Toward a new gold standard for early safety: automated temperature-controlled herg test on the patchliner®
Non-Clinical Safety, Pharma Research, F. Hoffmann-La Roche Ltd., Basel, Switzerland
The Patchliner®temperature-controlled automated patch clamp system was evaluated for
Ralf Franz Kettenhofen, Axiogenesis
testing drug effects on potassium currents through human ether-à-go-go related gene
AG, Germany
(hERG) channels expressed in Chinese hamster ovary cells at 35–37˚C. IC50 values for a
set of reference drugs were compared with those obtained using the conventional voltage
clamp technique. The results showed good correlation between the data obtained using
automated and conventional electrophysiology. Based on these results, the Patchliner®
Jean-Marie Chambard, Sanofi, France
represents an innovative automated electrophysiology platform for conducting the hERG
Aurore Colomar, Union chimique
assay that substantially increases throughput and has the advantage of operating at phys-
belge, Belgium
iological temperature. It allows fast, accurate, and direct assessment of channel function
*Correspondence:
Liudmila Polonchuk , Non-Clinical
to identify potential proarrhythmic side effects and sets a new standard in ion channel
Safety, Pharma Research, F.
research for drug safety testing.
Hoffmann-La Roche Ltd.,Grenzacherstr. 124, Building, 73/R.
Keywords: hERG, Patchliner®, automated patch clamp, physiological temperature
103b, CH-4070 Basel, Switzerland.
e-mail:
indicated a strong need for a high-quality electrophysiological
The cardiac proarrhythmic liability of new pharmacological enti-
method for early hERG profiling of a larger number of drug
ties is a major concern for pharmaceutical companies and regu-
latory agencies. Remarkably, many if not most compounds exert
Fortunately, automation has entered the area of experimen-
their arrhythmogenic effects through the same electrophysiologi-
tal electrophysiology over the past few years. The first automated
cal mechanism: blockade of repolarizing currents through human
patch clamp instruments focused on increasing throughput by the
ether-à-go-go related gene (hERG) K+ channels located in ven-
parallel formation of high-resistance seals using the planar patch
tricular myocytes ). As a
clamp at ambient temperature (reviewed in ). Var-
result,
in-vitro hERG assay has been introduced into the drug
ious companies have recently launched second-generation auto-
development process to test the proarrhythmic potential of small
mated platforms, including the Patchliner® (Nanion Technologies
molecules ).
GmbH, Munich, Germany), a fully automated patch clamp sys-
The assay evaluates the acute effects of new therapeutic agents on
tem with integrated temperature control. The planar NPC® chip
hERG channels stably expressed in mammalian cell lines (such
electrode combined with temperature control add-on is designed
as HEK293 and CHO cells). By directly measuring a compound's
to control giga-Ohm seal formation at 35–37˚C and accurately
ability to block hERG K+ channels it evaluates the proarrhyth-
maintain temperatures over an experiment. By recording from
mic liability associated with this fairly common arrhythmogenic
up to eight cells simultaneously at physiological temperature, the
Patchliner® claims to substantially increase through put without
The gold standard for assessing ion channel functionality is the
compromising data quality.
patch clamp technique. The high-resolution conventional man-
The objective of the present study was to transfer the hERG
ual method involves a glass micropipette filled with an ionic
assay at physiological temperature to the Patchliner® and eval-
solution that electrically connects an electrode wire to a small
uate the automated platform for its ability to correctly deter-
patch of cell membrane. Considerable expertise is needed and
mine potency and IC50 values for reference compounds including
throughput is low as only one cell can be recorded at a time.
known hERG blockers and negative controls.
