Optimal cutoff value of basal anti-mullerian hormone in iranian infertile women for prediction of ovarian hyper-stimulation syndrome and poor response to stimulation

Aghssa et al. Reproductive Health (2015) 12:85 DOI 10.1186/s12978-015-0053-4
Optimal cutoff value of basal anti-mullerianhormone in iranian infertile women forprediction of ovarian hyper-stimulationsyndrome and poor response to stimulation
Malek Mansour Aghssa1, Azam Manshadi Tarafdari1*, Ensieh Shahrokh Tehraninejad1, Mohammad Ezzati2,Maryam Bagheri1, Zahra Panahi1, Saeed Mahdavi3 and Mehrshad Abbasi4
Aim: We intended to establish the threshold of Anti-Mullerian Hormone (AMH) for detection of OvarianHyper-Stimulation Syndrome (OHSS) and poor response to treatment in Iranian infertile women.
Methods: Pre-stimulation menstrual cycle day-3 hormonal indices including basal AMH values were measured in105 infertile women aged 32.5 ± 4.3 years. Patients underwent long GnRH agonist Controlled Ovarian Hyperstimulation(COH) in a referral infertility center (Tehran, Iran). The gonadotropin dose was determined based on the age and basalserum Follicular Stimulating Hormone (FSH) level. The IVF/ICSI cycles were followed and the clinical and sonographicdata were recorded.
Results: Sixteen cases developed OHSS. The prevalence of PCOS was higher in subjects with OHSS [62.5 %(38.8-86.2) vs. 17 % (9.2-24.9)]. The patients with OHSS had higher ovarian follicular count [23.7 (3.2) vs. 9.1 (0.5);p < 0.05], collected oocytes [13.5 (1.9) vs. 6.9 (0.5); p < 0.05] and AMH level [7.9 (0.7) vs. 3.6 (0.3); p < 0.05]. BasalAMH level and oocyte yields (but not age, BMI, and PCOS) correlated with occurrence of OHSS; and only the AMHlevels were associated with poor ovarian response (oocytes yield ≤ 4). The optimal cutoff value for the predictionof OHSS was 6.95 ng/ml (area under the receiver operating characteristics curve: 0.86; CI: 0.78-0.95; sensitivity: 75 %;specificity: 84 %; odds ratio for occurrence of OHSS: 9 and p < 0.001). The optimal cut point to discriminate poorresponse (oocytes ≤4) was 1.65 ng/ml ( AUC : 0.8; CI: 0.69-0.91; sensitivity: 89 % specificity : 71 %; and OR = 23.8and P value <0.001).
Conclusions: Iranian women with basal AMH level > 6.95 ng/ml are at high risk of developing OHSS and thosewith AMH level < 1.65 ng/ml are poor responders.
Keywords: Assisted Reproductive Technology, Ovulation induction, Ovarian hyper-stimulation syndrome,Anti-mullerian hormone
* Correspondence: 1Vali-e-Asr Reproductive Health Research Center, Department of Obstetricsand Gynecology, Valiasr Hospital, Tehran University of Medical Sciences,1419433141 Tehran, IranFull list of author information is available at the end of the article
2015 Aghssa et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium,provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver applies to the data made available in this article, unless otherwise stated.
Aghssa et al. Reproductive Health (2015) 12:85
of the gonadotropin and diagnosed and managed OHSS
Anti-Mullerian Hormone (AMH) is a granulosa cell
irrespective of patients' participation in the study. The fol-
derived hormone secreted from pre-antral and small an-
licle count (≥14 mm) based on sonographic examination
tral follicles. AMH substantially inhibits the initiation of
on the day of HCG administration, the number of re-
primordial follicle growth and contributes to normal
trieved oocytes, and the final outcome of the IVF/ICSI
folliculogenesis by enhancing the role of FSH in cyclic
cycle were recorded.
