Ags/001/cap
Application of Tixel for Transdermal Delivery
Amnon Sintov, PhD
Ben-Gurion University of the Negev, Israel
Maja A. Hofmann, PD Dr. med
Charité, Universitatsmedizin Berlin, Germany
ABSTRACT
Background: Tixel is a novel device for thermal fractional skin treatments with low pain in
ablative and non-ablative modes.
Objective: This study is aimed to evaluate the drug permeation properties of skin treated with the
Tixel system in non-ablative mode.
Methods: Tixel S tip at 400oC was applied on skin for 9
ms. Skin permeability was evaluated by in
vivo confocal microscopy for visualization of the human skin channels as well as by a diffusion cell
system using excised porcine ear skin. Verapamil hydrochloride 1% was used as a hydrophilic
model drug known to have a poor absorption across mammal skin.
Results: Decomposition of the stratum corneum after Tixel treatment was visualized by confocal
microscopy. A matrix of open channels is created painlessly without bleeding in the epidermis
allowing diffusion of topical fluorezceine within 2 minutes down to the upper dermis in channels
that have been formed 6 hours earlier by Tixel. At the same parameters, skin permeability to
Verapamil was measured and compared to untreated skin. After 24 hours, the cumulative amount of
drug that had penetrated across the skin was 20 fold higher than across the untreated skins.
Conclusions: Fractional Tixel effectively opens the skin for transdermal delivery of compounds
with no pain, no bleeding and no downtime.
Keywords: verapamil, transdermal delivery, permeation, Franz cell, fluorescent
Transdermal delivery constitutes a large market for both aesthetic and medical applications.
The skin is a highly impermeable barrier that allows only small lipophilic compounds to penetrate
into it (1). Disruption of the skin outer layer, the stratum corneum, increases its permeability. A
number of approaches have been developed in order to disrupt the stratum corneum such as
mechanical, chemical or physical (2, 3, 4). One successful method to increase permeability is by
heat. Prausnitz et al showed in references 5 and 6 that short exposure to high temperature
dramatically increases skin permeability and that thermal damage can be localized to the stratum
corneum without damaging deeper tissue. A strong positive correlation between temperature and
skin permeability was found. Thermal ablation of skin that selectively ablated the Stratum
Corneum, dramatically increased skin permeability for transdermal drug delivery. Above 360ᴼC,
transdermal flux increased by 3 orders of magnitude, associated with decomposition and
vaporization of keratin to create micron-scale holes. Finally the authors claimed that a hundred
micron diameter pores should allow passage of a wide range of hydrophilic and macromolecular
drugs although at different diffusion rates.
The Tixel fractional treatment device is a novel thermal resurfacing system which can generate
ablative as well as non-ablative micropores in the skin (8,9). Superheating water molecules in the
skin tissues with a high conductivity metallic tip is effective in damaging the skin cells in a safe,
precise and predictable manner.
Tixel's D tip creates ablative micropores with similar properties to
fractional CO₂ lasers. Tixel's S tip can generate non-ablative "dressed" micropores with underlying
thermal damage extending down to the papillary dermis.
This study focuses on thermal decomposition of the stratum corneum using Tixel to increase skin
permeability by heat. The channels formed in the skin have been evaluated by histology and by in
vivo confocal microscopy. Permeability of the skin was tested with a small fluorescent dye using
confocal imaging as well as by diffusion cell using a hydrophilic molecular model, Verapamil
Hydroxide with 491.06Da.
2.1 The Tixel
The Tixel (Novoxel, Israel) is a thermo-mechanical system for fractional ablation. It applies a tip,
which is made of metallic, gold plated biocompatible materials (Fig. 1). The tip is fixated at the
distal section of the Tixel handpiece which is equipped with a linear motor (Fig. 1). The tip's active
surface consists of an array of 81 (9x9) pyramids evenly spaced within a boundary area of 1x1cm.