In addition, accurate drug safety assessment requires a highlysensitive temperature-controlled patch clamp since the current
MATERIALS AND METHODS
kinetics is temperature-dependent and inappropriate experimen-
CELL CULTURE
tal temperature may compromise the pharmacological proper-
The CHO crelox hERG cell line (ATCC reference Nr. PTA-6812)
ties of the hERG current Thus thor-
was generated and validated at Roche ).
ough analysis of hERG liabilities under physiological condi-
Recombinant hERG K+ channels originally cloned from human
tions has only been possible to date for advanced compounds
heart were stably expressed in CHO cells grown in sterile tissue
using the conventional manual patch clamp technique. How-
flasks in DMEM/F-12 (1:1) medium (Invitrogen, USA) supple-
ever, the high attrition rate due to cardiovascular adverse effects
mented with 10% (v/v) heat-inactivated fetal calf serum (FCS)
Patchliner® hERG assay
(Hyclone, USA) and 500 μg/ml gentamycin solution (Gibco, UK)at 35–37˚C in 5% CO2.
Confluent cell cultures were subcultured every 2–3 days using
Accumax (Innovative Cell Technologies Inc., USA). Before elec-trophysiological experiments, cells were treated with Accumax for3–5 min at 42˚C, harvested, and centrifuged at 1000 rpm for 1 min.
The pellet was gently resuspended in the extracellular solution anddirectly used for electrophysiological recording.
SOLUTIONS
The extracellular solution contained (mM): NaCl 150; KCl 4;
CaCl2 1.2; MgCl2 1; HEPES 10; pH 7.2–7.6 with NaOH, osmolar-
ity 290–330 mOsm. The internal solution contained (mM): KCl,
50; KF, 60; NaCl, 10; HEPES, 10; EGTA, 20; pH 7.0–7.4 with KOH,
osmolarity 260–300 mOsm.
All solutions were filtered through 0.2 μM GP Millipore Express
PLUS membrane and stored in aliquots at +2–8˚C.
COMPOUNDS
Olanzapine was synthesized by Roche (Basel, Switzerland). Amio-
darone, astemizole, bepridil, clemastine, haloperidol, terfena-
dine, and verapamil were purchased from Sigma (USA), cis-
apride from Research Diagnostic Inc. (USA), E-4031 from
Wako Chemicals (Japan), quinidine from Aldrich Chemicals
(USA), d,l-Sotalol from Bristol-Myers-Squibb (USA), amoxi-
cillin, fluoxetine, isradipine, and tamoxifen from USP (USA),
glyburide/glibenclamide from Calbiochem (USA), ibuprofen from
ICN Biomedicals Inc. (USA), moxifloxacin from APIChem Tech-
nologies Co. (China), and erythromycin and captopril from Fluka
(Switzerland).
Selection of test concentrations for each compound was done
based on the hERG potency data and the solubility in the extracel-lular solution. Stock solutions of compounds were freshly preparedin DMSO. Test solutions were made such that solvent concen-
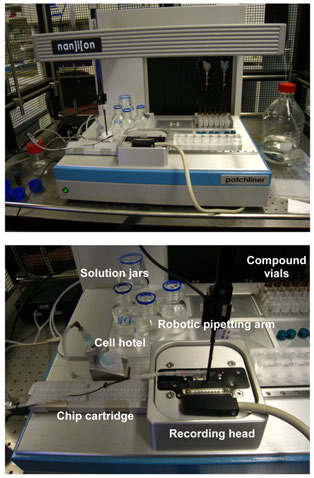
FIGURE 1 The Patchliner® is shown at the top panel and a closer view
trations were kept constant throughout the experiment (0.1%).
of the working area is presented below.
Compound solutions were transferred to 4 mL glass vials that wereplaced in the compound plate holder of the patch clamp system
for a few minutes and currents were steady, IKhERG amplitude andkinetics were recorded under control conditions (vehicle control)
AUTOMATED PATCH CLAMP PROCEDURES ON THE PATCHLINER®
for 3–5 min. Thereafter, a single test item concentration was added
On the day of the experiment, an aliquot of the cell suspension in
and incubated for at least 3 min until a new steady state current
the extracellular solution was placed in the cell hotel
level was reached (see and for the experimental pro-
Cells were added to each chip well and attracted to the holes by
cedure). At the end of the compound incubation, a 1 μM solution
suction applied by a pressure controller. Once a stable whole-cell
of standard IKhERG blocker E-4031 was applied twice to each cell
configuration was obtained, the experimental protocol was ini-
for 1 min to suppress IKhERG entirely.