recruitment of follicles Clinically, AMH can serve asa reliable ovarian reserve marker independent of go-
Controlled ovarian hyperstimulation protocol
nadotropins levels A particularly helpful aspect of
Participants were recruited among those who underwent
AMH, when used as an ovarian reserve marker, is that
long GnRH agonist protocol irrespective of age, gonado-
its serum levels remain relatively constant during normal
tropin dosage, and history of OHSS or antral follicular
menstrual cycles The reported variability during
count. From day 21 of the pre-stimulation menstrual
the menstrual cycles is not possibly clinically influential
cycle, the patient received a daily subcutaneous injection
In 2002, Seifer et al. underscored the association of
of Boucerelin acetate (Superfact, Hoecht AG, Frankfurt,
AMH levels with ovarian response to Controlled Ovar-
Germany). The gonadotropin (GonalF, Serono, Switzerland)
ian Hyperstimulation (COH) The recent meta-
was added on day 2 or 3 of the IVF/ICSI cycle. Dosing
analysis by Broer et al. highlighted 9 studies employing
was determined by the chief treating gynecologist of the
AMH to predict excessive responses during COH
center mainly based on the age and basal FSH levels
While the ability to predict excessive ovarian stimulation
(ranged between 150 and 225 IU/day). Dosage of gonado-
using basal AMH values is established, the optimal
tropin was adjusted based on the degree of ovarian re-
threshold of AMH to predict Ovarian Hyper-Stimulation
sponse in interval sonographic examinations (data not
Syndrome (OHSS) is controversial and subjected to this
collected for this report). Patients were examined by daily
trans-vaginal sonography starting on day 7 of stimulation.
HCG (250 mgr; Ovitrelle, Merck, Serono) was injec-
ted subcutaneously when the sonographic examination
A total of 105 infertile couples undergoing COH enrolled
showed a minimum of two 18 mm follicles. Oocyte
in this study. They were visited in a private referral infer-
pickup was performed from posterior vaginal fornix
tility center (Tehran, Iran) between March 2010 and
34 to 36 hours after HCG administration. Embryo
February 2011. Subjects with any known systemic diseases
was transferred within 2 days of oocyte pickup and
or endocrine disorders including diabetes, hypothy-
the patient received 400 mg of cyclogest (Alpharma,
roidism, hyper-prolactinemia, and those who were receiv-
Barnstaple, UK) every 12 hours during the first
ing levothyroxine or cabergoline were excluded. Data of
12 weeks of gestation and 2 mg of estradiol valereate
the first attempt was recorded for the patients who under-
every 12 hours for 2 weeks. Serum βHCG was mea-
went more than one IVF/ICSI cycle. The corresponding
sured 15 days after embryo transfer. Biochemical
demographic and infertility related data of the participants
pregnancy was defined as βHCG value > 50 mIU/ml.
were collected and the baseline pre-stimulation AMH,
Sonographic examination was performed 2 weeks later
FSH, LH, testosterone, dehydroepiandrosterone sulfate,
to confirm the presence of gestational Sac and then
TSH, and prolactin plasma levels were measured on the
2 weeks afterward to confirm viability of the embryo.
third day of the previous menstrual cycle. Pre-stimulation
The OHSS related symptoms and signs, including
cycle(s) was/were induced in those without regular men-
abdominal distension and discomfort, nausea, vomiting,
ses with intramuscular injection of 100 mg progestron in
diarrhea, ascites, plural effusion, edema, oliguria, hyper-
oil (Iran Hormone, Tehran, Iran) and maintained with
coagulative state, serum creatinine of 1.0-1.5 mg/ml,
daily OCP from day 3 in those without history of regular
hemoconcentration, and electrolyte imbalance were moni-
menses after withdrawal bleed. Serum AMH was mea-
tored. Suspected OHSS cases were examined by sonog-
raphy to determine ovarian enlargement and ascites ].
Ireland, Inc., Galway, Ireland) with functional sensitivity of
OHSS and the severity of the condition were defined ac-
0.2 ng/ml and intra and inter-assay coefficient of variabil-
cording to the Navot et al. []. Those patients experien-
ity of 8 and 12 %, respectively. Samples were centrifuged
cing OHSS were managed by cycle cancellation, coasting,
and the assays were done in serums after performing cali-
or freezing the embryo for future IVF cycles with or with-
brations according to the manufacturer instructions.
out additional cabergoline (dostinex) therapy.
