The effectiveness and safety of treatments used for polycystic ovarian syndrome management in adolescents: a systematic review and network meta-analysis protocol

Al Khalifah et al. Systematic Reviews (2015) 4:125 DOI 10.1186/s13643-015-0105-4
The effectiveness and safety of treatmentsused for polycystic ovarian syndromemanagement in adolescents: a systematicreview and network meta-analysis protocol
Reem A. Al Khalifah1,2,3, Iván D. Flórez1,4* Brittany Dennis1, Binod Neupane1, Lehana Thabane1,5,6and Ereny Bassilious2
Background: Polycystic ovarian syndrome (PCOS) is a common reproductive endocrine disease that is seen amongadolescent women. Currently, there is limited evidence to support treatment options leading to considerable variationin practice among healthcare specialists. The objective of this study is to review and synthesize all the available evidenceon treatment options for PCOS among adolescent women.
Methods/design: We will conduct a systematic review of all randomized controlled trials evaluating the use ofmetformin, oral contraceptive pills as monotherapy, or as combination with pioglitazone, spironolactone, flutamide,and lifestyle interventions in the treatment of PCOS in adolescent women ages 11 to 19 years. The primary outcomemeasures are menstrual regulation and change hirsutism scores. The secondary outcome measures include acnescores, prevalence of dysglycaemia, BMI, lipid profile, total testosterone level, and adverse events. We will performliterature searches through Ovid Medline, Ovid Embase, and Cochrane Central Register of Controlled Trials (CENTRAL),and gray literature resources. Two reviewers will independently screen titles and abstracts of identified citations, reviewthe full texts of potentially eligible trials, extract information from eligible trials, and assess the risk of bias and quality ofthe evidence independently. Results of this review will be summarized narratively and quantitatively as appropriate. Wewill perform a multiple treatment comparison using network meta-analysis to estimate the pooled direct and indirecteffects for all PCOS interventions on outcomes if adequate data is available.
Discussion: PCOS treatment poses a clinical challenge to the patients and physicians. This is the first systematic reviewand network meta-analysis for PCOS treatment in adolescents. We expect that our results will help improve patient care,unify the treatment approaches among specialists, and encourage research for other therapeutic options.
Systematic review registration: PROSPERO
Keywords: Adolescents, Polycystic ovarian syndrome, Hirsutism, Menstrual irregularity, Acne, Body mass index,Dysglycaemia
* Correspondence: 1Department of Clinical Epidemiology & Biostatistics, McMaster University,Juravinski Site, G Wing, 2nd Floor, 711 Concession Street, Hamilton, ON L8V1C3, Canada4Department of Pediatrics, University of Antioquia, Medellín, ColombiaFull list of author information is available at the end of the article
2015 Al Khalifah et al. Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0International License which permits unrestricted use, distribution, andreproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link tothe Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiverapplies to the data made available in this article, unless otherwise stated.
Al Khalifah et al. Systematic Reviews (2015) 4:125
pills (OCP) to control symptoms of hyperandrogenism
Polycystic ovarian syndrome (PCOS) is a common repro-
or metformin therapy in patients with impaired glucose
ductive endocrine disease encountered among adolescents
tolerance or features of metabolic syndrome [. How-
and young women [. Its prevalence varies between 1.8
ever, there is significant variability in clinical practice,
and 15 % depending on the diagnostic criteria used and
depending on whether the physician and patient's primary
ethnicity . Patients with PCOS can present with a
goal of treatment is to treat the symptoms of hyperandro-
constellation of symptoms including chronic anovulation
genism or the features of metabolic syndrome
(amenorrhea, oligomenorrhea, irregular menstrual cycles),
Additionally, in clinical practice anti-androgenic medica-
clinical features of hyperandrogenism (acne and hirsut-
tions such as spironolactone, flutamide, and insulin sensi-
ism), biochemical evidence of hyperandrogenism, polycys-
tizing agents such as pioglitazone are used as add-on
tic ovaries on ultrasound, and features of metabolic
therapy when OCP or metformin fail to produce the
syndrome. Oligomenorrhea is the presenting feature in
clinically desired outcomes yet the Endocrine
about 75 % of cases [, while hirsutism and acne are
Society guidelines do not comment on their use in
present in 60–70 % of cases and contribute to psycho-
the adolescent population.
logical distress in adolescent patients .