tiated. K+ currents were measured with a patch–voltage-clamp
IKhERG amplitudes were recorded at four to five drug concen-
technique in the whole-cell configuration at 35–37˚C using the
trations. The hERG current was measured as the average current
QuadroEPC-10 amplifier (HEKA Elektronik GmbH, Germany)
from five sweeps collected at the end of vehicle or compound addi-
and associated software (Patch MasterPro). Currents were low-
tion. After subtracting current levels from that of full block (i.e.,
pass filtered using the internal Bessel filter of the EPC-10 and
positive control) they were compared to the vehicle control values
digitized at 50 kHz. Series resistance was typically 2–4 MΩ and
(taken as 100%) to define fractional blocks. The fractional block
values at single concentrations were saved in the appropriate.xls
Cells were held at a resting voltage of −80 mV and stimulated
file generated by PatchControlHT software (Nanion). Data were
by a voltage pattern to activate hERG channels and conduct out-
expressed as mean ± SEM for each drug concentration and were
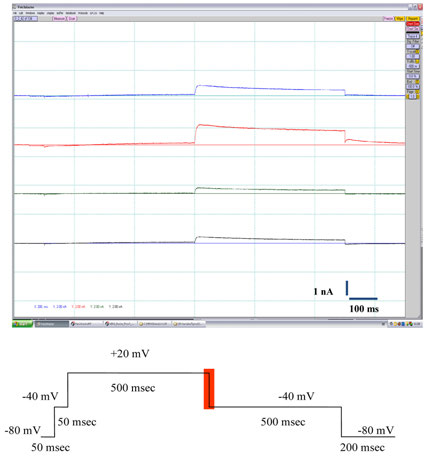
ward IKhERG current bottom panel), at a stimulation
used to determine the concentration–response relationship for the
frequency of 0.1 Hz (6 bpm). The reported current amplitudes
test compounds. The effect of the solvent (DMSO) on the hERG
represent the maximal peak tail current. After the cells stabilized
K+ current was studied in a vehicle control group.
Patchliner® hERG assay
FIGURE 2 The top panel shows typical outward K+ current traces elicited in CHO-hERG cells by the voltage pulse depicted at the bottom panel (the
area for the peak current measurement is highlighted in red).
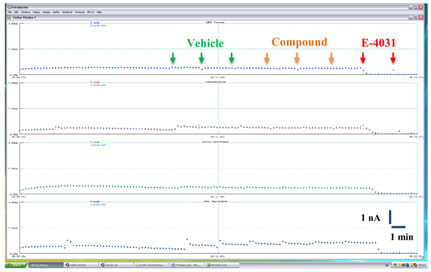
FIGURE 3 Time course of hERG K+ current amplitude recorded in a
exposed sequentially to vehicle (0.1% v/v DMSO), compound (0.1% v/v
representative vehicle control experiment on the Patchliner®. Cells were
DMSO), and 1 μM E-4031. Arrows indicate compound additions.
Patchliner® hERG assay
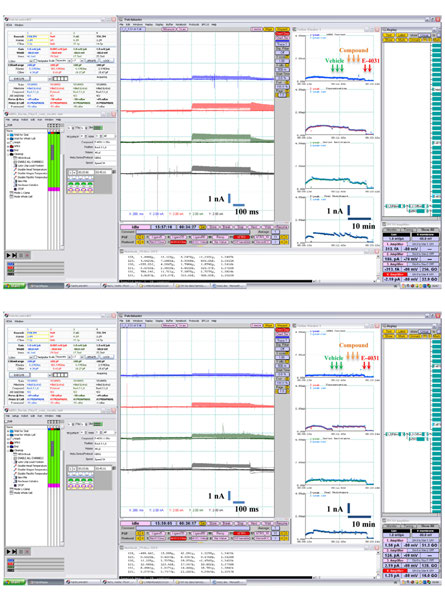
FIGURE 4 Examples of pharmacological experiments on the
hERG K+ current to the right. Arrows indicate compound additions for
Patchliner®. Recordings from four cells were performed in parallel.
