Jammonline.com
J Arch Mil Med. 2015 August; 3(3): e30057. DOI: 10.5812/jamm.30057 Published online 2015 August 24.
Assessment of Consensus-Based Pharmacological Therapies in Irritable
Seyed Reza Abtahi 1,*; Parvin Zareian 11Department of Physiology and Pharmacology, School of Medicine, AJA University of Medical Sciences, Tehran, IR Iran
*Corresponding author: Seyed Reza Abtahi, Department of Physiology and Pharmacology, School of Medicine, AJA University of Medical Sciences, Tehran, IR Iran. Tel: +98-2188337909,
Received: May 18, 2015
; Revised: May 26, 2015
; Accepted: June 8, 2015
Context: The irritable bowel syndrome (IBS) is a symptom-based gastrointestinal (GI) disease with the presence of symptoms such as
abdominal pain and abnormal intestinal activities. It is a frequent GI problem encountered by physicians. The purpose of this paper was
to review and assess some of the current and emerging pharmacological therapies for this syndrome.
Evidence Acquisition: In the present study, data on the IBS were principally collected via Google Scholar and PubMed, followed by articles
in journals and libraries.
Results: The pathophysiology of the IBS has yet to be fully elucidated. Global medical attempts, including pharmacological therapy and
herbal remedies, aim at curing and/or subsiding pain, flatulence, diarrhea, and constipation.
Conclusions: There is a need for new drugs in the setting of pharmacological therapy for the IBS. A new medical approach should include
both novel and traditional drugs in order to reach to a desirable outcome for patients and improve their quality of life.
Keywords: Diagnosis; Irritable Bowel Syndrome; Physiopathology; Pharmacotherapy
1. Context
The irritable bowel syndrome (IBS) is a functional gas-
organic disease (7). The Manning criteria were improved
trointestinal (GI) tract disturbance affecting a consider-
and published as the Rome I criteria in 1990. These new
able portion of the general population and is responsible
criteria were more detailed and contained more useful
for a large number of visits to physicians. The salient
definitions of the syndrome (8). A decade later, the Rome
characteristic of the IBS is recurrent abdominal pain/
I criteria were revised and upgraded into the Rome II
discomfort with a concurrent disturbance in defecation
criteria in order to suggest a relation between pain and
(1). The IBS encompasses a wide array of physiological
stool consistency (8, 9). Ultimately, the Rome III criteria
and psychological signs and symptoms which affect the
were presented in 2006 (10). The Rome III is a precise and
cerebro-intestinal regulation, GI tract activity, and viscer-
more specified modification of the Rome II criteria. In the
al perception (1, 2). The symptoms include altered bowel
Rome III, pain must be confirmed at least 3 days a month
habits, without any organic pathology (3). Considerable
in the previous 3 months (10). Hence, it is now possible to
evidence suggests that most patients with the IBS appear
determine the exact prevalence and incidence of the IBS
to have an enhanced perception of the overdistension of
in accordance with the Rome criteria and forge compat-
their rectum. This visceral hypersensitivity is presented
ibility in the studies conducted in this field.
by an increased intensity of sensations, intolerable intes-
The IBS exerts a negative influence on the lifestyle and
tinal pain, and/or propagated viscerosomatic referral in
daily activity of many of its sufferers (11-14), but it is still
comparison to healthy subjects (4). Patients with the IBS
not clear whether it increases the patients' mortality and
demonstrate a number of other symptoms such as back
morbidity. What is clear, however, is that this syndrome
pain, migraine headaches, epigastric pain, dyspareunia,
places a substantial financial burden on health care sys-
and myalgia compatible with the possibility of central
tems. The diagnosis of the IBS is made based on the crite-
pain hypersensitivity mechanisms (5).
ria and exclusion of organic disease (10% - 15%) (11). The IBS
The IBS is a common gastrointestinal disease respon-
was once thought to be common in females exacerbated
sible for the patients' referral to GI tract specialists (6).
by anxiety and depression. Recent statistical analyses
The first symptom-based criteria for the evaluation of
have, nonetheless, suggested that the female gender is
the IBS were presented in 1978 by Manning et al. (7). The
no longer a risk factor (15).
Manning criteria classified patients with abdominal
The prevalence of the IBS is on the increase in devel-
pain on the basis of whether or not they suffered from
oped and particularly in developing countries. Pharma-
Copyright 2015, AJA University of Medical Sciences. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommer-
cial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/) which permits copy and redistribute the material just in noncommercial usages,
provided the original work is properly cited.
Abtahi SR et al.
cological agents are used widely in all countries; some of
havior approximately once a year (20, 21), and some suf-
these agents have proved their efficacy, while others have
fer from gastric emptying, especially of solid foods (24-
achieved partial clinical benefits (16). A review of psycho-
26), which is particularly considerable in patients with
logical factors in patients who respond to specific psy-
constipation (27, 28). Furthermore, emotional status and
chological treatment plays a key role in their follow-up
anxiety attenuate the contractility of the stomach and
(17). To ensure simplicity, the present study does not delve
lead to delayed emptying in comparison with healthy
into the details of statistical procedures.
individuals (29). The peristaltic activity of the small in-
testine is faster in the IBS-D than in the other two types
2. Evidence Acquisition
(30). Colonic distension (an intestino-intestinal inhibi-
tory reflex that decreases duodenal motility) is impaired
(31). Disturbed contractile activity is responsible for these
2.1. Methodology
clinical features (32, 33). In these patients, postprandial
The databases drawn upon for data acquisition in the
peristaltic hyperactivity and propagated motor response
present study were Google Scholar, PubMed, and librar-
are more severe than those in healthy individuals (34-37).
ies and journals of the medical universities of the Islamic
Republic of Iran as well as AJAUMS. The focus, however,
3.3. Bloating
was on the data published in the English language in the
Abdominal pain and abnormal gas handling as a result
last 25 years, and in particular the past 10 years.
of gas retention are very common. Abnormal contrac-
tile activity, accompanied by visceral hypersensitivity, is
3. Results
more important than abdominal distention due to intes-
tinal gas (33). It has been reported that patients suffering
3.1. Clinical Manifestation of Irritable Bowel Syn-
from bloating have disturbed transit of extrinsic excess
gas, which exacerbates their symptoms (25). Nutrition,
physical activity, and body posture are factors that may
The cardinal manifestations of the IBS consist of con-
alleviate this problem (38-42).
tinuous or relapsing abdominal pain and/or bloating, ac-
Ninety-six per cent of individuals suffer from abdomi-
companied by change in defecation behavior, in the lack
nal gas retention, which is more frequent in women (43).
of constitutional disorders probable to assess for these
However, this is not considered a differentiated character
symptoms. Subjects are observed for over 6 months to
but an important feature of the syndrome (10). The pa-
evaluate the presence of other diseases such as temporary
tients tend to complain of a diurnal starting of abdomi-
infections or the GI tract malignancy, which are detected
nal gas excess, especially postprandial, which usually
approximately within 6 months of symptom onset (18-20).
subsides or is relieved by the evening, which helps to de-
Based on the predominant defecation pattern, the IBS
termine the differential diagnosis of abdominal swelling
is sub-classified to diarrhea predominant (IBS-D) and
such as an ovarian cyst or ascites (44, 45). Distension is
constipation predominant (IBS-C). Additionally, a mixed
only consistent with bloating in the IBS-C patients (60%)
bowel pattern (IBS-M) with both loose and hard stool is
in comparison with the IBS-D patients (40%) (45).
noted (18-20).