To date, there is one systematic review and meta-
Three different diagnostic criteria have been used for
analyses in adolescents (in press) that identified low num-
the diagnosis of PCOS: the National Institutes of Health
ber of low quality evidence from head-to head trials and
(NIH), the Rotterdam, and the Androgen Excess Society
identified large number of trials that compared metformin
Criteria All of them require the presence of men-
to placebo, OCP to placebo, and other PCOS combination
strual cycle disturbance and presence of clinical and/or
therapy ]. A traditional meta-analysis can only evaluate
biochemical hyperandrogenism, while the last two re-
the direct treatment efficacy of two treatment approaches
quire the presence of polycystic ovarian morphology on
at a time while a network meta-analysis can provide effect
ultrasound To date, the preferred diagnostic cri-
estimates for all direct and indirect treatment comparisons
teria in adolescents are the NIH criteria [
[]. Therefore, we aim to conduct a network meta-
The etiology of PCOS is complex and not well under-
analysis to address the following objectives: (1) assess the
stood. Primary intrinsic ovarian pathology in combination
effectiveness and safety of using metformin and OCP as
with hypothalamic–pituitary–ovarian axis abnormalities
monotherapy in adolescents with PCOS; (2) assess the ef-
may lead to increased ovarian androgen secretion [,
fectiveness and safety of using metformin and/or OCP in
Insulin resistance with compensatory hyperinsulinemia
combination with pioglitazone, spironolactone, flutamide,
may also play a role as it can lead to direct stimulation of
and lifestyle interventions, as evaluated across multiple
ovarian and adrenal androgen secretion, which leads to
outcomes such as menstrual cycle regulation, improve-
decreased hepatic sex hormone binding globulin synthesis
ment in clinical and or biochemical evidence of hyperan-
and therefore, to an increased bioavailability of free testos-
drogenism, and metabolic profile in adolescents with
terone level ]. Insulin resistance is involved in the
PCOS; (3) evaluate the effectiveness of different formula-
development of cardiometabolic disturbances such as dys-
tions of OCPs on hirsutism and acne scores.
glycaemia, hyperlipidemia, and obesity and it hasbeen described that between 18 and 24 % of adolescents
with PCOS have some degree of abnormal glucose me-
This systematic review and network meta-analysis proto-
tabolism [–These patients are at increased risk
col is registered on PROSPERO International prospective
of type 2 diabetes, hypertension, myocardial infarction,
register of systematic reviews (CRD42015016148). The re-
angina, and psychiatric diseases in addition to
port will comply with the Preferred Reporting Items for
gynecological and obstetrical complications, such as in-
Systematic Review and Network Meta-Analysis Protocols
fertility, higher rate for pregnancy loss, gestational dia-
(PRISMA-P) [].
betes, premature delivery, as well as gynecological andnon-gynecological cancers . In addition to the
Eligibility criteria
aforementioned co-morbidities, patients with PCOS ex-
The search for studies will be limited to randomized
perience a low perceived health quality over lack of
clinical trials (RCT) (including all designs such as cross-
symptom improvement, primarily with weight control,
over, cluster, and patient-randomized clinical trials) asses-
hirsutism, acne, menstrual irregularity, and infertility as
sing the efficacy, effectiveness, or safety of different
inferred from qualitative studies
regimen for the treatment of PCOS that enrolled adoles-
Optimal first line treatment of PCOS in adolescents
cent girls ages 11–19 years. The definition of adolescent
remains controversial. Current Endocrine Society treatment
age group is based on the widely accepted World Health
guidelines first recommend lifestyle changes (dietary and
Organization definition for adolescent Studies that
exercise modification) followed by either oral contraceptive
include both adolescents and adults participants will be
Al Khalifah et al. Systematic Reviews (2015) 4:125
included in the review, and upon contact, we will ask au-
search of bibliographies of identified randomized controlled
thors to provide separate data for the adolescent partici-
trials and guidelines; (2) trials registries (Clinicaltrials.gov,
pants. If we are unable to obtain this information, we will
World Health Organization WHO International Clinical
include the study and we will conduct subgroup analyses
Trials Registry Platform Search Portal, controlled-trials.com
in order to assess the difference between studies which in-
and the National Institutes of Health database of funded
cluded only adolescents and studies which included both
studies for ongoing or unpublished trials); and (3) confer-
adolescents and adults. Sub-studies or secondary ana-
ences preceding and abstracts of the North American and
lysis of reported eligible studies will be excluded to
European Endocrine Society and The Society of Adolescent
avoid duplication.