vehicle (0.1% v/v DMSO), test item single concentration [0.3 μM
Original traces are shown in the middle with the on-line analysis
quinidine in (A) and 6 μM glibenclamide in (B)] and positive control
window showing the time course of a compound effect on the peak
(1 μM E-4031).
where C was the concentration, IC50 the 50% inhibitory concen-
If the effect of a drug on the currents was ≤20% at the highest
tration, h the Hill coefficient.
concentration tested, IC50 was reported as exceeding the highest
Concentration–response curves were fitted by non-linear
concentration value. If the effect of a drug on the currents exceeded
regression analysis using the XL fit add-in (IDBS Ltd.,
20% at the highest concentration tested, then concentration–
UK). Data were fitted using a four-parameter logistic model
response data from three to five cells were normalized to vehicle
[fit = (A + (B/(1 + ((x/C)∧D)))], where A = 0 and B = 100).
and 100% blocking levels and the resulting data fitted with the
Statistical analysis was performed using two-way ANOVA
following relationship:
with the Bonferroni post-test, applying the correction for mul-tiple comparison at a significance level of p < 0.05 with Graph-
Pad Prism 5 for Windows (GraphPad Software, USA). Cor-
relation between assays [Pearson's coefficient (r)] and values
Patchliner® hERG assay
for statistical significance (p) were determined using GraphPad
Table 1 Effects of 0.1% (v/v) DMSO on the hERG K+ current.
hERG current (% of control)
RESULTS
The Patchliner® work station shown in uses NPC-16
borosilicate glass chips with a microfluidic cartridge for parallel
seal formation and patch clamp recordings from up to eight dif-
ferent wells. In this study, a simultaneous recording from four cells
exposed to the same compound concentration was performed,
resulting in a four-point dose–response curve with one chip car-tridge, containing 16 recording wells in total. After priming, cellswere captured on the holes of the chip by applying continuousvacuum pressure. After allowing current to stabilize in the whole-cell configuration, a quality control check was performed beforestarting treatment to ensure that only recordings with true giga-Ohm-seals and a minimum current of 300 pA were included inthe experiment. The seal resistance varied between 3.9 ± 2.6 GΩat the start and 3.2 ± 2.1 GΩ at the end of the experiment while theaverage current amplitude was 0.9 ± 0.6 nA (mean ± SD, n = 297cells). Typical examples of the hERG current traces are shownin The recordings remained stable for over 30 minat physiological temperature enabling subsequent conduction ofcompound incubation followed by exposure to the positive con-trol at each concentration with an overall success rate >70%. Cellswere maintained in suspension for over 2 h in a cell hotel withno associated decline in patch properties. The sealing quality wasvery high with the giga-Ohm-seal formation and the whole-celltransition rates almost reaching 100% on the Patchliner®. On aver-
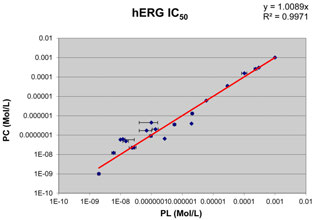
FIGURE 5 Correlation of the hERG IC
values obtained in the
age, three out of four cells maintained giga-Ohm-seals and stable
automated temperature-controlled Patchliner® system (PL) with those
currents until the end of the experiments.
measured using the conventional manual patch clamp (PC) at 37˚C.