The pyramids are 1.25 mm tall having a radius of about 100 microns at the edge. The back plane of
the tip is flat and is connected to a coin-size heater which is kept at a constant temperature of 400oC
during operation. When not in use, the tip is base-positioned at a distance of 2cm from the skin's
surface (home). The tip weighs 7grams and is re-usable. The system checks, validates, cleans,
sterilizes and exchanges tips automatically. Tip cleaning after treatment is performed within 5
minutes at 520oC, while the tip is still mounted on the handpiece and tip automatic self-sterilization
is performed within 3 minutes at 350oC. Tip cleaning, tip sterilization and tip biocompatibility have
all been validated. The handpiece weighs 270 grams. Two tip types are available: D with high
thermal conductivity and S with low thermal conductivity. When the user activates the handpiece,
the linear motor rapidly advances the tip which comes in brief contact with the tissue. Thermal
energy is transferred to the skin, creating micropores in it by evaporation. The tip recedes within a
precisely controlled distance and time to its home position, away from the tissue. The duration of
the pulse, i.e. time of contact between tip and skin, can range from 6ms to18ms. A double pulsing
mode is also enabled. The motor's displacement accuracy is in the range of 1-8 μm. A 14ms pulse
delivers a high energy pulse of 25 mJ/pore while a 10 ms pulse delivers a medium energy pulse of
15 mJ/pore and a 6ms pulse delivers a low energy pulse of 10 mJ/pore. The theoretical and
engineering foundations of the Tixel technology have previously been described in Lask et al, 2012
(7). The utilization of the Tixel does not require the use of protective eyewear or a smoke evacuator.
2.2 In vivo confocal microscopy of the human Tixel channel
Confocal imaging was performed using a fluorescent confocal microscope (Vivascope 1500
multilaser, Lucid Inc, Rochester, USA) on 6 volunteer's arm. A fluorescent probe, fluoreszein SE
thilo, was placed for 2 minutes on skin areas previously treated with Tixel (parameters: S Tip, 9ms
single pulses) 2 and 6 hours before and then sponged out. Permeation of fluorescence up to 200
microns deep in the skin layers was visualized.
2.3 Transdermal delivery of Verapamil on ex vivo pig skin by Franz cell
Full-thickness porcine skins were excised from fresh ears of white pigs (Kibbutz Lahav, Israel).
Only pieces that the TEWL levels were less than 10 g/m2/h were used. The animal protocol was
reviewed and approved by the Institutional Animal Care and Use Committee.
Excised skins were treated or untreated with Tixel (Tixel, NOVOXEL ltd, Israel). A S-Tip was
operated at 400oC for 6ms or 9ms.
Immediately after skin treatment, the permeability of Verapamil hydrochloride 1% (Euroasian
Chemicals Ltd., Mumbai, India; MfD: 02/2014) through porcine skin was determined in vitro with a
Franz diffusion cell system (Crown Bioscientific, Inc., Clinton, NJ) [9]. The diffusion area was
1.767 cm2 (15 mm diameter orifice), and the receptor compartment volume was 12 ml. The
solutions in the water-jacketed cells were thermostated at 37°C and stirred. Samples (2 ml) were
withdrawn from the receiver solution at predetermined time intervals (3, 5, 7, 9, 12, 20 and 24
hours) and kept at -20oC until analyzed by HPLC. Aliquots of 20 µl from each vial were injected
into HPLC system (Shimadzu VP series including diode-array detector for peak spectrum
identification), equipped with a pre-packed C18 column (Betasil C18, 5µm, 250X4.6mm,
ThermoHypersil, UK) heated to a temperature of 30°C. Data were expressed as the permeating drug
quantity per unit of the skin surface area, Qt/S (S = 1.767 cm2). Cumulative drug permeations (Qt)
were calculated as in [10].
The statistical differences were analyzed by the two-way unweighted means analysis of variance
(ANOVA) test. The differences among group means were considered significant for
p values <
0.05. Results are given as average ± standard deviation.
Histology
Treated skin samples were fixed in formalin, embedded in paraffin and stained for H&E for
histopathological examination.
Tixel's skin channel characterization
As shown in figure 2, tiny lesions are created in the skin by Tixel with the S tip at 9ms pulse
duration affecting the stratum corneum and epidermis. In some cases, the epidermis stays intact.
There is minimal damage to the dermis with minimal dermal coagulation. In vivo treatments were
without pain, erythema disappeared within a day on facial treatment sites.
Visualization of the channels by confocal microscopy shows a clean hole in the epidermis down to
the upper dermis (figure 3). Figure 4 shows stratum corneum decomposition.
Transdermal Delivery of fluorescein
The passage of a small fluorescent hydrophilic molecule trough channels created 2 and 6 hours
previously, at the same Tixel settings, on the in vivo human arm was tested by confocal
microscopy. The dye permeated through the stratum corneum barrier and diffused into the
epidermis and upper dermis in both cases (figure 5).