Medicine and Health. Search alerts are set up for monthly
The diagnosis of PCOS will be based on the known
notification, and the search will be repeated before the final
PCOS diagnostic criteria: Endocrine Society Guidelines,
manuscript submission to identify any new literature. We
NIH criteria, Rotterdam criteria, and the Androgen Excess
will contact the authors of unpublished work to establish
Society criteria We will exclude studies that in-
eligibility and methodological quality of the study.
cluded normal control participants or patients with othercauses of oligomenorrhea or hyperandrogenism, such as
hyperprolactinemia, thyroid dysfunction, androgen secret-
Two reviewers (RA and IF) will independently and in
ing tumors, or late-onset congenital adrenal hyperplasia.
duplicate screen the title and abstract available of identi-
We will include studies that evaluated single and/or
fiable articles to assess its eligibility. In case of disagree-
combined interventions, at any dose, such as metformin,
ment, the full text will be retrieved and reviewed
OCP, pioglitazone, spironolactone, flutamide, and life-
independently by one of the authors (EB), to resolve dis-
style interventions. In order to be included, the study
crepancy. We will refer to inclusion and exclusion cri-
will have had to report the effectiveness of one of these
teria during the screening process. Records of ineligible
interventions and the intervention effect on one or more
articles along with the reason for ineligibility will be
of the outcomes of interest.
saved for future reference. Eligible articles citations will
Our primary outcomes are menstrual cycle regulation
be saved in EndnoteX6 library. We will include the
and hirsutism scores. The secondary outcomes include
PRISMA flow diagram demonstrating the search and
acne scores, prevalence of dysglycaemia, BMI, total tes-
screening process (Fig. We will contact authors of
tosterone level, lipid profile (triglyceride, total choles-
primary studies during data extraction to provide any
terol, LDL, HDL), and adverse events; Table shows the
definitions of outcome measures. We chose not to re-port on pregnancy outcomes because it necessities chan-
ging the scope of the review to involve fertility induction
The study data will be collected in standardized online
medications. Hence, we will exclude studies that only
data extraction forms (Google forms) according to pre-
used fertility induction medications and which primary
specified instructions. The data extraction form will
outcome of interest was pregnancy.
include information pertaining to study background, lan-guage of publication, country, funding sources, confirm
Data sources and search strategy
study eligibility, participant ages, PCOS diagnostic cri-
We performed literature search through Ovid MEDLINE,
teria, the study design, number of intervention groups,
Ovid EMBASE, and Cochrane Central Register of Con-
intervention details, number of participants allocated to
trolled Trials (CENTRAL) from the database inception to
each intervention group, randomization, concealment of
January 2015 using combination of controlled terms, i.e.,
allocation, blinding, length of follow up, analysis type,
Medical Subject Heading (MeSH), Emtree terms, and
outcome definition, unit of measurement, ascertainment
free-text terms with various synonyms for polycystic
of the outcome, estimate of intervention effect with con-
ovarian syndrome (PCOS), adolescent, metformin, pio-
fidence interval, and missing follow up data. When stud-
glitazone, oral contraceptive pills, flutamide, and life-
ies measure outcomes at more than one time point, we
style interventions ).
will collect results for the last measurement point in the
We used the randomized controlled trial filter created
study. The data extraction form will be pilot tested by all
from McMaster University for Ovid Embase platform
reviewers independently before its use. Four reviewers
and the Cochrane library filter for Ovid Medline platform
will perform data extraction (RA, IF, EB, BD), working
These filters provide a good balance between sensi-
in pairs independently and in duplicate. In case of dis-
tivity and specificity. Our search strategy was developed in
agreement in assessing the methodological quality of the
liaison with an experienced librarian. No language, publica-
study, we will try to resolve it by consensus. If consensus
tion status, or date limit was used. Additionally, we per-
cannot be reached, a third designated reviewer from the
formed a gray literature search through (1) manual hand
team will be involved.