Samling of the hemolyzed samples were repeated and no
The ethical committee of the Faculty of Medicine
particular strategy for handling Heterophile antibody was
(Tehran University of Medical Sciences) approved the
employed. The chief gynecologist of the center (i.e.
study and waved the need for written informed consent.
MMA) selected the stimulation protocol, type, and dose
The data was handled and analyzed anonymously.
Aghssa et al. Reproductive Health (2015) 12:85
skewed. For the parametric tests the square root of the
Independent sample T-tests and Chi square tests were
AMH values was generated and employed (Kolmogorov-
used to compare correspondingly the difference of con-
Smirnov's P value of transferred data = 0.39).
tinuous values and the prevalence of categorical vari-ables between subjects with and without OHSS. A
binary logistic regression model (enter method) was de-
The subjects' characteristics are shown in Table Basal
signed to study the multivariate correlation of OHSS
AMH level as well as ovarian follicle and collected
with age, BMI, basal biochemical indices (including
oocytes counts but not age and BMI were higher in the
AMH), sonographic findings (follicle count), and clinical
subjects with OHSS. The mean basal AMH value of the
outcomes (retrieved oocytes). Additionally, a univariate
105 studied cycles was 4.2 ng/ml [SD: 3.3, median: 3.5,
general linear model (enter method) was employed to
inter-quartile range: 1.4-7.0, range: 0.05-15.0]. Sixteen
adjust the effect of covariates on the association of
patients presented with moderate or severe OHSS
OHSS and AMH level. Finally, a receiver operating char-
(15.2 %) out of whom 4 cycles were canceled, oocytes
acteristics (ROC) curve was created to classify subjects
were collected for subsequent IVF attempts in 7 cases
with and without OHSS according to AMH levels. Two
(with additional dostinex therapy in one patient), ovula-
different approaches were assessed to determine the op-
tion induction was coasted in 2 patients (with additional
timal cutoff value of AMH to classify OHSS: Youden
dostinex therapy in one patient), and two subjects were
index which is the maximum sensitivity - (1-specificity)
treated with dostinex alone. Two cases with severe
and the shortest distance on the ROC from the optimal
OHSS were managed in the hospital setting. Forty-two
sensitivity and specificity [(1 - sensitivity)2 + (1 - specifi-
(40 %; CI: 30.6-49.4 %) IVF/ICSI cycles led to clinical
city)2] [Sensitivity, specificity, positive likelihood
pregnancies and 37 (35.2 %; CI: 26.1-44.4 %) live births.
ratio [PLR; sensitivity / (1 - specificity)], and negative
The subjects with OHSS had a clinical pregnancy rate of
likelihood ratio [NLR; (1 - sensitivity) / specificity] of the
3/16 in the studied cycles with only 2 live births both in
cutoffs were also calculated A binary logistic re-
subjects treated with dostinex alone. In 4 patients (25 %)
gression model was also designed to predict poor re-
the OHSS occurred early and severely enough to prevent
sponse to stimulation with different variables including
oocyte collection and 2 patients, out of 89 subjects
AMH level. The response to stimulation was defined
(2.2 %), without OHSS had no oocytes yield.
poor ≤4 collected oocytes or good > 4 collected oocytes
Twenty-five subjects (23.8 %; CI: 15.7-32.0 %) had
in this model.
PCOS with higher prevalence of OHSS (40 % vs. 7.6 %;
The AMH values were not normally distributed
p < 0.001), younger age [30.5(0.7) vs. 33.3 (0.5); p =
(Kolmogorov-Smirnov's P value <0.001) and were positively
0.005] and higher basal AMH levels [7.1(0.7) vs. 3.3(0.3);
Table 1 The characteristics of the subjects with and without ovarian hyper-stimulation syndrome
Subjects without OHSS n = 89
Subjects with OHSS n = 16
Duration of infertility (yrs)
Duration of stimulation (days)
Number of follicles (HCG day)
Number of retrieved oocytes
Basal Anti-mullerian Hormone
Basal Luteinizing Hormone
Basal Follicle stimulating Hormone
Thyroid stimulating hormone
Basal Testosterone
Polycystic ovary syndrome
Clinical pregnancy
Data are mean or percentages and standard error from the mean or 95 % confidence intervals in the parentheses.