Transdermal Delivery of Verapamil
The penetration of verapamil hydrochloride increased about 5 and 20 times after skin had been
pretreated with Tixel TMA system for 6
ms and 9
ms, respectively (Q24 = 168.16± 93 µg/cm2 (n=8)
and 728 ±358 µg/cm2 (n=11) compared to Q24=33.75 ± 25.3 µg/cm2 (n=5) in untreated skin. The
statistical tests revealed highly significant differences between the experimental groups (p<0.05)
(Fig 6). In addition, lag time to get quantitative permeation across the skin decreased significantly
from 9-10 hours in untreated skin to 3-5 hours in Tixel-treated skin.
DISCUSSION AND CONCLUSIONS
Confocal microscopy confirmed that the Tixel, at low energy is able to disrupt the stratum corneum
and form channels in the skin that stay open for the diffusion of a fluorescent dye for up to 6 hours
post Tixel application, which could be useful for drug delivery and combination treatment with
cosmetic products. Treatment is painless with minimal crusting. Erythema clears within 24 hours.
REFERENCES
1. Mariko Egawa , Tetsuji Hirao and Motoji Takahashi. In vivo Estimation of Stratum Corneum
Thickness from Water Concentration Profiles Obtained with Raman Spectroscopy. Acta Derm
Venereol 2007; 87: 4–8.
2. Gratieri T, Alberti I, Lapteva M, Kalia YN. Next generation intra- and transdermal therapeutic
systems: using non- and minimally-invasive technologies to increase drug delivery into and
across the skin. Eur J Pharm Sci. 2013; 18; 50(5):609-22.
3. Lindsay R. Sklar, Christopher T. Burnett, _ Jill S. Waibel, Ronald L. Moy, and David M. Ozog,
Laser Assisted Drug Delivery: A Review of An Evolving Technology. Lasers in Surgery and
Medicine 2014; 46:249–262.
4. Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P and Stinchcomb AL. Challenges
and opportunities in dermal/transdermal delivery. Ther Deliv. 2010; 1(1): 109–131.
5. Park JH, Lee JW, Kim YC, Prausnitz MR. The effect of heat on skin permeability. Int J Pharm.
2008, 359(1-2):94-103
6. Lee JW, Gadiraju P, Park JH, Allen MG, Praunitz MR. Microsecond Thermal Ablation of Skin
for Transdermal drug Delivery. J Control Release 2011, 54(1):58-68
7. Fournier Fractional vaporization of tissue with an oscillatory
array of high temperature rods – Part I: Ex vivo study. J Cosmetic and Laser therapy 2012;
8. Elman M, Fournier N, Barneon G, Hofmann M, Bernstein MD, Lask G. Fractional Treatment of
Aging Skin with Tixel, a Clinical and histological Evaluation. J Cosm Therap, Submitted 2015.
9. Sintov AC, Brandys-Sitton R. Facilitated skin penetration of lidocaine: combination of a short –
term iontophoresis and microemulsion formulation. Int J Pharma 316 (2006) 58-67.
10. Sintov AC, Botner S. Transdermal drug delivery using microemulsion and aqueous systems:
influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous
vehicle systems. Int J Pharma 311 (2006) 55-62.