According to the Rome III criteria, the main manifesta-
3.4. Serotonin (5-HT) Role in Motility and Secretion
tions of the IBS are abdominal discomfort, which is obvi-
ously related to intestinal dysfunction and is alleviated
by defecation, and alteration in stool frequency or con-
It is obvious that serotonin functions as a crucial neu-
sistency. The most common clinical features are bloating,
rotransmitter in the GI tract and participates in the
abnormal stool form and frequency, abdominal cramp,
pathophysiological status of the IBS. Serotonin modu-
and mucus in feces. The patients tend to complain of the
lates the GI autonomic nervous system, which influences
intervals of remission and the exacerbation of the symp-
the enhancement or inhibition of the GI secretion and
toms (21, 22).
motility. Abnormality in the serotonin signaling path-
way gives rise to various annoying features of the disease
3.2. Abnormal Esophageal, Gastric, Small Intesti-
(46). Serotonin excess is consistent with the IBS-D, and
nal, and Colonic Motility
the inadequate secretion of serotonin is an important
factor in the manifestation of the IBS-C. What confirms
Large and small intestine activity is widely studied in
this hypothesis is that the postprandial blood levels of
the IBS. There are some reports on the esophagus and
serotonin in individuals with the IBS-D are elevated. In
stomach motility dysfunction. These malfunctions de-
addition, the platelet-depleted plasma 5-HT levels are
crease the lower esophageal sphincter pressure and lead
elevated before and after meals. The tissue level of 5-hy-
to abnormal contraction activity (23). Abnormal colon
droxyindoleacetic acid (5-HIAA)/5-HT ratio is attenuated
activity is known as the spastic and irritable colon (8).
in individuals with the IBS-C, and there is a remarkable
Twenty per cent of individuals alter their defecation be-
deficit in high plasma 5-HT fed levels in individuals with
J Arch Mil Med. 2015;3(3):e30057
Abtahi SR et al.
the IBS-C (46). Serotonin is gathered in the special cells
larly specialized for the management of this syndrome
(enterochromaffin cells) of the GI system and plays a cru-
in recent years. Therapeutic protocols recommend that
cial role in the contractile activity, visceral sensitivity, and
the patient's predominant complaint such as pain, con-
gut secretion (47).
stipation, and diarrhea be addressed first and foremost.
For the treatment of the symptoms of the IBS-D in fe-
Multiple drugs are used for the management of this syn-
males, the 5-HT3 antagonists are used widely. Ischemic
drome, albeit with low effect on subsiding pain and gas
colitis is an untoward effect and limits the prescription of
retention. Therapeutic goals emphasize on the control
these agents. Moreover, the 5-HT4 agonists have been ap-
of the autonomic nervous system in the GI tract in order
proved for use in the management of the IBS-C in women
to improve bowel habits following the relaxation of the
as well as in the other constipation categories.
smooth muscle cells. This intervention attenuates the
Serotonin is responsible for visceral hypersensitivity
visceral noxious perception signaling. In the motility dys-
and contractile activity, and also participates in the mod-
function of the GI tract, increasing or decreasing intesti-
ulation of the GI tract secretion and absorption (48, 49).
nal contractile activity is crucial (36, 50, 51). The chemical
It has been suggested that the 5-HT4 receptor agonists
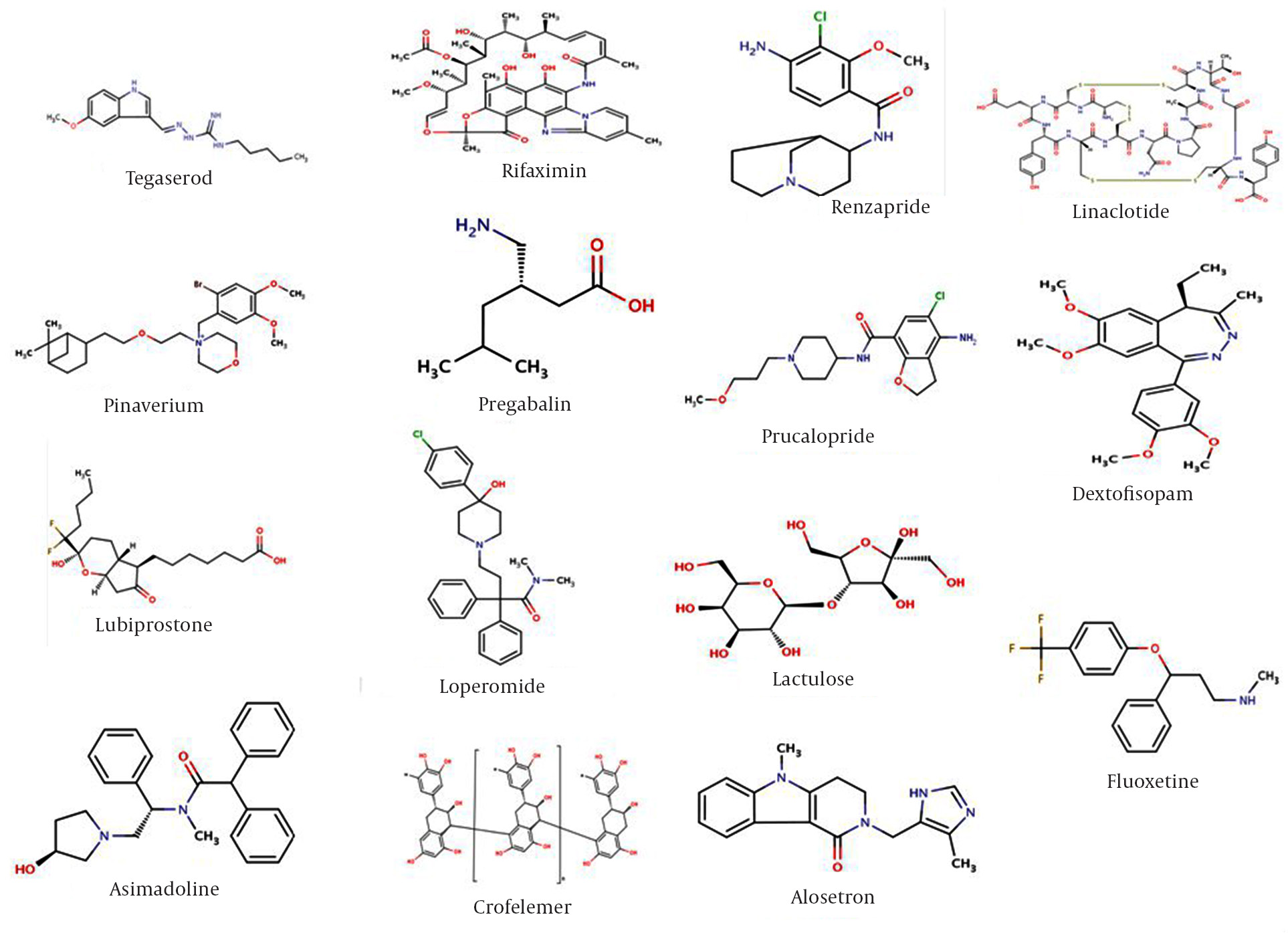
structures of some agents are depicted in Figure 1.