† indicates significant difference between the values of subjects with and without ovarian hyper-stimulation syndrome (T-test or Chi squared test; p < 0.05).
Aghssa et al. Reproductive Health (2015) 12:85
p < 0.001]. Their pregnancy rate, however, was not statis-
The total administered gonadotropin dose (but not
tically different from other patients [28(10.4-45.6) vs.
total days of stimulation) correlated inversely with the
44.3(33.3-55.3); p = 0.1].
OHSS occurrence (OR for every additional 75 inter-
The binary logistic regression model was designed
national unit = 0.8, CI: 0.7-0.9; p = 0.002).
with age, BMI, number of previous OSCs, PCOS, FSH
After adjustment for the effect of age, BMI, and PCOS;
level, number of retrieved oocytes, and basal AMH level
the AMH values were higher in the subjects with
(square root) as independent variables to predict the
consequent OHSS than in those without OHSS [7.7
OHSS (dependent variable). The number of retrieved
(0.7) ng/ml vs. 4.7(0.4) ng/ml; df = 5, F (11.9) = 14.2,
oocytes [OR: 1.3(95 CI: 1.1-1.6), Wald: 8.0, and P =
eta2 = 0.1; P = 0.001]. No significant interaction effect
0.004] and basal AMH value [OR: 5.4 (95 CI: 1.1-27.9),
was detected for the presence of PCOS on the asso-
Wald: 4.1, and P = 0.04] (but not PCOS, age, and BMI)
ciation of AMH levels and OHSS [F (11.9) =2.4 and
were significant independent predicting factors of OHSS.
The square root of basal AMH levels were moderately
AMH levels classified subjects with and without OHSS
correlated with the number of retrieved oocytes (r = 0.5;
with an area under the ROC curve (AUC) of 0.86 (0.78-
p < 0.001). Another model was defined with follicle
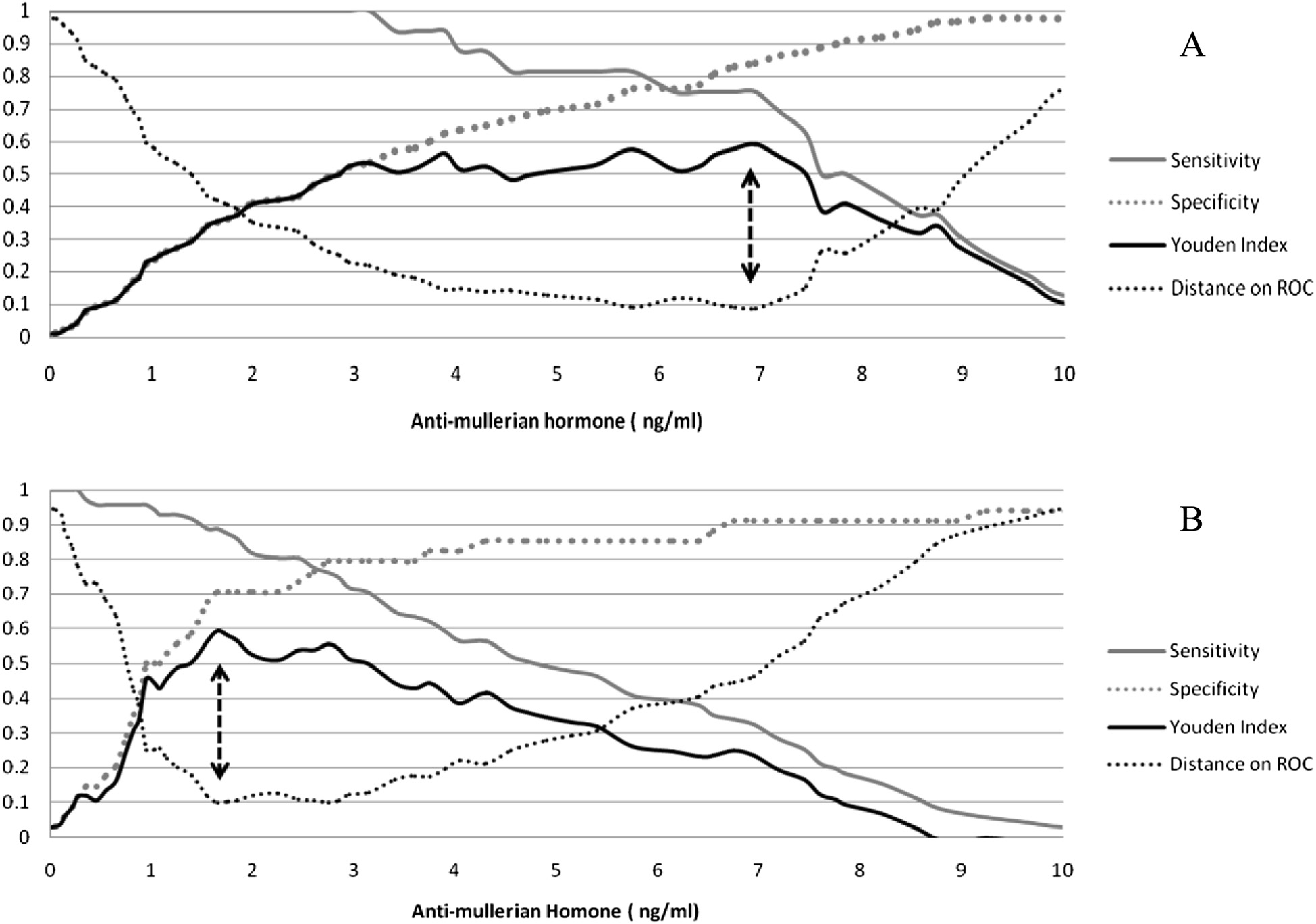
0.95; Fig. the best AMH cutoff value to predict OHSS
count instead of collected oocytes count (considering
was 6.95 ng/ml (sensitivity: 75 %, specificity: 84 %, PLR:
significant co-linearity). In this model, in addition to
4.7, NLR: 0.3; Fig. panel a). Patients with AMH values
AMH level the follicle count was significantly associated
higher than 6.95 ng/ml experienced a higher frequency
with OHSS [OR: 1.5 (95 CI: 1.1-1. 9), Wald: 8.9, and
of OHSS (5.1 % vs. 46.2 %; OR = 9, CI: 1.3-59.7, Chi2 P