Al Khalifah et al. Systematic Reviews (2015) 4:125
Table 1 Outcome measures
Measurement of variable (units)
Statistical estimates andmeasurement of associationof this outcome
Menstrual regulation
Number of girls achieved regular menses
Number of cycles per year
Mean difference ± SD
Ferriman Gallawey score
Mean difference ± SD
Lesion counting or grading
Standardized mean difference ± SD
The rate of occurrence of T2DM, impaired glucose tolerance,
and impaired fasting glucose assessed by oral glucose tolerancetest and/or fasting blood glucose, and/or HBA1c
Mean difference ± SD
Total testosterone level
Mean difference ± SD
Total cholesterol
Mean difference ± SD
Mean difference ± SD
Mean difference ± SD
Mean difference ± SD
Number of girls developed:
1- GI: all GI related adverse events:
• Nausea• Vomiting• Diarrhea• Constipation• Abdominal pain• Flatulence• Gastritis• GI bleeding
2- thrombosis: all vascular events related to thrombusformation such as:
• Deep venous thrombosis• Stroke• Myocardial infarction• Pulmonary embolism
3- serious: adverse events that are of majormorbidity such as:
• Any bleeding not including GI bleed• Lactic acidosis• Liver failure• Renal failure• Vasculitis• Electrolyte imbalance• Agranulocytosis• Photosensitivity• Hypertension• Pancreatitis• Anaphylaxis• Chorea• Depression
Al Khalifah et al. Systematic Reviews (2015) 4:125
Table 1 Outcome measures (Continued)
4- minor: adverse events that are of minormorbidity such as:
• Headache• Fatigue• Beast tenderness• Vaginal bleeding (spotting)• Edema• Weight gain• Metallic taste• Muscle cramp• Rash• Fever• Hot flashes• Glucose intolerance• Infection
OR odds ratio, T2DM type 2 diabetes mellitus, BMI body mass index, LDL low-density lipoprotein, HDL high-density lipoprotein, GI gastrointestinal
Assessment of risk of bias in included studies
risk," or "unclear risk." We will further categorize the "un-
Two independent reviewers will assess each included study
clear risk" to "probably low risk" or "probably high risk" in
for risk of bias using the modified Cochrane handbook for
order to give a better understanding of the unclear risk of
systematic reviews of interventions tool [, which as-
bias score. We will rate the overall risk of bias score for
sesses six elements: (1) sequence generation, (2) allocation
each study as "high risk" if the study meets more than two
concealment, (3) blinding of participants, personnel and
criteria for high risk of bias, "moderate risk of bias" if the
outcome assessors, (4) completeness of follow up, (5) se-
study meets one to two criteria for high risk of bias, and
lective outcome reporting, and 6) presence of other biases.
"low risk of bias" if the study does not meet any high risk
Each domain will be assigned a score of "low risk," "high
of bias criteria [].
Standard direct comparisonsWe will perform a pairwise meta-analysis using R soft-ware. Effect estimates and their 95th confidence interval(CI) will be calculated using risk ratio (RR) for binaryoutcomes and mean difference for continuous outcomesif they are reported using the same metrics; otherwise,estimates reported using different metrics will be con-verted into standardized mean difference (SMD). Wewill pool all direct evidence using random-effect meta-analysis with the maximal likelihood (ML) estimatorWe will assess for heterogeneity by estimating thevariance between studies using the chi-square test andquantify it using the I2 test statistic. We will interpretthe I2 using the thresholds set forth by the CochraneCollaboration
The network meta-analysisGiven that many of the treatment combinations availableto treat PCOS were not compared in head-to-head stud-ies, a network meta-analysis (NMA) will be necessary toprovide effect estimates for all indirect comparisonsWe will perform a multiple treatment comparison
Fig. 1 The primary selection process
to estimate the pooled direct, indirect, and the network
Al Khalifah et al. Systematic Reviews (2015) 4:125
estimates (mixed evidence from direct and indirect esti-
hierarchical regression model but considers multiple com-
mates) for all PCOS interventions on outcomes if the as-
ponents of an intervention as dummy variables in the same
sumptions of homogeneity and similarity are judged to
model. Hence, the analysis allows the estimation of the ef-
be reasonable. Effect estimates will be presented along
fects and ranking of a combination of all possible and ap-
with their corresponding 95 % credibility intervals (CrIs);
propriate components. Thus, it is possible to explore such
these are the Bayesian analog of 95 % CIs. However,
a combination that could have been the best for the treat-
mixed evidence will only be used if the consistency as-
ment of PCOS but has never been tested before in any
sumption is met.
trial. Further, such an analysis allows the assessment of
We will fit a Bayesian random-effect hierarchical
additive or multiplicative (interaction) effects between two
model with non-informative priors using vague normal
or more components if sufficient data are available. We
distribution (mean 0, variance 10,000) and adjusting for
will re-analyze the data under complex intervention ap-
correlation between effects in multi-arm trials. We will
proach as well [to assess if there exists a potentially bet-
ter combination of components which have been ever or
Monte-Carlo (MCMC) simulation technique running
never assessed.
the analysis in four parallel chains. We will use a series
We will perform the Bayesian network meta-analysis
of 100,000 burn-in simulations to allow convergence
in JAGS (version 3.4.0) or WinBUGS software (version
and then a further 20,000 simulations (succeeding
1.4.3, MRC Biostatistics Unit, Cambridge, UK) inter-
50,000 simulations saved at an interval of 10 in each
facing through R software.
chain) to produce the outputs. We will assess modelconvergence using Gelman and Rubin diagnostic test ].