enhance the release of water and electrolytes in the small
intestine (49).
3.6. Drugs Acting on Central Nervous System
3.5. Consensus-Based Pharmacological Treatment
Some drugs function within the central nervous system
for Irritable Bowel Syndrome
(CNS). Dextofisopam is an R-enantiomer of tofisopam
that binds to a specific site of the gamma-aminobutyric
The pharmacological management of the IBS, albeit cru-
acid (GABA) receptors, inhibiting nerve signaling, and its
cial, presents the prescriber with a major therapeutic di-
advantages in the management of the IBS-D have been
lemma. There have been no highly specific drugs particu-
previously approved (52).
Figure 1. Chemical Structures of Some Agents
J Arch Mil Med. 2015;3(3):e30057
Abtahi SR et al.
3.7. Kappa (κ)-Opioid Receptor Agonists
the United States food and drug administration (FDA).
Visceral pain control in the GI system is a target for drug
This drug is recommended for female patients who suf-
prescription. Peripheral κ-opioid receptor agonists block
fer from the relapsing symptoms of the IBS-D (67), and a
the signaling of the afferent pain perception from the in-
meta-analysis study has demonstrated its advantages in
testines with no untoward effects such as dependency and
females with the IBS-D (68, 69). Alosetron is more effec-
constipation, which are observed in μ opioid agonists.
tive than placebo in relieving visceral hypersensitivity
Kappa receptors are distributed in the stomach, large
and improving abnormal defecation behavior, stool con-
intestine, and cerebrum (53, 54). Asimadoline, a selective
sistency, and colonic spasm; nevertheless, the drug is ab-
κ-opioid receptor agonist, possesses a low blood-brain
solutely contraindicated in constipation (68-70). Cilanse-
barrier penetration potency and a negligible concentra-
tron is a newer similar agent for the management of the
tion level in the CNS. Its analgesic effect is modulated
IBS-D and is prescribed for 3 to 6 months to treat noxious
by the attenuation of nerve excitement (55, 56). Many
visceral perception and disturbed bowel habits in all pa-
human studies have shown that asimadoline decreases
tients (67, 71). The 5-HT4 receptor agonists modulate the
intestinal pain perception with no serious side effects
visceral afferent function. The probable mechanism is
(53-57). The potential and favorable role of the drug in the
the release of acetylcholine via the presynaptic 5-HT4 re-
management of the IBS has led to further research (57),
ceptor on the cholinergic neurons. Tegaserod is a strong,
and statistical studies have shown defined and promis-
bearable aminoguanidine indole selective partial ago-
ing advantages in the control of visceral noxious percep-
nist at the 5-HT4 receptor and has been approved for the
tion and disturbed bowel habits in the D-IBS (58).
management of the IBS-C (72). This agent has been evalu-
ated in well-extended studies (16, 72) and has prokinetic
effects in the small and large intestines (72-74). Tegaserod
3.8. Antispasmodic Drugs
accelerates the upper and lower intestine contractile ac-
Anticholinergic agents inhibit the muscarinic recep-
tivity, induces prokinetic effects on the stomach, enhanc-
tors in the GI tract, decelerating the propagated contrac-
es bowel secretion, and improves constipation in women
tile activities before and after meals, particularly in the
with the IBS-C (75). The advantages are those associated
IBS-D (36). The efficacy of antispasmodic agents has been
with defecation frequency (76). Some studies have shown
evaluated by several meta-analyses (59-62).
that this drug enhances the quality of life (72, 74). The
Hyoscine, peppermint oil, and cimetropium are an-
most common untoward effect of tegaserod (6 mg twice
tispasmodic and relax the smooth muscle cells of the
daily) is predictably diarrhea (77). Prucalopride is a newer
GI system (anticholinergic). Colonic spasm is a noxious
5-HT4 receptor agonist and has acceptable advantages in
symptom and a target of antispasmodic gents (63).
the management of the IBS-C. In Europe, this drug is rec-
The nonspecific antagonists of the muscarinic recep-
ommended for the treatment of recurrent constipation
tor are dicyclomine and hyoscyamine (tertiary amines)
in females (78).
as well as glycopyrrolate (quaternary ammonium com-
pound) and methscopolamine. The low lipid solubility of
3.10. 5-HT4 Receptor Agonists/5-HT3 Receptor An-
the latter two compounds decreases the brain-blood bar-
rier passage and lessens the CNS untoward effects such as
drowsiness and nervousness (64).
Renzapride is a mixed serotonin receptor agonist/an-
Mebeverine is a musculotropic antispasmodic agent
tagonist. It may be more effective than a single agent and
and a derivative of hydroxybenzamide. It has a direct ef-
is prescribed for the therapeutic management of the IBS-
fect on smooth muscle cells and blocks Na+, K+, and Ca2+
C. This drug facilitates motility thanks to its 5-HT4 recep-
channels (64). Mebeverine has no serious side effects and
tor agonist mechanism, although its efficacy in the treat-
is used before meals. The efficacy of the drug in compari-
ment of the IBS has yet to be documented (79).
son with placebo has been confirmed (64).