P = 0.003]. A binary logistic regression model was de-
value < 0.001). In subjects without OHSS, those with
fined with independent variables identical to those of
AMH values over 6.95 (n = 14) compared to those with
the previous models excluding collected oocyte or fol-
AMH levels below 6.95 received significantly smaller
licle counts. AMH level (square root) was the only
gonadotropine doses per day [157 (17) IU vs. 197 (46);
predictor of the response to stimulation [OR: 0.25 (95
p < 0.005] with higher collected oocytes [9.4 (1.0) vs. 6.4
CI: 0.11-0.59), Wald: 10.3, and P = 0.001].
(0.5); p < 0.05] and the equal follicle count [11.1 (0.7) vs.
Fig. 1 Age adjusted anti-mullerian hormone is higher in those with consequent ovarian hyper-stimulation syndrome in patients with and withoutpolycystic ovary syndrome
Aghssa et al. Reproductive Health (2015) 12:85
Fig. 2 The Receiver Operating Characteristics (ROC) curves of basal anti-mullerian hormone values to predict ovarian hyper-stimulation syndrome.
AURC: Area Under the ROC Curve
8.7 (0.7); p < 0.067]. The cutoff value of AMH with the
responders to ovarian stimulation (post-test probability:
best prediction of poor response to controlled ovarian
75 %; OR = 23) and those with AMH levels >6.95
stimulation (oocytes ≤4) was 1.65 ng/ml with AUC of
(almost the upper quartile) are at a higher risk for OHSS
0.8 (0.69-0.91) and sensitivity and specificity of 89 %
(post-test probability: 46 %; OR = 9). With subclass ana-
and 71 %, respectively (Fig. panel b). A poor response
lysis of the administered gonadotropin dose, we suggest
rate in those with an AMH value below and above
that milder ovarian stimulation in high risk patients has
the cut point (1.65) was 75 % and 13.7 %, respectively
no detrimental effect on the COH main outcome (re-
(OR = 23.8, CI: 6.0-94.1, Chi2 P value <0.001). The
trieved oocyte count). Thus, we believe that based on
cut points for detection of OHSS and poor response
the proposed cut point of basal AMH levels (i.e. 6.95;
were substantially the same after exclusion of subject
roughly the upper quartile), reduced stimulation would
with PCOS (data not shown).