The Bayesian model provides flexibility for moderate
In case there is significant heterogeneity and inconsistency,
levels of treatment heterogeneity, sampling variability,
we will use meta-regression to explain the heterogeneity,
and incoherence This model introduces a ran-
provided we have enough data to do so; otherwise, we will
dom effect representing any changes in the observed
perform subgroup analyses. We will perform meta-
treatment effect that may be due to the comparison
regression using study level covariates: methodological
being made We will interpret variability in this
quality (high risk of bias versus low risk of bias), partici-
random effect as incoherence [We will use the
pant's average age, BMI status (obese and/or overweight
node-splitting method to detect incoherence between
BMI ≥25 kg/m2 versus normal <25 kg/m2), homeostatic
direct and indirect evidence within a closed loop as
model assessment (HOMA-IR) (high and moderate ≥3
well as identify loops with large inconsistency [
versus low <3), medication dose, length of treatment
We will measure the goodness-of-fit of the model using
(≥3 months versus <3 months), use of ultrasound to docu-
the deviance information criterion (DIC)
ment polycystic ovaries (used versus not used), and studies
To ensure interpretability of the NMA results, we will
that included young adults versus adolescents only to
present the network geometry, the results with probabil-
examine the improvement or change in model fit after co-
istic statements, and also the estimates of interventions
variates are included into the model. We will also perform
effects and corresponding 95 % CrIs, as well as forest
a subgroup analysis to evaluate the effectiveness of differ-
plots. We will first rank the intervention and report each
ent oral formulations of contraceptive pill on changes of
interventions' probability of ranking first (being the best
hirsutism and acne scores.
treatment) as well as the surface under the cumulativeranking curve (SUCRA) values High SUCRA values
Rating the confidence in estimates of the effect in NMA
are expected for the best treatments, and low SUCRA
The confidence in the estimates (quality of evidence) for
values are expected for the worst treatments.
each reported outcome will be assessed independently
The above analysis assumes that the interventions are
by two reviewers (RA, IF) using the Grading of Recom-
competing (suitable when most components forming an
mendations Assessment, Development, and Evaluation
intervention are pharmaceutical and hence cannot go to-
Working Group (GRADE Working Group) approach;
gether), so that each combination is considered to be a
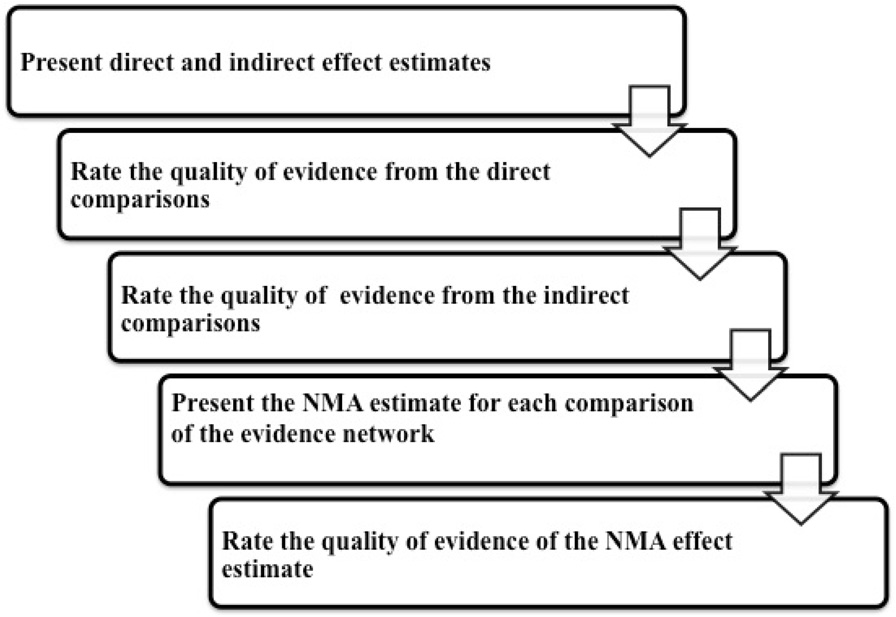
see Fig. for the flow of quality assessment The
separate treatment. For example, a combination of com-
quality of evidence is categorized by GRADE into four
ponents of metformin, flutamide, and exercise forming an
levels: high quality, moderate quality, low quality, and
intervention and another combination of metformin and
very low quality. For the direct comparisons, we will as-
exercise forming another intervention are treated as com-
sess and rate each outcome based on the five GRADE
peting interventions and assesses whether one combination
categories: risk of bias, imprecision, inconsistency, indir-
is better than another. However, it is possible to perform
ectness, and publication bias
the network meta-analysis treating these combinations as
For the assessment of confidence in the estimates ob-
complex intervention [Such an analysis fits a similar
tained in the NMA, we will use the recent approach
Al Khalifah et al. Systematic Reviews (2015) 4:125
Table 2 Search strategy: Ovid MEDLINE(R) in-process and othernon-indexed citations, Ovid MEDLINE(R) Daily and OvidMEDLINE(R) 1946 to January 29, 2015
Fig. 2 The quality assessment flow diagram
young adult*.mp.