FIGURES and LEGENDS
Figure 1: The Tixel handpiece and its metallic tip
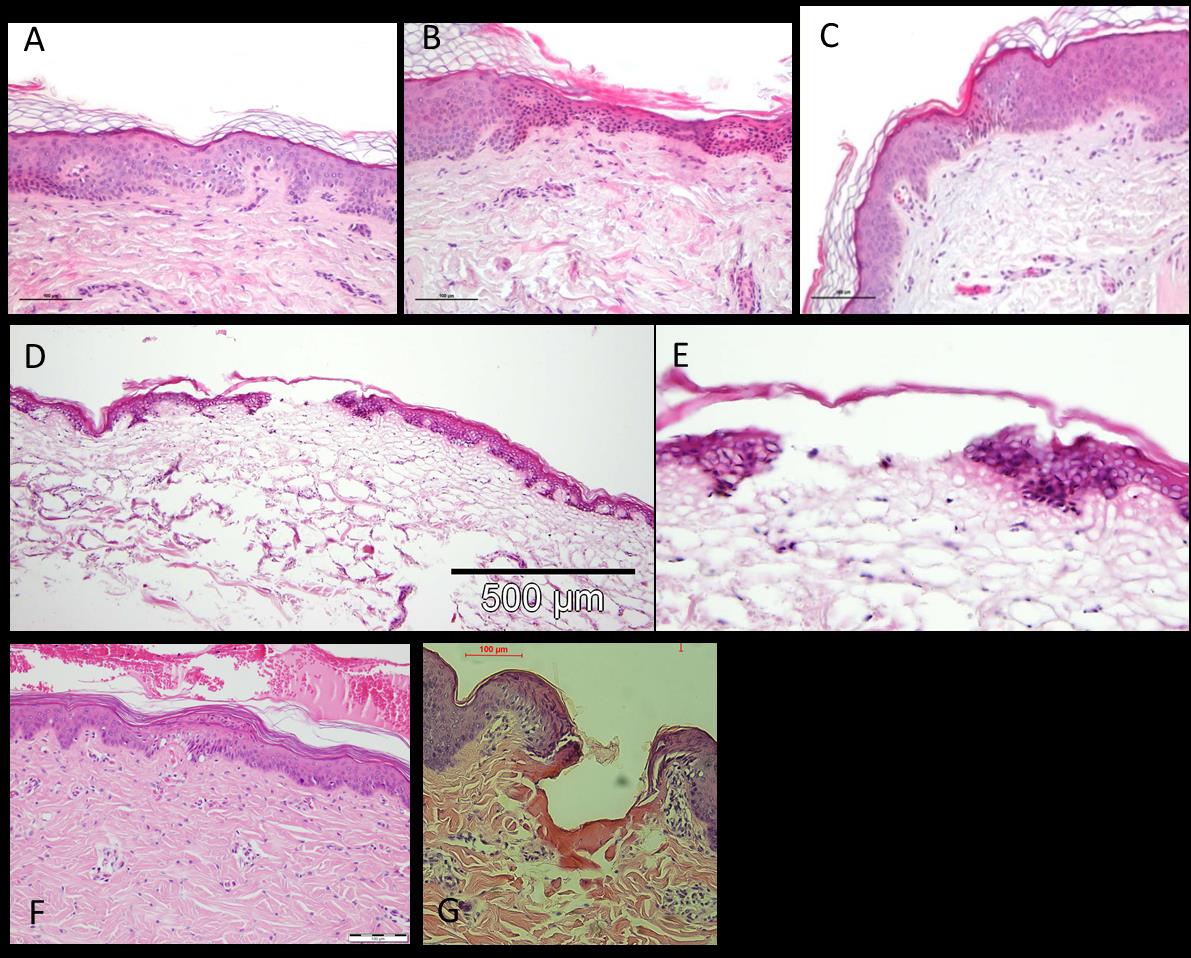
Figure 2: Skin histologies immediately after Tixel treatment (S tip, 9 ms pulse). (A, B, C) - in vivo
treatment of a male 61 years. (D & E) – ex vivo treatment of a freshly excised human skin. (F) in
vivo treatment of a 5 weeks pig. (G) ex vivo treatment of pig's ear.
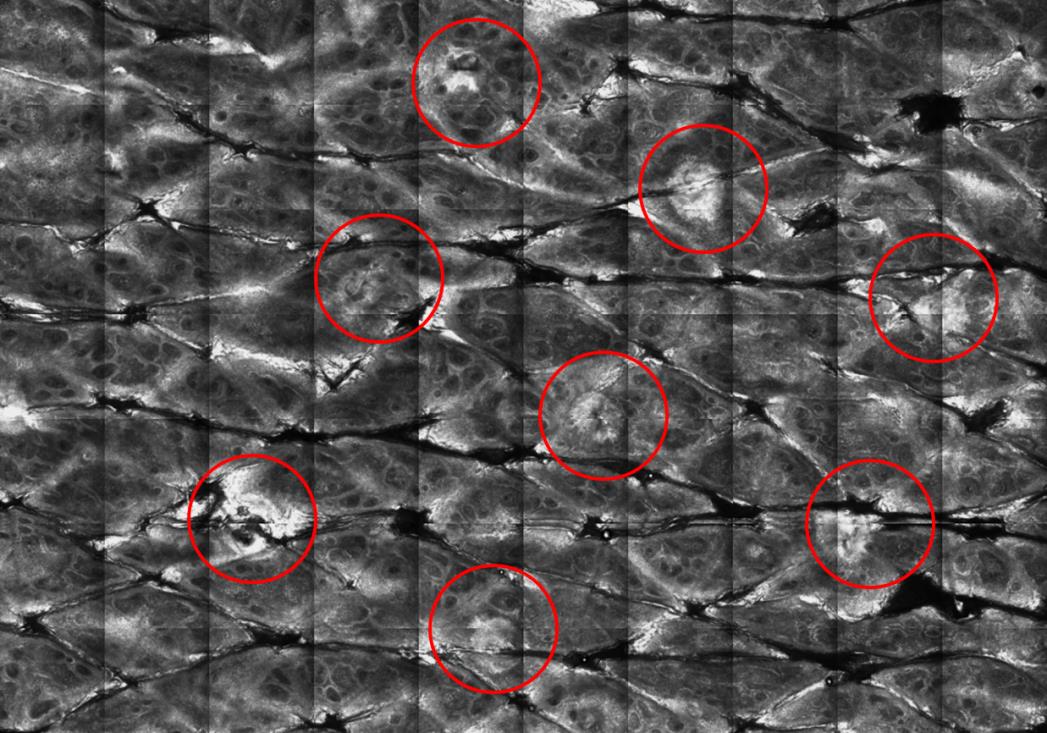