3.11. Aminobutyric Acid Analogue
3.9. 5-HT3 Receptor Antagonists
Pregabalin is a novel, second-generation γ-aminobutyric
acid (GABA) analog (α2δ ligand). It attenuates nerve ac-
The 5-HT3 receptor acts in the sensitization of the spi-
tion potential and blocks the release of excitatory neu-
nal sensory neurons, nausea stimulating vagal nerve,
rotransmitters associated with depolarization-induced
and peristaltic reflexes (49, 65). Alosetron is a potent
calcium influx (80, 81). The effectiveness of pregabalin in
5-HT3 receptor antagonist and is more effective than an-
the management of pain with different origins has been
tispasmodic agents (49). It inhibits basal secretion in the
demonstrated (80, 81).
healthy jejunum (66) and is prescribed to improve the
quality of life in the IBS-D females (67). Ischemic colitis
3.12. Drugs Affecting Chloride Channels in Gastro-
and constipation are the untoward effects of alosetron,
intestinal Tract
and they resulted in the withdrawal of this drug from the
market in 2000. Alosetron was reintroduced in 2002 by
Some agents act on the chloride channels by activating
J Arch Mil Med. 2015;3(3):e30057
Abtahi SR et al.
or inhibiting the efflux of chloride ions into the lumen
peripheral anticholinergic and non-SSRI effects. TCAs are
of the GI tract. This phenomenon results in the mainte-
prescribed in a various neurotic and visceral pain (89).
nance of isoelectric equilibrium and isotonic environ-
The drugs may change pain perception (90), especially
ment by moving water in the GI tract lumen and sodium
during severe stress (93), in addition to their antidepres-
efflux subsequently. Accelerated intestinal secretion and
sant or antianxiety effects (90). Many studies have shown
excess fluid volume provide a new opportunity for the
that low-dose TCAs effectively decrease symptoms. Ami-
management of the patients suffering from prolonged
triptyline, trimipramine, desipramine, clomipramine,
constipation and the IBS-C (82, 83).
and doxepin possess the pharmacological efficacy and
potency to relieve pain. Mianserin is an SSRI and has simi-
3.13. Activators
lar effects. The initial analgesic effects of these drugs have
led to the continuous use of these agents in individu-
Lubiprostone activates the chloride ions channels. The
als with the IBS-D (94). The untoward effects of TCAs are
FDA recommends this agent for the management of the
parasympatholytic symptoms such as constipation and
IBS-C in females (82, 83). In addition, linaclotide is anoth-
blared vision, observed in 30% of subjects (90). These un-
er activator of the chloride ions channels and functions
toward effects often lead to drug discontinuation; it is,
as a guanylate cyclase-C agonist. This agent is an activa-
therefore, essential that prescribers counsel the patients
tor of the cystic fibrosis transmembrane regulator (CFTR)
about these side effects and explain the drug function
chloride ion channels (84). There have been promising
and the need for its consumption for 1 to 4 weeks (94, 95).
results in the use of lubiprostone for the management of
It is recommended that TCAs be used for 6 to 12 months
the IBS-C (85-87).
and then tapered (93, 96).
3.14. Inhibitors
3.17. Selective Serotonin Re-Uptake Inhibitors
The K channels and the 3Na/2K pump develop an elec-
trochemical driving force for the release ofchloride,
The effect of SSRIs on the GI tract motility is more pro-
which is followed by the secretion of sodium and water.
nounced than that on decreasing visceral pain. Parox-
The CFTR regulates the chloride ion channels. CFTR is
etine accelerates gastric accommodation in healthy in-
an inhibitory enterocytes membrane component and
dividuals and does not affect fasting gastric compliance
blocks GI secretion.
(45, 97). SSRIs are widely used and have no serious untow-
Crofelemer interacts with the CFTR inhibitory function.
ard effects in the management of psychiatric disorders
It also possesses advantages in the management of the
(98, 99). In four studies, the therapeutic standard dose
IBS-D and enhances secretion (88).
regimen of SSRIs was applied in the IBS and improvement
in lifestyle and global good advantages were observed. In
addition, there was no considerable effect on noxious vis-
ceral perception (90, 97, 98). In another clinical study, 84%
The application of antidepressants in a considerable
of the subjects who received SSRIs (vs. 37% on placebo)
part of gastrointestinal disorders is accepted. The anal-
wanted to continue with the drug. SSRIs have convenient
gesic effect of some of these drugs is responsible for the
advantages in patients with somatization (100, 101).
relief of pain in these patients. The concurrent existence
of anxiety and depression with the IBS is remarkable. An-
3.18. Fiber and Laxatives
tidepressants are effective in relieving the visceral pain
in the IBS through modulating the interactions between
Constipation is a frequent complaint in the IBS-D. Many
the central and enteric nervous systems (46, 84, 89-91).
different drugs with naturally herbal-derived ingredi-
Antidepressants are categorized as selective serotonin
ents and/or chemical origins are applied to relieve this
re-uptake inhibitors (SSRIs), tricyclics and related antide-
symptom. Fiber such as bran and methylcellulose helps
pressants, and monoamine oxidase inhibitors (MAOIs).
form bulky feces and improve large intestinal motility.
The drugs include duloxetine, flupentixol, mirtazapine,
The most important laxatives are divided into four
reboxetine, tryptophan, and venlafaxine (84, 91, 92).
groups: fecal softeners (e.g. liquid paraffin); stimulant
It should also be considered that, in addition to antide-
laxatives (e.g. bisacodyl); osmotic laxatives (e.g. meth-
pressants therapy, psychological management has a cru-
ylcellulose); and bulk-forming laxatives (e.g. lactulose).
cial role in the IBS treatment.
There is no sign of intestinal damage in the long-term
prescription of laxatives, but the side effect of laxatives is
3.16. Tricyclic Antidepressants
hypokalemia. The untoward effects of stimulant laxatives
are dependency and tachyphylaxis (102-104).
Functional gastrointestinal disorders (FGIDs) and the
IBS are the therapeutic targets of tricyclic antidepres-
sants (TCAs). In a comprehensive study, one third of the
3.19. Antidiarrheal Drugs
subjects used an antidepressant, although the purpose of
The analogues of the opioid receptors in the GI tract
taking the drug was not obvious (92). TCAs are drugs with
such as diphenoxylate and loperamide inhibit intesti-
J Arch Mil Med. 2015;3(3):e30057
Abtahi SR et al.
nal motility and secretion. The anti-diarrheal effect of
emerging therapies in the IBS based upon the evolving
loperamide is higher than its anti-spasmodic effect (105,
understanding of the pathophysiology of this disorder.