be a safe and the reasonable method of preventing
No significant association was found between basal
OHSS and its consequences. This approach may decrease
AMH level and the outcome of the IVF/ICSI procedure
the severe OHSS cases with its unfavorable consequences
(clinical pregnancy vs. failed cycle).
including hospitalization and cycle cancellation Afteradjustment for AMH levels, other pre-stimulation vari-
ables including age, BMI, and PCOS did not correlate with
Our results indicate an association between extreme
OHSS or poor response to COH.
AMH levels and OHSS and poor response to controlled
Our optimal cutoff value of AMH for the prediction of
ovarian stimulation independent of the effect of age,
OHSS is higher than the cut points suggested by previ-
BMI, and a history of PCOS. According to our findings,
ous studies (i.e. 1.6 ng/ml 2.1 ng/ml 3.4 ng/ml
subjects with an AMH level <1.65 (almost the lower
3.5 ng/ml ] and 4.8 ng/ml Our results
quartile of AMH values) are more likely to be poor
are in line with the result of La Marca et al. with
Aghssa et al. Reproductive Health (2015) 12:85
Fig. 3 Sensitivity, specificity, Yuden index and distance to the optimal point on the ROC curve for the basal anti-mullerian hormone levels topredict ovarian hyper-stimulation syndrome (panel a) and poor response to the IVF cycle (panel b). Arrows indicate the most effective thresholdvalue of AMH (6.95 and 1.65 ng/ml for ovarian hyper-stimulation syndrome and poor response to ovarian stimulation, respectively) correspondingto both maximum Youden index and shortest distance on the ROC curve
cutoff value of 7 ng/ml (75th percentile) for the classifi-
[]. Generally there is good correlation between the new
cation of exaggerated responses. The threshold value of
(i.e. Beckman-Coulter) and old AMH assay kits .
the study by Lee et al. was numerically lower but again
In our study 15 % of cases had moderate to severe
it was the 75th percentile of the AMH values of the stud-
OHSS. Moderate OHSS is reported in 1 to 14 % of sub-
ied population. Ebner et al. also reported the best
jects with less than 1 % severe OHSS cases We had
ovarian response was achieved when the basal AMH
higher frequency of diagnosed OHSS compared to the
value was between the 25th and 75th percentiles with
studies by Lee et al. (8 %), Nardo (10 %) and Aramvit
reduced oocyte quality of patients in the top quartile
et al. (12 %). Nevertheless, the prevalence of severe cases
(AMH level > 4.5 ng/ml). The cutoff point suggested by
leading to hospitalization was relatively low . The
Nardo et al. (3.5 ng/ml) is presumably the upper quartile
risk of excessive ovarian response is a function of the
of non-PCOS normal responders to COH. The differ-
COH protocol and the health characteristics of the par-
ences between the AMH thresholds for higher OHSS
ticipants (including age and the prevalence of PCOS).
risk noted in this study as compared to other studies
One may expect to encounter less exaggerated responses
may be explained by the differences between the studied
in the short COH protocols and in populations with
populations (age, threshold for treatment of infertility, or
lower PCOS. The prevalence of PCOS was high among
frequency of PCOS), the definition of OHSS, and the
the infertile population of this study (i.e. 23.8 %). The
different AMH measurement methods. Roughly - also
prevalence of PCOS among subjects undergoing COH
not limited by the remarkable difference between the
varies from 4 to 22 % The risk of OHSS in
AMH readings of two older ELISA kits i.e. DSL and
our study was dramatically high in patients with PCOS
Immunotech - the patients with upper quartile AMH
(40 %), which is consistent with the study by Aramvit
values are at high risk for exaggerated ovarian response
et al. in which about 45 % of the PCOS subjects
Aghssa et al. Reproductive Health (2015) 12:85
experienced OHSS Interestingly the incidence of
Washington Hospital Center, Washington, DC, USA. 3Saeed Medical
OHSS associated with AMH high levels independent of
Laboratories, Tehran, Iran. 4Department of Nuclear Medicine, Valiasr Hospital,Tehran University of Medical Sciences, Tehran, Iran.
the diagnosis of PCOS. Also after adjustment for the ef-fect of AMH there was no correlation between PCOS
Received: 2 August 2014 Accepted: 4 July 2015
The clinical pregnancy rate was comparable and rather
high in our study (40 %) as compared with the results of
Nardo et al. (24 %) ], Kini et al. (34 %) ], and Lee
Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-müllerian hormone as a
et al. (41 %) . Relatively higher pregnancy rates ac-
marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20–4.