recommended by the GRADE working group We
will assess and rate the confidence in all the indirect com-
parisons, if available, obtained from first order loops fol-
lowing the five GRADE categories used for assessing the
direct comparisons in addition to the intransitivity assess-
exp Polycystic Ovary Syndrome/
ment. Then, we will rate the confidence in each NMA ef-fect estimate using the higher quality rating when both
direct and indirect evidence are present. However, the es-
timate can be rated down for incoherence
exp Hyperandrogenism/
PCOS treatment in adolescents poses clinical chal-
lenges to patients and physicians. To our best know-
ledge, our study will be the first NMA in adolescents
(polycystic ovar* adj3 (syndrome or disorder or disease)).tw.
to investigate the effectiveness and safety of using
metformin and OCP as monotherapy as well as in
combination with pioglitazone, spironolactone, fluta-mide, or lifestyle interventions.
Our planned approach for this review has many
strengths. We will implement a wide search strategy that
included published and unpublished work. As adolescent
women share some similar physiology with adult women
and in an effort to overcome publication bias, we also
exp Contraceptives, Oral/
plan to include studies that included adolescents andyoung adults. Additionally, we aim to report on many
patient important outcomes as inferred from previous
qualitative research. Similar to previous systematic re-
views in adults with PCOS, we anticipate that we will
identify studies which use different definitions of PCOS,
various definitions for outcome measures of interest,
and small sample sizes These factors may pose po-tential limitations to our study.
We hope that this review will provide hierarchical evi-
dence to improve patient care, help unify the treatment
approaches among specialists, and encourage research
exp spironolactone/
for new therapeutic options.
Al Khalifah et al. Systematic Reviews (2015) 4:125
Table 2 Search strategy: Ovid MEDLINE(R) in-process and other
Table 2 Search strategy: Ovid MEDLINE(R) in-process and other
non-indexed citations, Ovid MEDLINE(R) Daily and Ovid
non-indexed citations, Ovid MEDLINE(R) Daily and Ovid
MEDLINE(R) 1946 to January 29, 2015 (Continued)
MEDLINE(R) 1946 to January 29, 2015 (Continued)
exp hyperandrogenism/
exp Health Promotion/
exp oral contraceptive agent/
randomized controlled trial.pt.
Controlled clinical trial.pt.
clinical trials as topic.sh.
14 and 24 and 52 and 60
exp spironolactone/
exp pioglitazone/
Embase 1974 to 2015 January 27
exp health promotion/
exp diet therapy/
young adult*.mp.
Double blind.tw.
Single blind.tw.
exp ovary polycystic disease/
14 and 49 and 54 and 57
Al Khalifah et al. Systematic Reviews (2015) 4:125
Table 2 Search strategy: Ovid MEDLINE(R) in-process and other
Author details1Department of Clinical Epidemiology & Biostatistics, McMaster University,
non-indexed citations, Ovid MEDLINE(R) Daily and Ovid
Juravinski Site, G Wing, 2nd Floor, 711 Concession Street, Hamilton, ON L8V
MEDLINE(R) 1946 to January 29, 2015 (Continued)
1C3, Canada. 2Department of Pediatrics, Division of Endocrinology andMetabolism, McMaster University, Hamilton, Ontario, Canada. 3Department of
Cochrane central: search up to 30/01/2015 20
Pediatrics, King Saud University, Riyadh, Saudi Arabia. 4Department of
#1 MeSH descriptor: [Adolescent] explode all trees
Pediatrics, University of Antioquia, Medellín, Colombia. 5Department ofPediatrics, McMaster University, Hamilton, Ontario, Canada. 6Department of
#2 MeSH descriptor: [Child] explode all trees
Pediatrics and Anesthesia, McMaster University, Hamilton, Canada.
#3 MeSH descriptor: [Young Adult] explode all trees
Received: 15 July 2015 Accepted: 25 August 2015
#4 young adult.tw
Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary
#7 MeSH descriptor: [Polycystic Ovary Syndrome] explode all trees
syndrome in women in China: a large community-based study. Hum
#8 Stein Leventhal Syndrome or Polycystic Ovar* or PCOS. tw
Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, et
#9 MeSH descriptor: [Hirsutism] explode all trees
al. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril.