106). Loperamide has negligible side effects. A combina-
The chief complaint of the IBS patients is abdominal
tion of diphenoxylate and atropine (co-phenotrope) is
pain. A percentage of these patients present with aggra-
available, but loperamide is preferred. Another agent is
vated pain sensitivity to gut distention (visceral hyper-
codeine phosphate, which is not convenient due to its
risk of dependency (107). Both co-phenotrope and lop-
Various pharmacological agents have been used in the
eramide may be prescribed for the management of this
management of the IBS, but with limited efficacy for the
syndrome (107, 108).
symptom-based approach. Consensus-based pharmaco-
logical therapy in the IBS advocates the use of traditional
3.20. Antibiotics
and/or novel drugs that have an equivalent effect on pain,
flatulence, diarrhea, and constipation. The prescribed
Sometimes bacterial gastroenteritis results in the devel-
medications consist of TCAs, SSRIs, antispasmodics, cen-
opment of the IBS, as has been demonstrated in a large
trally acting agents, κ-opioid receptor agonists, 5-HT3
number of studies (109). The lactulose hydrogen breath
receptor agonists and antagonists, aminobutyric acid
test is useful and is positive in 70% of the IBS patients
analog, and agents acting on the chloride channels in
(110). In the absence of bacteria in the small intestine,
the GI tract. In addition, antimotility drugs may subside
lactulose fermentation does not occur and it reaches the
diarrhea and laxatives may alleviate constipation. Finally,
large intestine without any chemical conversion (111). The
not only do probiotics offer considerable potential in the
presence of small intestinal bacterial overgrowth is sug-
treatment of FGIDs, but also there is now new evidence to
gestive for starting chemotherapy (109).
support their efficacy in the management of the IBS.
It is recommended to prescribe wide-spectrum antibi-
otics such as clarithromycin, ciprofloxacin, amoxicillin,
metronidazole, and doxycycline for 10 days. Thirty-three
of these patients became asymptomatic (112). Rifaximin
The authors would like to thank the IT Service of AJA uni-
has also been demonstrated to be efficacious (113, 114).
versity of medical sciences for supporting and providing
data acquisition facilities.
3.21. Probiotics
Lilly and Stillwell were the first scientists to introduce
Seyed Reza Abtahi was responsible for the study concept
probiotics in 1965. These agents modulate the intestinal
design and the primary writing of the article. Parvin Zare-
flora (115). Of all FGIDs, the IBS has enjoyed most attention
ian was responsible for the review of the article and study
in terms of the possible role of the intestinal flora in the
pathogenesis of probiotics in its therapy (116). Disequilib-
rium within the normal flora is suggested to play a part
in the pathogenesis of the IBS (114).
Probiotics are microbial-derived factors that stimulate
1. Camilleri M, Chang L. Challenges to the therapeutic pipeline for
irritable bowel syndrome: end points and regulatory hurdles.
the growth of other organisms (117). Probiotics offer a less
effective way for altering the bowel flora. Randomized,
2. Schwetz I, Bradesi S, Mayer EA. The pathophysiology of irritable
placebo-controlled trials of probiotics have shown ben-
bowel syndrome. Minerva Med. 2004;95(5):419–26.
3. Hammer J, Eslick GD, Howell SC, Altiparmak E, Talley NJ. Diag-
efits with respect to some symptoms, especially bloating
nostic yield of alarm features in irritable bowel syndrome and
and flatulence.
functional dyspepsia. Gut. 2004;53(5):666–72.
Probiotics Bifidobacteria and Lactobacilli species have
4. Talley NJ, Spiller R. Irritable bowel syndrome: a little understood
been shown to improve the IBS symptoms (116). Bifido-
organic bowel disease? Lancet. 2002;360(9332):555–64.
5. Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral
bacterium infantis (Bifidobacteria would displace the
and cutaneous pain in the irritable bowel syndrome. Pain.
proteolytic bacteria, which cause diarrhea) has shown
benefit by attenuating the hypersensitivity of the im-
6. Harvey RF, Salih SY, Read AE. Organic and functional disorders in
mune response (116, 118, 119).
2000 gastroenterology outpatients. Lancet. 1983;1(8325):632–4.
7. Saito YA, Locke GR, Talley NJ, Zinsmeister AR, Fett SL, Melton L3.
A comparison of the Rome and Manning criteria for case iden-
tification in epidemiological investigations of irritable bowel
syndrome. Am J Gastroenterol. 2000;95(10):2816–24.
The IBS is the most common gastrointestinal disorder
8. Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review:
seen in primary care. The pathophysiology of the IBS is
the efficacy of treatments for irritable bowel syndrome--a Euro-
pean perspective. Aliment Pharmacol Ther. 2006;24(2):183–205.
complex and unclear. Both central and peripheral factors,
9. Boyce PM, Talley NJ, Burke C, Koloski NA. Epidemiology of the
including psychosocial factors, abnormal GI motility and
functional gastrointestinal disorders diagnosed according to
secretion, and visceral hypersensitivity, are thought to
Rome II criteria: an Australian population-based study. Intern
contribute to the symptoms of the IBS.
10. Drossman DA. The functional gastrointestinal disorders and the
The present review discussed some of the current and
Rome III process. Gastroenterology.. USA: Wiley; 2010. pp. 1377–90.
J Arch Mil Med. 2015;3(3):e30057
Abtahi SR et al.
11. Lovell RM, Ford AC. Global prevalence of and risk factors for ir-
sponse in patients with irritable bowel syndrome during mental
ritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepa-
tol. 2012;10(7):712–721 e4.
33. Fink RN, Lembo AJ. Intestinal Gas. Curr Treat Options Gastroenterol.
12. Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, et al.
Impairment in work productivity and health-related quality of
34. Narducci F, Bassotti G, Granata MT, Pelli MA, Gaburri M, Palumbo
life in patients with IBS. Am J Manag Care. 2005;11(1 Suppl):S17–26.
R, et al. Colonic motility and gastric emptying in patients with ir-
13. Sun SX, Dibonaventura M, Purayidathil FW, Wagner JS, Dabbous
ritable bowel syndrome. Effect of pretreatment with octylonium
O, Mody R. Impact of chronic constipation on health-related
bromide. Dig Dis Sci. 1986;31(3):241–6.
quality of life, work productivity, and healthcare resource use:
35. Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motil-
an analysis of the National Health and Wellness Survey. Dig Dis
ity abnormality in patients with irritable bowel syndrome
exhibiting abdominal pain and diarrhea. Am J Gastroenterol.
14. Chang L. Review article: epidemiology and quality of life in
functional gastrointestinal disorders. Aliment Pharmacol Ther.
36. Sullivan MA, Cohen S, Snape WJ. Colonic myoelectrical activity in
2004;20 Suppl 7:31–9.
irritable-bowel syndrome. Effect of eating and anticholinergics.
15. Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of
N Engl J Med. 1978;298(16):878–83.
enterochromaffin cell hyperplasia, anxiety, and depression in
37. Rogers J, Henry MM, Misiewicz JJ. Increased segmental activ-
ity and intraluminal pressures in the sigmoid colon of patients
16. Andrews EB, Eaton SC, Hollis KA, Hopkins JS, Ameen V, Hamm LR,
with the irritable bowel syndrome. Gut. 1989;30(5):634–41.
et al. Prevalence and demographics of irritable bowel syndrome:
38. Serra J, Azpiroz F, Malagelada JR. Gastric distension and duode-
results from a large web-based survey. Aliment Pharmacol Ther.
nal lipid infusion modulate intestinal gas transit and tolerance
in humans. Am J Gastroenterol. 2002;97(9):2225–30.
17. Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel
39. Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, et
syndrome in general practice: prevalence, characteristics, and
al. Symptoms and visceral perception in patients with pain-
predominant irritable bowel syndrome. Am J Gastroenterol.
18. Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE,
Hughes AO. Defecation frequency and timing, and stool form in
40. Dainese R, Serra J, Azpiroz F, Malagelada JR. Effects of physical
the general population: a prospective study. Gut. 1992;33(6):818–24.
activity on intestinal gas transit and evacuation in healthy sub-
19. Mearin F, Balboa A, Badia X, Baro E, Caldwell E, Cucala M, et al.
jects. Am J Med. 2004;116(8):536–9.
Irritable bowel syndrome subtypes according to bowel habit:
41. Dainese R, Serra J, Azpiroz F, Malagelada JR. Influence of body
revisiting the alternating subtype. Eur J Gastroenterol Hepatol.
posture on intestinal transit of gas. Gut. 2003;52(7):971–4.
42. Passos MC, Serra J, Azpiroz F, Tremolaterra F, Malagelada JR. Im-
20. Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The im-
paired reflex control of intestinal gas transit in patients with ab-
pact of irritable bowel syndrome on health-related quality of
dominal bloating. Gut. 2005;54(3):344–8.
43. Houghton LA, Whorwell PJ. Towards a better understanding of
21. Hahn B, Watson M, Yan S, Gunput D, Heuijerjans J. Irritable bowel
abdominal bloating and distension in functional gastrointesti-
syndrome symptom patterns: frequency, duration, and severity.
nal disorders. Neurogastroenterol Motil. 2005;17(4):500–11.
Dig Dis Sci. 1998;43(12):2715–8.
44. Van Oudenhove L, Demyttenaere K, Tack J, Aziz Q. Central ner-
22. Swarbrick ET, Hegarty JE, Bat L, Williams CB, Dawson AM. Site of
vous system involvement in functional gastrointestinal disor-
pain from the irritable bowel. Lancet. 1980;2(8192):443–6.
ders. Best Pract Res Clin Gastroenterol. 2004;18(4):663–80.
23. Whorwell PJ, Clouter C, Smith CL. Oesophageal motil-
45. Vahedi H, Merat S, Rashidioon A, Ghoddoosi A, Malekzadeh R.
ity in the irritable bowel syndrome. Br Med J (Clin Res Ed). 1981;
The effect of fluoxetine in patients with pain and constipation-
predominant irritable bowel syndrome: a double-blind random-
24. Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rec-
ized-controlled study. Aliment Pharmacol Ther. 2005;22(5):381–5.
tal perception is a biological marker of patients with irritable
46. Spiller R. Serotonin and GI clinical disorders. Neuropharmacol-
bowel syndrome. Gastroenterology. 1995;109(1):40–52.
25. van Wijk HJ, Smout AJ, Akkermans LM, Roelofs JM, ten Thije OJ.
47. Camilleri M, Andresen V. Current and novel therapeutic op-
Gastric emptying and dyspeptic symptoms in the irritable bowel
tions for irritable bowel syndrome management. Dig Liver Dis.
syndrome. Scand J Gastroenterol. 1992;27(2):99–102.
26. van der Voort IR, Osmanoglou E, Seybold M, Heymann-Mon-
48. McLean PG, Borman RA, Lee K. 5-HT in the enteric nervous sys-
nikes I, Tebbe J, Wiedenmann B, et al. Electrogastrography as a
tem: gut function and neuropharmacology. Trends Neurosci.
diagnostic tool for delayed gastric emptying in functional dys-
pepsia and irritable bowel syndrome. Neurogastroenterol Motil.
49. Gershon MD, Tack J. The serotonin signaling system: from basic
understanding to drug development for functional GI disorders.
27. Caballero-Plasencia AM, Valenzuela-Barranco M, Herrerias-
Gutierrez JM, Esteban-Carretero JM. Altered gastric empty-
50. Patrizi F, Freedman SD, Pascual-Leone A, Fregni F. Novel therapeu-
ing in patients with irritable bowel syndrome. Eur J Nucl Med.
tic approaches to the treatment of chronic abdominal visceral
28. Welgan P, Meshkinpour H, Ma L. Role of anger in antral motor ac-
51. Trinkley KE, Nahata MC. Medication management of irritable
tivity in irritable bowel syndrome. Dig Dis Sci. 2000;45(2):248–51.
bowel syndrome. Digestion. 2014;89(4):253–67.
29. Kumar D, Wingate D. Irritable bowel syndrome: a paroxysmal
52. Leventer SM, Raudibaugh K, Frissora CL, Kassem N, Keogh JC,
motor disorder. Lancet. 1986;1(8481):620–1.
Phillips J, et al. Clinical trial: dextofisopam in the treatment of
30. Muller-Lissner S, Koch G, Talley NJ, Drossman D, Rueegg P, Dung-
patients with diarrhoea-predominant or alternating irritable
er-Baldauf C, et al. Subject's Global Assessment of Relief: an ap-
bowel syndrome. Aliment Pharmacol Ther. 2008;27(2):197–206.
propriate method to assess the impact of treatment on irritable
53. Camilleri M. Novel pharmacology: asimadoline, a kappa-opi-
bowel syndrome-related symptoms in clinical trials. J Clin Epide-
oid agonist, and visceral sensation. Neurogastroenterol Motil.
31. Fukudo S, Kanazawa M, Kano M, Sagami Y, Endo Y, Utsumi A, et
54. Shekhar C, Whorwell PJ. Emerging drugs for irritable bowel syn-
al. Exaggerated motility of the descending colon with repetitive
drome. Expert Opin Emerg Drugs. 2009;14(4):673–85.
distention of the sigmoid colon in patients with irritable bowel
55. Delgado-Aros S, Chial HJ, Cremonini F, Ferber I, McKinzie S, Bur-
syndrome. J Gastroenterol. 2002;37 Suppl 14:145–50.
ton DD, et al. Effects of asimadoline, a kappa-opioid agonist, on
32. Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H,
satiation and postprandial symptoms in health. Aliment Pharma-
Simren M. Altered visceral perceptual and neuroendocrine re-
col Ther. 2003;18(5):507–14.
J Arch Mil Med. 2015;3(3):e30057
Abtahi SR et al.