Van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong
company higher exaggerated ovarian stimulation rates
FH, et al. Serum antimullerian hormone levels best reflect the reproductive
possibly due to stronger protocols or possibly higher
decline with age in normal women with proven fertility: a longitudinal
prevalence of younger PCOS patients.
study. Fertil Steril. 2005;83(4):979–87.
van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, Jong FH, et al.
Our study suffers from certain flaws: above all the
Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve.
OHSS was mainly diagnosed based on the decision of
Hum Reprod. 2002;17:3065–71.
the chief researcher who decided also for the future
La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al.
Anti-Mullerian hormone measurement on any day of the menstrual cycle
treatment of the patients. This reduces the extend the
strongly predicts ovarian response in assisted reproductive technology.
results could be extrapolated. The sample size of the
Hum Reprod. 2007;22:766–71.
study was also rather small, hence the conclusion re-
Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER,Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual
garding the pregnancy rate after assisted reproduction
cycle do not show substantial fluctuation. J Clin Endocrinol Metab.
cycles should be interpreted with caution; however, this
result adds information regarding the ethnic group being
La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerianhormone throughout the human menstrual cycle. Hum Reprod.
studied here. Also we employed long down-regulated
protocol because at the time of study antagonist agents
Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA,
were unavailable to us. Lastly, since the measurement of
Schliep K, et al. Biological variability in serum anti-Müllerian hormonethroughout the menstrual cycle in ovulatory and sporadic anovulatory
AMH by Gen II assay is subject to the complement
cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764–72.
interference which may overestimate or underestimate
Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular
the actual AMH values, further studies with the use of
serum mullerian-inhibiting substance levels are associated with ovarianresponse during assisted reproductive technology cycles. Fertil Steril.
new protocols even in the already studied populations
Broer SL, Do'lleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJM.
AMH and AFC as predictors of excessive response in controlled ovarianhyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17(1):46–54.
Golan A, Ron-EL R, Herman A, Sofer Y, Weinraub Z, Caspi E. Ovarian
We suggest that infertile patients undergoing COH with
hyperstimulation syndrome: An update review. Obstet Gynecol Survey.
top and low quartile basal AMH values are at high risk
Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel
for OHSS and poor ovarian response, respectively. Re-
reproductive technologies: prevention and treatment. Fertil Steril.
duced stimulation dosage in high risk subjects for OHSS
had no detrimental effect on the final outcome. Our
Perkins NJ, Schisterman EF. The Inconsistency of "Optimal" Cut-points UsingTwo ROC Based Criteria. Am J Epidemiol. 2006;163(7):670–5.
study indicated that AMH was a superior predictor to
McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of
traditional factors including age, BMI, and PCOS. Also
Metabolic Markers To Identify Overweight Individuals Who Are Insulin
considering that AMH is available before the stimula-
Resistant. Ann Intern Med. 2003;139:802–9.
Lainas GT, Kolibianakis EM, Sfontouris IA, Zorzovilis IZ, Petsas GK, Tarlatzi TB,
tion, it is superior to the number of oocytes yielded.
et al. Outpatient management of severe early OHSS by administration ofGnRH antagonist in the luteal phase: an observational cohort study.
Competing interest
Reprod Biol Endocrinol. 2012;31:10–69.
The authors declare that they have no competing interest.
Riggs RM, Duran EH, Baker MW, Kimble TD, Hobeika E, Yin L, et al.
Assessment of ovarian reserve with anti-Mullerian hormone: a
Authors' contributions
comparison of the predictive value of anti-Mullerian hormone,
MMA and EST conceived the study, MMA also carried out the clinical
follicle-stimulating hormone, inhibin B, and age. Am J Obstet Gynecol.
procedures and made the clinical decisions and they interpreted the results.