Vehicle effect (0.1% v/v DMSO) on the hERG current was
studied in time-matched vehicle control groups Thesecontrol measurements were performed once a week during the val-
The hERG potencies of all reference drugs were correctly iden-
idation period. The largest reduction in the outward K+ current
tified. Amiodarone, astemizole, cisapride, clemastine, E-4031, and
magnitude (mean ± SEM) produced by the subsequent applica-
terfenadine were by far the strongest hERG inhibitors of the com-
tion of a vehicle alone to the CHO cells expressing recombinant
pounds tested, with IC50 values in the low and mid-nanomolar
hERG K+ channels was 6 ± 1% This indicates that expo-
range. Some drugs, such as olanzapine, moxifloxacin, sotalol,
sure to solvent may produce only a slight significant effect on the
tamoxifen, and verapamil, showed an intermediate effect on the
current with time. Therefore, the relative current values were cor-
hERG current. Captopril, ibuprofen, and glibenclamide did not
rected appropriately to the mean vehicle effect at each test item
affect the hERG current up to the highest concentration tested.
concentration as follows:
Comparison of the results showed statistically significant cor-relation (r = 0.9989, p < 0.0001) between the data obtained at
Icorrected (%) =
physiological temperature on the Patchliner® and those using the
conventional patch clamp rigat the same temperature
where I was the relative current amplitude.
To validate the utility of the Patchliner® for the hERG assay, the
actions of reference drugs on the hERG current were examined
The hERG test has become the routine in vitro approach for assess-
at physiological temperature. A set of 21 compounds includ-
ing the cardiac safety of new drugs in early development. Since
ing known hERG blockers and negative controls was evaluated.
some compounds exhibit temperature-dependent potency shifts
Examples of pharmacological experiments on the Patchliner® are
), performing experiments at physiological temper-
shown in Obtained IC50 values were plot-
ature improves the relevance of pharmacological data and ensures
ted against those obtained using the conventional voltage clamp
more accurate cardiac risk assessment in vivo. Until recently,
technique. Manual patch clamp data for reference drugs were
the manual patch clamp was the only technique for conduct-
either generated in-house using the CHO–hERG cells and identical
ing temperature-controlled electrophysiological measurements.
experimental conditions (solutions and voltage step protocol) or
However, its low throughput hampered functional early profiling
taken from published literature. Literature hERGIC50 values were
of large compound series for proper clinical candidate selection.
carefully selected to match such key experimental parameters as
The availability of a predictive cost–effective hERG assay based on
temperature, expression system, and voltage protocol.
efficient high-resolution technology is thus of major importance
Patchliner® hERG assay
Table 2 Effects of reference drugs on the hERG K+ current.
Test conc. PL (μM)
hERGIC50 PL (μM)
hERG IC50 PC (μM)
Mean ± SE
0.070 (Ridley et al., 2004)
0.024 (Walker et al., 1999)
0.012 (Ridley et al., 2006)
0.01 (Du et al., 2011)
29 (Alexandrou et al., 2006)
0.1 (Yuill et al., 2004)
0.096 (Kirsch et al., 2004)
0.136 (Kirsch et al., 2004)
for the pharmaceutical industry. A recent publication by Golden
In addition to the standard test, the Patchliner® could be a
et al. described a first attempt to evaluate the hERG chan-
useful tool for studying temperature-dependent potency shifts
nel physiology at 37˚C using an automated sub-giga-Ohm planar
and new mechanisms of compound action, e.g., the kinetics of
patch clamp IonFlux system from Fluxion with a satisfactory out-
recovery after full inhibition of the hERG current that may pro-
come. The present study aimed to evaluate the Nanion Patchliner®,
vide valuable guidance for drug candidate identification
a novel automated electrophysiological workstation, for hERG
). An additional advantage of the system is that experiments
testing under physiological conditions.
can be preprogrammed and executed in a stand-alone mode.