56. Delvaux M, Beck A, Jacob J, Bouzamondo H, Weber FT, Frexinos J.
A, et al. A randomised controlled trial assessing the efficacy and
Effect of asimadoline, a kappa opioid agonist, on pain induced
safety of repeated tegaserod therapy in women with irritable
by colonic distension in patients with irritable bowel syndrome.
bowel syndrome with constipation. Gut. 2005;54(12):1707–13.
Aliment Pharmacol Ther. 2004;20(2):237–46.
78. Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Pru-
57. Szarka LA, Camilleri M, Burton D, Fox JC, McKinzie S, Stanislav T,
calopride (Resolor) in the treatment of severe chronic constipa-
et al. Efficacy of on-demand asimadoline, a peripheral kappa-
tion in patients dissatisfied with laxatives. Gut. 2009;58(3):357–65.
opioid agonist, in females with irritable bowel syndrome. Clin
79. Ford AC. Renzapride in IBS: is efficacy in the eye of the beholder?
Aliment Pharmacol Ther. 2010;32(1):113–4.
58. Mangel AW, Hicks GA. Asimadoline and its potential for the treat-
80. Ben-Menachem E. Pregabalin pharmacology and its relevance to
ment of diarrhea-predominant irritable bowel syndrome: a re-
clinical practice. Epilepsia. 2004;45 Suppl 6:13–8.
view. Clin Exp Gastroenterol. 2012;5:1–10.
81. Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD.
59. Mozaffari S, Nikfar S, Abdollahi M. Metabolic and toxicological
Effect of a second-generation alpha2delta ligand (pregabalin)
considerations for the latest drugs used to treat irritable bowel
on visceral sensation in hypersensitive patients with irritable
syndrome. Expert Opin Drug Metab Toxicol. 2013;9(4):403–21.
bowel syndrome. Gut. 2007;56(9):1218–25.
60. Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of
82. Lacy BE, Levy LC. Lubiprostone: a novel treatment for chronic
the irritable bowel syndrome: a systematic review of random-
constipation. Clin Interv Aging. 2008;3(2):357–64.
ized, controlled trials. Ann Intern Med. 2000;133(2):136–47.
83. Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and safety of
61. Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth
lubiprostone in patients with chronic constipation. Dig Dis Sci.
muscle relaxants in the treatment of irritable bowel syndrome.
Aliment Pharmacol Ther. 2001;15(3):355–61.
84. Clouse RE, Lustman PJ. Use of psychopharmacological agents for
62. American College of Gastroenterology Task Force on Irritable
functional gastrointestinal disorders. Gut. 2005;54(9):1332–41.
Bowel S, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR,
85. Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG,
Schoenfeld PS, et al. An evidence-based position statement on
et al. Linaclotide for irritable bowel syndrome with consti-
the management of irritable bowel syndrome. Am J Gastroen-
pation: a 26-week, randomized, double-blind, placebo-con-
terol. 2009;104 Suppl 1:S1–35.
trolled trial to evaluate efficacy and safety. Am J Gastroenterol.
63. Dann RHD, Brunton LL. Irritable bowel syndrome. Goodman and
Gilman's Manual of pharmacology and therapeutics. 2 ed. Califor-
86. Lembo AJ, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA,
nia: Mc GrawHill; 2014. pp. 812–3.
et al. Efficacy of linaclotide for patients with chronic constipa-
64. Kruis W, Weinzierl M, Schussler P, Holl J. Comparison of the thera-
tion. Gastroenterology. 2010;138(3):886–95 e1.
peutic effect of wheat bran, mebeverine and placebo in patients
87. Corsetti M, Tack J. Linaclotide: A new drug for the treatment of
with the irritable bowel syndrome. Digestion. 1986;34(3):196–201.
chronic constipation and irritable bowel syndrome with consti-
65. Camilleri M. Pharmacology and clinical experience with alos-
pation. United European Gastroenterol J. 2013;1(1):7–20.
etron. Expert Opin Investig Drugs. 2000;9(1):147–59.
88. Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an anti-
66. Lucak S. Irritable bowel syndrome and ischemic colitis: evidence
secretory antidiarrheal proanthocyanidin oligomer extracted
supporting the increased use of alosetron. Therap Adv Gastroen-
from Croton lechleri, targets two distinct intestinal chloride
channels. Mol Pharmacol. 2010;77(1):69–78.
67. Lesbros-Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum
89. Jackson JL, O'Malley PG, Tomkins G, Balden E, Santoro J, Kroen-
AL. Meta-analysis: The treatment of irritable bowel syndrome.
ke K. Treatment of functional gastrointestinal disorders
Aliment Pharmacol Ther. 2004;20(11-12):1253–69.
with antidepressant medications: a meta-analysis. Am J Med.
68. Imtiaz M, Qadir MI. Management of irritable bowel syndrome.
Management of irritable bowel syndrome. 2011;2(2):11–4.
90. Adeyemo MA, Chang L. New treatments for irritable bowel syn-
69. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron
drome in women. Womens Health (Lond Engl). 2008;4(6):605–22.
in irritable bowel syndrome: a meta-analysis of randomized con-
91. Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF,
trolled trials. Neurogastroenterol Motil. 2003;15(1):79–86.
et al. International survey of patients with IBS: symptom features
70. Chey WD, Chey WY, Heath AT, Dukes GE, Carter EG, Northcutt A,
and their severity, health status, treatments, and risk taking to
et al. Long-term safety and efficacy of alosetron in women with
achieve clinical benefit. J Clin Gastroenterol. 2009;43(6):541–50.
severe diarrhea-predominant irritable bowel syndrome. Am J
92. Drossman DA. Beyond tricyclics: new ideas for treating patients
with painful and refractory functional gastrointestinal symp-
71. Caras S, Krause G, Biesheuvel E, Steinborn C. Cilansetron shows
toms. Am J Gastroenterol. 2009;104(12):2897–902.
efficacy in male and female non-constipated patients with irri-
93. Mertz H, Fass R, Kodner A, Yan-Go F, Fullerton S, Mayer EA. Ef-
table bowel syndrome in a United States study. Gastroenterology.
fect of amitriptyline on symptoms, sleep, and visceral percep-
tion in patients with functional dyspepsia. Am J Gastroenterol.
72. Bracco A, Jonsson B, Ricci JF, Drummond M, Nyhlin H. Economic
evaluation of tegaserod vs. placebo in the treatment of patients
94. Vahedi H, Merat S, Momtahen S, Kazzazi AS, Ghaffari N, Olfati G,
with irritable bowel syndrome: an analysis of the TENOR study.
et al. Clinical trial: the effect of amitriptyline in patients with
diarrhoea-predominant irritable bowel syndrome. Aliment Phar-
73. Novick J, Miner P, Krause R, Glebas K, Bliesath H, Ligozio G, et al. A
randomized, double-blind, placebo-controlled trial of tegaserod
95. Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind place-
in female patients suffering from irritable bowel syndrome with
bo-controlled trial of amitriptyline for the treatment of irritable
constipation. Aliment Pharmacol Ther. 2002;16(11):1877–88.
bowel syndrome in adolescents. J Pediatr. 2008;152(5):685–9.