AMT participated in the data collection, analysis, interpretation and
Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and
manuscript drafting. MB and ZP participated in data collection and clinical
FSH: prediction of live birth and extremes of response in stimulated cycles–
procedures. SM performed the laboratory measurements and interpretations
implications for individualization of therapy. Hum Reprod. 2007;22:2414–21.
and drafted the related parts. ME participated in manuscript drafting and
Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum
interpretations. MA analyzed the data, participated in the interpretations and
anti-Mullerian hormone and estradiol levels as predictors of ovarian
drafted the manuscript. All authors read and approved the final manuscript.
hyperstimulation syndrome in assisted reproduction technology cycles.
Hum Reprod. 2008;23:160–7.
Eldar-Geva T, Ben Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T,
1Vali-e-Asr Reproductive Health Research Center, Department of Obstetrics
et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol
and Gynecology, Valiasr Hospital, Tehran University of Medical Sciences,
following FSH stimulation and ovarian ultrasonography as predictors of IVF
1419433141 Tehran, Iran. 2Department of Obstetrics and Gynecology,
outcome. Hum Reprod. 2005;20:3178–83.
Aghssa et al. Reproductive Health (2015) 12:85
Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al.
Circulating basal anti-Mullerian hormone levels as predictor of ovarianresponse in women undergoing ovarian stimulation for in vitro fertilization.
Fertil Steril. 2009;92:1586–93.
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarianresponse to controlled ovarian hyperstimulation: anti-Mullerian hormoneversus small antral follicle count (2–6 mm). J Assist Reprod Genet.
2009;26:319–25.
Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G.
Basal level of anti-Müllerian hormone is associated with oocyte quality instimulated cycles. Hum Reprod. 2006;21(8):2022–6.
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al.
Anti-Mullerian hormone (AMH) as a predictive marker in assistedreproductive technology (ART). Hum Reprod Update. 2010;16(2):113–30.
Li HW, Ng EH, Wong BP, Anderson RA, Ho PC, Yeung WS. Correlationbetween three assay systems for anti-Müllerian hormone (AMH)determination.J. J Assist Reprod Genet. 2012;29(12):1443–6.
Garcia-Velasco JA. How to avoid ovarian hyperstimulation syndrome: a newindication for dopamine agonists. Reprod BioMed Online. 2009;12:71–5.
Kahnberg A, Enskog A, Brannstrom M, Lundin K, Bergh C. Prediction ofovarian hyperstimulation syndrome in women undergoingin vitrofertilization. Acta Obstetricia et Gynecologica. 2009;88:1373–81.
Aramwit P, Pruksananonda K, Kasettratat N, Jammeechai K. Risk factors forovarian hyperstimulation syndrome in Thai patients using gonadotropins forin vitro fertilization. J Health-Syst Pharm. 2008;65:1148–53.
Kini S, Raymond HW, Morrell D, Pickering S, Thong KJ. Anti-mullerianhormone and cumulative pregnancy outcome in in-vitro fertilization.
J Asist Reprod Genet. 2010;27:449–56.
Ward G. AMH – where are we up to? Pathology. 2015;47(S1):s16.
Submit your next manuscript to BioMed Central
and take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution
Submit your manuscript at www.biomedcentral.com/submit
Source: http://old.reproductive-health-journal.com/content/pdf/s12978-015-0053-4.pdf
a bayeux.fr un été à Bayeux Les Médiévales : Un nouveau complexe de loisirs avenue de la Vallée des Près Un jardin pour Salomé Le 19 mai dernier a été inauguré le Jardin de Salomé, baptisé en hommage à Salomé Girard, jeune Bayeusaine victime de l'attentat survenu à EmbEllir la villE
This leaflet describes the various ways in which Barrett's Oesophagus is treated. There are two aimsin treating Barrett's Oesophagus: to relieve the symptoms of acid reflux and to prevent it developinginto cancer. Treatment of acid reflux soon as you get symptoms. People with Barrett's Oesophagus Rennies and Tums, and most of often have bad acid reflux but,