The Patchliner® has been successfully introduced as a high-
Thus the results of the present study indicate that the Patchliner®
quality medium throughput automated planar patch clamp plat-
can be successfully used to produce high-quality data similar to
form for ion channel research in drug discovery and safety (review
those obtained by means of the current gold standard, the manual
in A unique feature is its ability to conduct
patch clamp. The system is unique in combining the features of
stable recordings at different temperatures in the 22–60˚C range.
high-quality patch clamp recordings with increased physiological
The results of the present study showed that the Patchliner®
relevance of the collected data, resulting in the enhanced safety
gives high-quality patch clamp recordings in CHO-hERG cells,
profiling of new chemical entities.
with a high success rate of giga-Ohm-seals lasting up to anhour at 37˚C. Such performance makes the system a powerful
tool for studying hERG channel pharmacology at physiological
The Patchliner® workstation provides stable currents and high-
quality giga-Ohm-seal recordings at 37˚C. Based on the validation
In this work, a systematic comparison of diverse panel of refer-
results, this automated electrophysiology workstation can be used
ence drugs spanning a broad hERG potency range was performed
for conducting an advanced hERG assay for new compounds at
to validate the Patchliner® for temperature-controlled hERG assay.
physiological temperature and may eventually become a new gold
The obtained IC50s values were compared with their manual patch
standard for ion channel research in safety pharmacology.
clamp counterparts. The main finding was that the potency datagenerated on the Patchliner® matched those obtained by the con-
ventional method (Pearson r = 0.9989, p < 0.0001). It is impor-
The author would like to thank the Nanion Technologies GmbH
tant to note that among the drugs tested, the inhibitory effects
team, especially Drs. David Guinot and Alison Haythornthwaite,
of d,l-sotalol and erythromycin ) were cor-
for their help. I am also grateful to Drs. Silvio Albertini, Claudia
rectly identified at 37˚C. The Patchliner® system allowed fast and
McGinnis, Susanne Mohr, and Thomas Weiser from Roche Non-
accurate electrophysiological characterization of ion channels, e.g.,
Clinical Safety for supporting the acquisition and validation of the
for determining the IC50 values of ion channel blockers.
system, and to Fabian Häusermann for technical assistance.
Patchliner® hERG assay
in cardiac safety: screening hERG
dronedarone: resistance to muta-
A. A., Kozlowski, R. Z., and Han-
Alexandrou, A., Duncan, R., Sullivan, A.,
cell lines and novel compounds with
tions of the S6 residues Y652 and
cox, J. C. (2004). Potent inhibition of
Hancox, J., Leishman, D., Witchel,
the ion works HTTM system. J.
F656. Biochem. Biophys. Res. Com-
human cardiac potassium (HERG)
H., and Leaney, J. (2006). Mecha-
Biomol. Screen. 10, 832–840.
mun. 325, 883–891.
channels by the anti-estrogen agent
nism of hERG K+ channel block-
International Conference on Harmon-
Sanguinetti, M. C., and Tristani-Firouzi,
clomiphene-without QT interval
ade by the fluoroquinolone antibi-
isation. (2005). ICH S7B: the non-
M. (2006). hERG potassium chan-
prolongation. Biochem. Biophys. Res.
otic moxifloxacin. Br. J. Pharmacol.
clinical evaluation of the poten-
nels and cardiac arrhythmia. Nature
Commun. 318, 556–561.
147, 905–916.
tial for delayed ventricular repo-
440, 463–469.
Du, C. Y., El Harchi, A., Zhang, Y. H.,
larization (QT interval prolonga-
Swinney, D. C. (2009). The role of bind-
Orchard, C. H., and Hancox, J. C.
tion) by human pharmaceuticals.
ing kinetics in therapeutically use-
Conflict of Interest Statement: The
(2011). Pharmacological inhibition
CHMP/ICH/423/02. Fed. Regist. 70,
ful drug action. Curr. Opin. Drug
author declares that the research was
of the HERG potassium channel is
Discov. Devel. 12, 31–39.
conducted in the absence of any com-
modulated by extracellular but not
Kirsch, G. E., Trepakova, E. S., Brime-
Vandenberg, J. I., Varghese, A., Lu, Y.,
mercial or financial relationships that
intracellular acidosis. J. Cardiovasc.
combe, J. C., Sidach, S. S., Erick-
Bursill, J. A., Mahaut-Smith, M. P.,
could be construed as a potential con-
Electrophysiol .22, 1163–1670.
son, H. D., Kochan, M. C., Shyjka,
and Huang, C. L. (2006). Tem-
flict of interest.