Figure 3: Decomposition of the stratum corneum (red circles) as viewed by in vivo confocal
microscopy on human skin. Treatment setting : S tip, 9ms pulse.
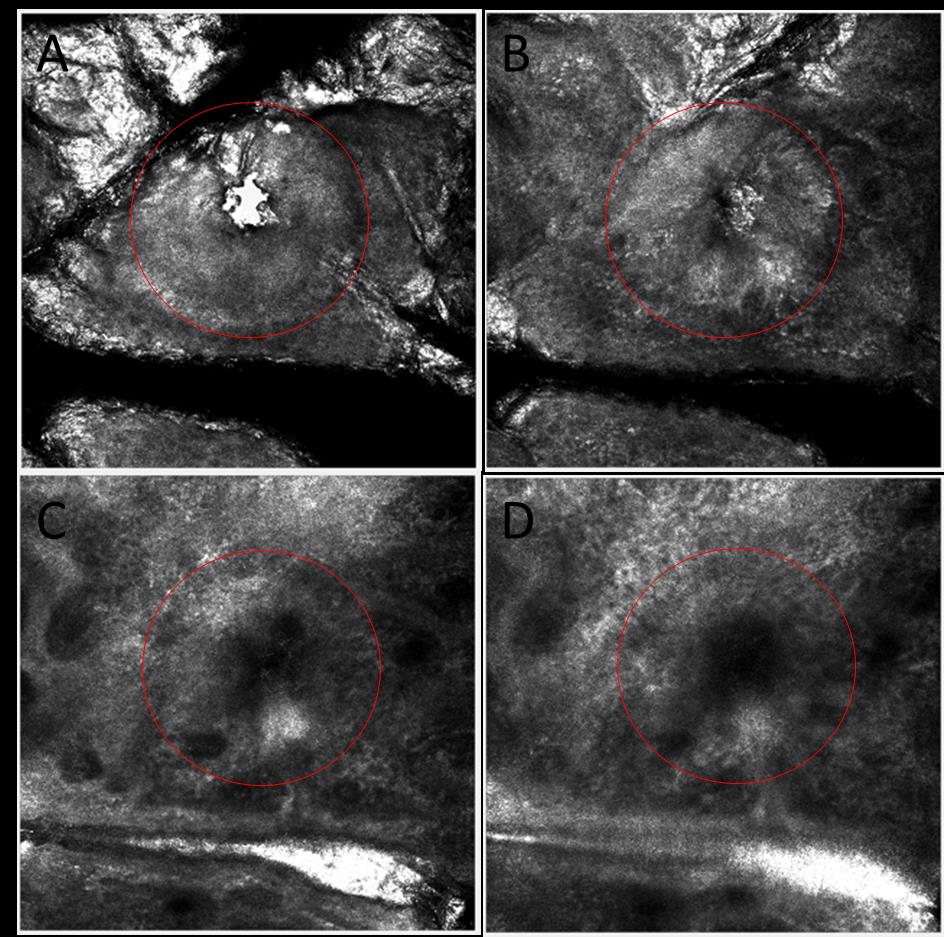
Figure 4: Clean channel visualized by in vivo confocal microscope on human skin. Red circle
shows the channel (S tip, 9ms pulse). Red circle shows the channel. (A) skin surface. (B) Epidermis
layer. (C, D) Upper dermis layer.
