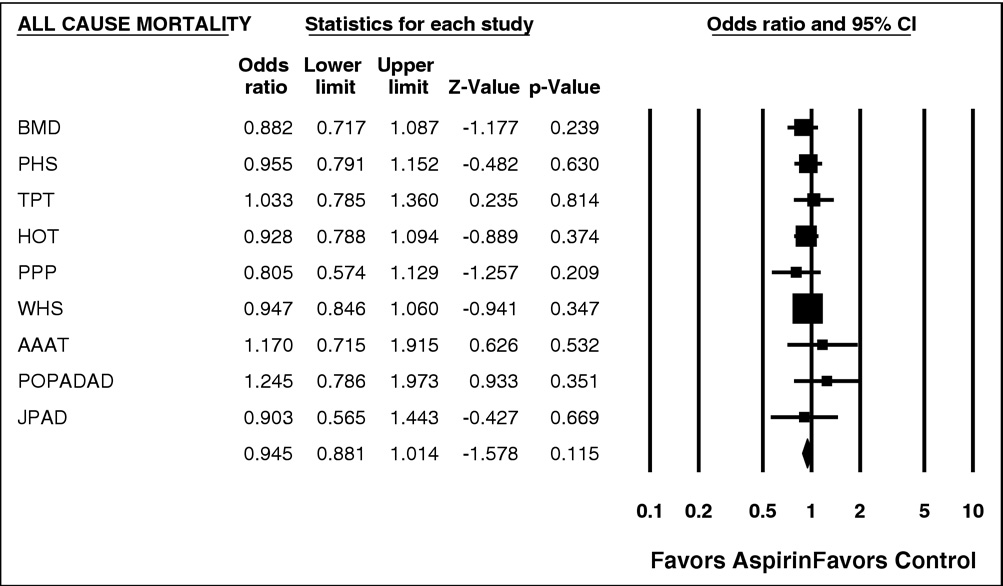
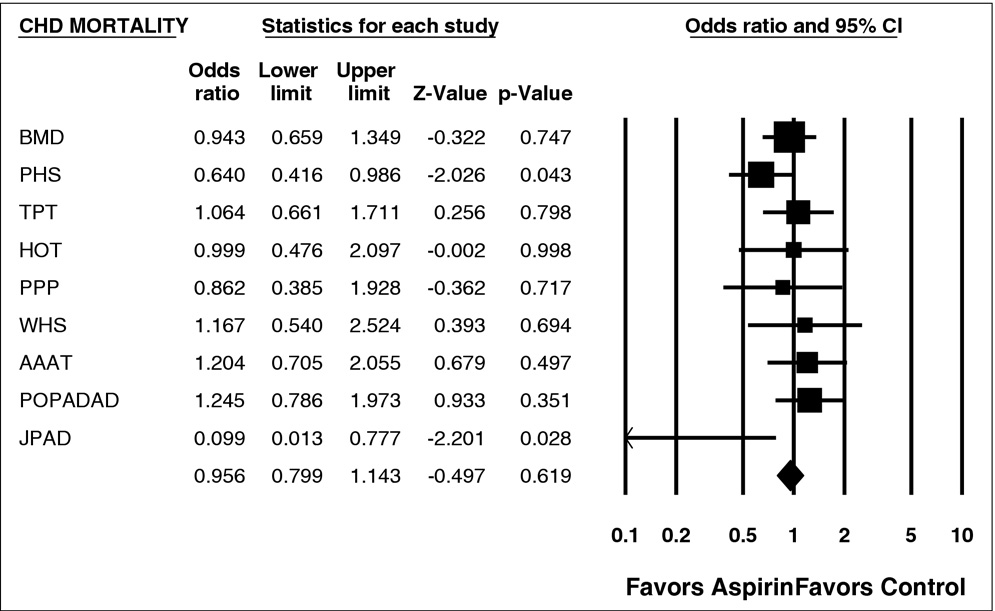
effect on CHD, stroke, CV mortality, and all-cause mortality,
However, the 3 most recent studies on the use of aspirin in
but was highly significant for overall CV events.
primary prevention for the most part were statistically in-
In the ATTC antiplatelet therapy decreased the
Although all these studies were underpow-
combined outcome of any serious vascular event by about
ered, there was a need to reassess the use of aspirin in this
25%, nonfatal MI by about 33%, nonfatal stroke by 25%,
setting. We present a meta-analysis of all trials published to
and vascular mortality by about 16%. Our results are for the
date assessing the effect of aspirin in primary CV prevention.
most part consistent with the ATTC's results. The ATTC
Preventive Cardiology/Meta-Analysis of Aspirin in Primary Prevention
Figure 3. Forest plot of all-cause mortality. CI ⫽ confidence interval.
Figure 4. Forest plot of CHD mortality. CI ⫽ confidence interval.
caution that in primary prevention aspirin is of uncertain net
tained with the random-effects model, which accounts for
value, because the reduction in occlusive events needs to be
the randomness of the effects across studies.
weighed against any increase in major bleeds. Bleeds or
The HOT and WHS are relatively larger than the other
major bleeds were not our focus here, and we agree with the
studies, accounting for approximately 59% of the sample.