Farre, C., Haythornthwaite, A., Haar-
L. M., Lacerda, A. E., and Brown,
perature dependence of ether-a-go-
mann, C., Stoelzle, S., Kreir, M.,
A. M. (2004). Variability in the
go-related gene K+ currents. Am.
Received: 16 August 2011; accepted: 06
George, M., Brüggemann, A., and
measurement of hERG potassium
J. Physiol. Cell Physiol. 291, C165–
January 2012; published online: 26 Janu-
Fertig, N. (2009). Port-a-patch and
channel inhibition: effects of tem-
Patchliner: high fidelity electrophys-
perature and stimulus pattern. J.
Walker, B., Singleton, C., Bursill,
Citation: Polonchuk L (2012) Toward a
iology for secondary screening and
Pharmacol. Toxicol. Methods 50,
J., Wyse, K., Valenzuela, S., Qiu,
new gold standard for early safety: auto-
safety pharmacology. Comb. Chem.
M., Breit, S., and Campbell, T.
mated temperature-controlled hERG test
High Throughput Screen. 12, 24–37.
Möller, C. (2010). Impact of new tech-
(1999). Inhibition of the human
on the PatchLiner®. Front. Pharmacol.
Golden, A. P., Li, N., Chen, Q.,
nologies for cellular screening along
ether-a-go-go-related gene (HERG)
3:3. doi:
Lee, T., Nevill, T., Cao, X., John-
the drug value chain. Drug Discov.
potassium channel by cisapride:
This article was submitted to Frontiers
son, J., Erdemli, G., Ionescu-Zanetti,
Today 15, 384–390.
affinity for open and inactivated
in Pharmacology of Ion Channels and
C., Urban, L., and Holmqvist,
Ridley, J., Milnes, J., Hancox, J., and
Channelopathies, a specialty of Frontiers
M. (2011). IonFlux: a microfluidic
Witchel, H. (2006). Clemastine, a
in Pharmacology.
patch clamp system evaluated with
conventional antihistamine, is a high
Yao, J. A., Du, X., Lu, D., Daharsh, E.,
Copyright 2012 Polonchuk. This is an
human ether-à-go-go related gene
potency inhibitor of the HERG K+
and Atterson, P. (2005). Estimation
open-access article distributed under the
channel physiology and pharmacol-
channel. J. Mol. Cell Cardiol. 40,
of potency of hERG channel block-
terms of the
ogy. Assay Drug Dev. Technol. 9,
ers: impact of voltage protocol and
which per-
Ridley, J., Milnes, J., Witchel, H.,
temperature. J. Pharmacol. Toxicol.
mits non-commercial use, distribution,
Guthrie, H., Livingston, F. S., Gubler, U.,
and Hancox, J. (2004). High affin-
Methods 52, 146–153.
and reproduction in other forums, pro-
and Garippa, R. (2005). A place for
Yuill, K. H., Borg, J. J., Ridley, J. M.,
vided the original authors and source are
ade by the antiarrhythmic agent
Milnes, J. T., Witchel, H. J., Paul,
Source: http://www.nanion.cn/uploadfiles/upload/1406175464.pdf
The future of worming STARTS here! Dual active worm control Dual active worm control Introducing a novel strategy for sustainable worm control in sheep. STARTECT® is the first dual active wormer. It gives an additive effect for improved worm control, which can enhance animal performance. Containing a novel active ingredient derquantel, and a potent ML abamectin it not only kills worms resistant to other products, but used routinely it can help delay wormer resistance, protecting your flock from drench failure now and into the future.
Typhoid fever perforation in Kathmandu, Nepal - A retrospective study of risk factors and antibiotic treatment Author: Isabel Vigmo, Medical Student at the Sahlgrenska Academy University of Gothenburg, Sweden Supervisors: Yogendra Singh, MD, PhD, Professor of Surgical Oncology Department of Surgery, Tribhuvan University Teaching Hospital, Kathmandu, Nepal Göran Kurlberg, MD, PhD, Associating Professor of Surgery