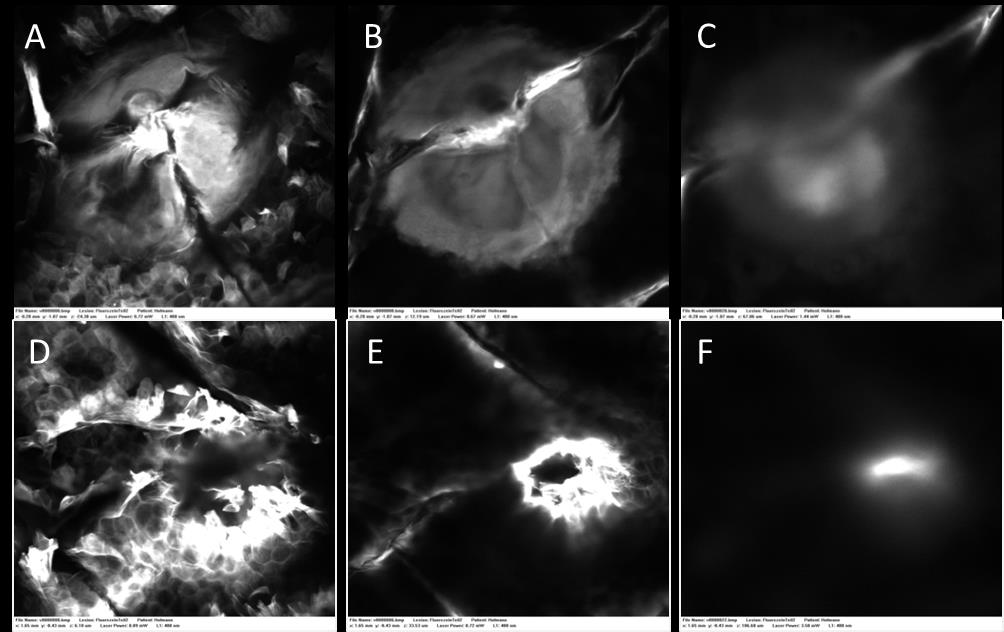
Figure 5: In vivo fluorescent confocal microscopy of Tixel (S Tip, 9ms single pulse) channel on
human skin. Diffusion of the fluorescent compound is visualized through the epidermis and upper
dermis. Upper row - Tixel was applied on the skin 2 hours prior to imaging, (A) 36μm deep, (B)
54μm deep, (C) 101μm deep. Lower row - Tixel was applied on the skin 6 hours prior to imaging,
(D) 30μm deep, (E) 67μm deep, (F) 113μm deep.
Figure 6: Verapamil delivery through Tixel pretreated skin at 6ms and 9ms pulse and untreated
skin. The penetration of verapamil hydrochloride increased about 5 and 20 times after skin had been
pretreated with Tixel TMA system for 6 ms and 9ms, respectively (Q24 = 168.16± 93 µg/cm2 (n=8)
and 728 ±358 µg/cm2 (n=11) compared to Q24=33.75 ± 25.3 µg/cm2 (n=5) in untreated skin. The
statistical tests revealed highly significant differences between the experimen0tal groups (p<0.05).
In addition, lag time to get quantitative permeation across the skin decreased significantly from 9-
10 hours in untreated skin to 3-5 hours in Tixel-treated skin.
Source: http://www.novoxel.co.il/wp-content/uploads/2015/07/Application-of-Tixel-for-Transdermal-Delivery-A-Draft1.pdf
AgentSheets®: an Interactive Simulation Environment with End-User Programmable Agents Alexander Repenning AgentSheets Inc., Gunpark Drive 6560, Boulder, Colorado, 80301, email: [email protected] Center for LifeLong Learning and Design, Department of Computer Science, University of Colorado at Boulder, Campus Box 430, Boulder, Colorado 80309-0430, email: [email protected]
Fenofibrate (Lipidil) for dyslipidaemia • Statins are first line for people with existing cardiovascular disease. • Fenofibrate is an alternative to gemfibrozil in hypertriglyceridaemia, or in mixed dyslipidaemia when elevated triglyceride concentration is the predominant disorder. • Monitor for myopathy when fenofibrate is used in combination with a statin, as the risk is