#10 MeSH descriptor: [Hyperandrogenism] explode all trees
Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and
#11 hyperandrogen*. tw
cardiometabolic risk of polycystic ovary syndrome under differentdiagnostic criteria. Hum Reprod. 2012;27(10):3067
#12 Hirsutism. tw
Roe AH, Dokras A. The diagnosis of polycystic ovary syndrome in
#13 #6 or #7 or #8 or #9 or #10 or #11 or #12
adolescents. Rev Obstet Gynecol. 2011;4(2):45–51.
Kumarapeli V, Seneviratne Rde A, Wijeyaratne C. Health-related quality of life
#14 metformin or glucophage or pioglitazone or oral contraceptive pill
and psychological distress in polycystic ovary syndrome: a hidden facet in
or OCP or Ortho Tri-Cyclen or Ortho-CyclenCyclessa or Mircette or
South Asian women. BJOG. 2011;118(3):319–28.
Yasmin or diane or Desogen or Flutamide or spironolactone or
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group.
Revised 2003 consensus on diagnostic criteria and long-term health risks
#15 MeSH descriptor: [Metformin] explode all trees
related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS
#16 MeSH descriptor: [Contraceptives, Oral, Hormonal] explode all trees
Society criteria for the polycystic ovary syndrome: the complete task force
#17 MeSH descriptor: [Spironolactone] explode all trees
report. Fertil Steril. 2009;91(2):456–88.
Zawadski J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome:
#18 MeSH descriptor: [Flutamide] explode all trees
towards a rational approach. In: Dunaif A, Givens J, Haseltine F, Merriam G,
#19 MeSH descriptor: [Life Style] explode all trees
editors. Polycystic Ovary Syndrome. 1st ed. Boston: Blackwell ScientificPublications; 1992. p. 377–84.
#20 #14 or #15 or #16 or #17 or #18 or #19
Hardy TS, Norman RJ. Diagnosis of adolescent polycystic ovary syndrome.
Steroids. 2013;78(8):751
#21 #7 and #13 and #20 in Trials
Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, etal. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society
clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92.
BMI: body mass index; DIC: deviance information criterion; GRADE: Grading of
Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–36.
Recommendations Assessment, Development and Evaluation; NIH: National
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome:
Institutes of Health; NMA: network meta-analysis; OCP: oral contraceptive pill;
etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31.
PCOS: polycystic ovarian syndrome; RCT: randomized controlled trials;
Pauli JM, Raja-Khan N, Wu X, Legro RS. Current perspectives of insulin
T2DM: type two diabetes mellitus.
resistance and polycystic ovary syndrome. Diabet Med. 2011;28(12):1445–54.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanismand implications for pathogenesis. Endocr Rev. 1997;18(6):774–800.
Competing interests
Rehme MF, Pontes AG, Goldberg TB, Corrente JE, Pontes A. Clinical
The authors declare that they have no competing interests.
manifestations, biochemical, ultrasonographic and metabolic of polycysticovary syndrome in adolescents. Rev Bras Ginecol Obstet. 2013;35(6):249–54.
Li L, Chen X, He Z, Zhao X, Huang L, Yang D. Clinical and metabolic features
Authors' contributions
of polycystic ovary syndrome among Chinese adolescents. J Pediatr Adolesc
RA conceptualized and designed the study, drafted and critically reviewed
the manuscript, and approved the final manuscript as submitted. IF
Bekx MT, Connor EC, Allen DB. Characteristics of adolescents presenting to a
conceptualized and designed the study, critically reviewed the manuscript,
multidisciplinary clinic for polycystic ovarian syndrome. J Pediatr Adolesc
and approved the final manuscript as submitted. BD designed the study,
drafted and critically reviewed the manuscript, and approved the final
Gooding HC, Milliren C, St Paul M, Mansfield MJ, DiVasta A. Diagnosing
manuscript as submitted. EB conceptualized the study, designed the study,
dysglycemia in adolescents with polycystic ovary syndrome. J Adolesc Health.
critically reviewed the manuscript, and approved the final manuscript as
submitted. LT designed the study, critically reviewed the manuscript, and
Flannery CA, Rackow B, Cong X, Duran E, Selen DJ, Burgert TS. Polycystic
approved the final manuscript as submitted. BN drafted and critically
ovary syndrome in adolescence: impaired glucose tolerance occurs across
reviewed the manuscript and approved the final manuscript as submitted.
the spectrum of BMI. Pediatr Diabetes. 2013;14(1):42–9.