ATTC that gastrointestinal bleeds, strokes, and heart attacks
Thus, the meta-analysis accommodates for this difference by
may not be equivalent, as we examine these end points
assigning them greater weight (sample size) than the other
separately. See for a summary of gastrointestinal
studies. In addition, these 2 studies, when weighted accord-
bleeds across studies.
ingly in the assessment of study size bias, did not contribute
In our study, there was heterogeneity across studies for
significantly to any bias, as presented in However,
several outcomes. Possible sources of this heterogeneity
weighting can also take into account other information in
include patient selection and randomization, baseline dis-
studies, such as length of follow-up, the detail of patient char-
ease severity, management of intercurrent outcomes (such
acteristics, information on entry, and eligibility criteria. This
as bleeding, gastritis, and hypertension), and treatment strat-
information may vary across studies and, if available, will be
egies. However, the overall difference between aspirin and
scored or weighted differently in each study.
placebo, as shown in this meta-analysis, is not affected by
A limitation of our study is that it is a meta-analysis of
significant heterogeneity, because similar results were ob-
the results of published studies. Thus, we did not have the
The American Journal of Cardiology (www.ajconline.org)
Figure 5. Forest plot of stroke events. CI ⫽ confidence interval.
Figure 6. Forest plot of total CHD. CI ⫽ confidence interval.
ability to do thorough cross-study checks that can be done
Gastrointestinal bleeding for the 9 study meta-analysis
using the raw data. Furthermore, the overall size of oursample and the differing cohorts within each study lend
convincing evidence to the advantage of aspirin over pla-
cebo or no aspirin for decreasing the risk for CV events in
a range of patients. However, the benefits of primary pre-
vention with aspirin must be considered in relation to the
potential risks on a patient-by-patient basis.
1. Bartolucci AA, Howard G. Meta-analysis of data from the six primary
prevention trials of cardiovascular events using aspirin. Am J Cardiol
2006;98:746 –750.
Preventive Cardiology/Meta-Analysis of Aspirin in Primary Prevention
2. Fowkes FGR, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC,
of individual participant data from randomized clinical trials. Lancet
Sandercock PA, Fox KA, Lowe GD, Murray GD, for the Aspirin for
Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of car-
6. United States Preventive Services Task Force. Aspirin for the primary
diovascular events in a population screened for a low ankle brachial
prevention of cardiovascular events: recommendation and rationale.
index. JAMA 2010;303:841– 848.
Ann Intern Med 2002;136:157–160.
3. Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott RL,
7. Egger M, Smith D, Schneider M, Minder C. Bias in meta-analysis
Bancroft J, MacEwan S, Shepard J, Macfarlane P, Morris A, Jung R,
detected by a simple graphical test. BMJ 1997;315:629 – 634.
Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G,
8. Egger M, Juni P, Bartlett C, Sterne J. How important are comprehen-
McKnight J, O'Brien I, Semple C, Petrie J, Gordon D, Pringle S,
sive literature searches and the assessment of trial quality in systematic
MacWalter R; Prevention of Progression of Arterial Disease and Di-
reviews? Empirical study. Health Technol Assess 2003;7:1– 4.
abetes Study Group; Diabetes Registry Group; Royal College of Phy-
9. Irwig L, Macaskill P, Berry G, Glasziou P. Bias in meta-analysis
sicians Edinburgh. The Prevention of Progression of Arterial Disease
detected by a simple graphical test. Graphical test is itself biased. BMJ
and Diabetes (POPADAD) trial: factorial randomized placebo con-
trolled trial of aspirin and antioxidants in patients with diabetes and
10. Sterne J, Egger M, Smith D. Investigating and dealing with publication
asymptomatic peripheral arterial disease. BMJ 2008;337:a1840.
and other biases in meta-analysis. BMJ 2001;323:101–105.
4. Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N,
11. Macaskill P, Walter S, Irwig L. A comparison of methods to detect
Jinnouchi H, Sugiyama S, Saito Y, for the Japanese Primary Preven-
publication bias in meta-analysis. Stat Med 2001;20:641– 654.
tion of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Inves-
12. Schwarzer G, Antes G, Shumacher M. Inflation of type I error rate in
tigators. Low-dose aspirin for primary prevention of atherosclerotic
two statistical tests for the detection of publication bias in metaanalysis
events in patients with type 2 diabetes: a randomized controlled trial.
with binary outcomes. Stat Med 2002;21:2465–2477.
JAMA 2008;300:2134 –2141.
13. Harbord R, Egger M, Sterne J. A modified test for small study effects
5. Antithrombotic Trialists' Collaboration. Aspirin in the primary and
in meta-analysis of controlled trials with binary endpoints. Stat Med
secondary prevention of vascular disease: collaborative meta-analysis
Source: http://jacemedical.com/articles/Aspirin%20does%20not%20decrease%20deaths%20from%20heart%20attacks%20or%20all-cause%20mortality.pdf
New Release Dana Pickel Dana Pickel GYMINI® Sunny Day GYMINI® Belle journée GYMINI® Zonnige Dag GYMINI® Día de Sol GYMINI® Dia Ensolarado Guía de instrucciones PLEASE KEEP THIS INSTRUCTION GUIDE AS IT CONTAINS IMPORTANT INFORMATION. READ CAREFULLY.
The Neurobehavioral Pharmacology of Ketamine: Implications for Drug Abuse, Addiction, and Psychiatric Disorders Keith A. Trujillo, Monique L. Smith, Brian Sullivan, Colleen Y. Heller, Cynthia Garcia, and Melvin Bates Ketamine was initially developed in the 1960s as a safer alternative to phencyclidine (PCP1) for anes- Ketamine was developed in the early 1960s as an anesthetic