74. Reilly MC, Barghout V, McBurney CR, Niecko TE. Effect of tegas-
96. Mertz HR. Irritable bowel syndrome. N Engl J Med. 2003;
erod on work and daily activity in irritable bowel syndrome with
constipation. Aliment Pharmacol Ther. 2005;22(5):373–80.
97. Tack J, Broekaert D, Coulie B, Fischler B, Janssens J. Influence of
75. McLaughlin J, Houghton LA. The rationale, efficacy and safety
the selective serotonin re-uptake inhibitor, paroxetine, on gas-
evidence for tegaserod in the treatment of irritable bowel syn-
tric sensorimotor function in humans. Aliment Pharmacol Ther.
drome. Expert Opin Drug Saf. 2006;5(2):313–27.
76. Muller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E,
98. Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Parox-
Nault B, et al. Tegaserod, a 5-HT(4) receptor partial agonist, re-
etine to treat irritable bowel syndrome not responding to high-
lieves symptoms in irritable bowel syndrome patients with ab-
fiber diet: a double-blind, placebo-controlled trial. Am J Gastroen-
dominal pain, bloating and constipation. Aliment Pharmacol Ther.
99. Creed F, Fernandes L, Guthrie E, Palmer S, Ratcliffe J, Read
77. Tack J, Muller-Lissner S, Bytzer P, Corinaldesi R, Chang L, Viegas
N, et al. The cost-effectiveness of psychotherapy and parox-
J Arch Mil Med. 2015;3(3):e30057
Abtahi SR et al.
etine for severe irritable bowel syndrome. Gastroenterology.
109. Thabane M, Kottachchi DT, Marshall JK. Systematic review and
meta-analysis: The incidence and prognosis of post-infectious irri-
100. Aragona M, Bancheri L, Perinelli D, Tarsitani L, Pizzimenti A, Con-
table bowel syndrome. Aliment Pharmacol Ther. 2007;26(4):535–44.
te A, et al. Randomized double-blind comparison of serotonergic
110. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal
(Citalopram) versus noradrenergic (Reboxetine) reuptake inhib-
bacterial overgrowth reduces symptoms of irritable bowel syn-
itors in outpatients with somatoform, DSM-IV-TR pain disorder.
drome. Am J Gastroenterol. 2000;95(12):3503–6.
Eur J Pain. 2005;9(1):33–8.
111. Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD,
101. Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers AM, Jans-
Thomas MC. The lactulose breath hydrogen test and small intes-
sens J. A controlled crossover study of the selective serotonin
tinal bacterial overgrowth. Am J Gastroenterol. 1996;91(9):1795–803.
reuptake inhibitor citalopram in irritable bowel syndrome. Gut.
112. Talley NJ. Pharmacologic therapy for the irritable bowel syn-
drome. Am J Gastroenterol. 2003;98(4):750–8.
102. Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Sys-
113. Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. Ri-
tematic review: the role of different types of fibre in the treat-
faximin therapy for patients with irritable bowel syndrome
ment of irritable bowel syndrome. Aliment Pharmacol Ther.
without constipation. N Engl J Med. 2011;364(1):22–32.
114. Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a
103. Francis CY, Whorwell PJ. Bran and irritable bowel syndrome:
nonabsorbed oral antibiotic (rifaximin) on the symptoms of the
time for reappraisal. Lancet. 1994;344(8914):39–40.
irritable bowel syndrome: a randomized trial. Ann Intern Med.
104. Attar A, Lemann M, Ferguson A, Halphen M, Boutron MC, Flourie
B, et al. Comparison of a low dose polyethylene glycol electrolyte
115. Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of
solution with lactulose for treatment of chronic constipation.
probiotics in the treatment of irritable bowel syndrome: a sys-
tematic review. Am J Gastroenterol. 2009;104(4):1033–49.
105. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loper-
116. Quigley EM, Flourie B. Probiotics and irritable bowel syndrome: a
amide and placebo in management of irritable bowel syndrome
rationale for their use and an assessment of the evidence to date.
(IBS). Dig Dis Sci. 1984;29(3):239–47.
106. Lavo B, Stenstam M, Nielsen AL. Loperamide in treatment of irri-
117. Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probi-
table bowel syndrome--a double-blind placebo controlled study.
otic mixture alleviates symptoms in irritable bowel syndrome
Scand J Gastroenterol Suppl. 1987;130:77–80.
patients: a controlled 6-month intervention. Aliment Pharmacol
107. Palmer KR, Corbett CL, Holdsworth CD. Double-blind cross-
over study comparing loperamide, codeine and diphenoxyl-
118. Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-
ate in the treatment of chronic diarrhea. Gastroenterology.
blind, randomized study on the efficacy of Lactobacillus planta-
rum 299V in patients with irritable bowel syndrome. Eur J Gastro-
108. Williams AJ, Merrick MV, Eastwood MA. Idiopathic bile acid
malabsorption--a review of clinical presentation, diagnosis, and
119. Korpela R, Niittynen L. Probiotics and irritable bowel syndrome.
response to treatment. Gut. 1991;32(9):1004–6.
Microb Ecol Health Dis. 2012;23.
J Arch Mil Med. 2015;3(3):e30057
Source: http://jammonline.com/46053.pdf
Toward Fulfilling the Promise of Molecular Medicine in Fragile X Syndrome Dilja D. Krueger and Mark F. Bear The Picower Institute for Learning and Memory, Howard Hughes Medical Institute,Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology,Cambridge, Massachusetts 02139; email: [email protected] Annu. Rev. Med. 2011. 62:411–29 First published online as a Review in Advance on
CSIRO PUBLISHING Historical Records of Australian Science, 2013, 24, 242– Mollie Elizabeth Holman 1930–2010 Elspeth M. McLachlanA,C and G. David S. HirstB A Neuroscience Research Australia, Randwick, NSW 2031, Australia. B86 Caroline Street, South Yarra, Vic. 3141, Australia. CCorresponding author. Ema Mollie Holman was a biophysicist whose work on the autonomic nervous system and the innervation of smooth muscle was seminal in advancing knowledge of its behaviour at a cellular level. She was particularly known for her technical expertise in microelectrode recording of membrane potential from single smooth muscle cells, and the interpretation of their electrical activity, both spontaneous and in response to transmitters released from their autonomic nerves.