All authors read and approved the final manuscript.
Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A.
Screening for abnormal glucose tolerance in adolescents with polycysticovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1017–23.
Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, et al. Diabetes
We would like to thank Mrs. Neera Bhatnagar, McMaster Health Sciences
and cardiovascular events in women with polycystic ovary syndrome: a
Librarian for her invaluable assistance in refining the search strategy.
20-year retrospective cohort study. Clin Endocrinol. 2013;78(6):926–34.
Al Khalifah et al. Systematic Reviews (2015) 4:125
Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a
Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE working Group
woman's long-term health using data linkage. J Clin Endocrinol Metab.
approach for rating the quality of treatment effect estimates from network
meta-analysis. BMJ. 2014;349:g5630.
Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS.
Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising
A meta-analysis of pregnancy outcomes in women with polycystic ovary
drugs versus the combined oral contraceptive pill for hirsutism, acne and
syndrome. Hum Reprod Update. 2006;12(6):673–83.
risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic
Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast
ovary syndrome. Cochrane Database Syst Rev 2007;1:CD005552.
cancer in women with polycystic ovary syndrome: a systematic review andmeta-analysis. Hum Reprod Update. 2014;20(5):748–58.
Shen CC, Yang AC, Hung JH, Hu LY, Tsai SJ. A nationwide population-basedretrospective cohort study of the risk of uterine, ovarian and breast cancerin women with polycystic ovary syndrome. Oncologist. 2015;20(1):45–9.
Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer amongwomen with polycystic ovary syndrome: a Danish cohort study. GynecolOncol. 2015;136(1):99–103.
Jones GL, Hall JM, Lashen HL, Balen AH, Ledger WL. Health-related qualityof life among adolescents with polycystic ovary syndrome. J Obstet GynecolNeonatal Nurs. 2011;40(5):577–88.
Crete J, Adamshick P. Managing polycystic ovary syndrome: what ourpatients are telling us. J Holist Nurs. 2011;29(4):256–66.
Nasiri Amiri F, Ramezani Tehrani F, Simbar M, Montazeri A, MohammadpourThamtan RA. The experience of women affected by polycystic ovary syndrome: aqualitative study from Iran. Int J Endocrinol Metab. 2014;12(2):e13612.
Conway G, Dewailly D, Diamanti-Kandarakis E, et al. European survey ofdiagnosis and management of the polycystic ovary syndrome: results of theESE PCOS Special Interest Group's Questionnaire. Eur J Endocrinol.
2014;171(4):489–98.
Auble B, Elder D, Gross A, Hillman JB. Differences in the management ofadolescents with polycystic ovary syndrome across pediatric specialties.
J Pediatr Adolesc Gynecol. 2013;26(4):234–8. Date of Publication: August2013.; 2013.
AlKhalifah R, Florez ID, Thabane L, Dennis B, Bassilious E. Metformin versusoral contraceptive pills for the management of polycystic ovarian syndromein adolescents: systematic review and meta-analysis. PROSPEROInternational prospective register of systematic reviews2015.
Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and networkmeta-analysis. BMJ (Clinical research ed). 2013;346:f2914.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al.
Preferred reporting items for systematic review and meta-analysis protocols(PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;349:g7647.
Sacks D; Canadian Paediatric Society. Age limits and adolescents. Paediatrics& Child Health. Nov 2003;8(9):577.
Search Filters for MEDLINE in Ovid Syntax and the PubMed translation.
2015.
Collaboration TC. The Cochrane handbook for systematic reviews ofinterventions version 5.1.0. 2011.
Guyatt G, Busse J. Commentary on tool to assess risk o f bias in cohortstudies.
Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis ofinconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267–70.
Gelman A, Rubin DB. Inference from iterative simulation using multiplesequences. Stat Sci. 1992;7:457–72.
Lumley T. Network meta-analysis for indirect treatment comparisons. StatMed. 2002;21(16):2313–24.
Higgins J. Identifying and addressing inconsistency in network meta-analysis.
Paper presented at: Cochrane comparing multiple interventions methodsgroup Oxford training event 2013.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed
Submit your next manuscript to BioMed Central
treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44.
and take full advantage of:
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numericalsummaries for presenting results from multiple-treatment meta-analysis: an
• Convenient online submission
overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71.
Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment
• Thorough peer review
comparison meta-analysis of complex interventions: psychological
• No space constraints or color figure charges
interventions in coronary heart disease. Am J Epidemiol. 2009;169(9):1158–65.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE
• Immediate publication on acceptance
guidelines: 1. Introduction-GRADE evidence profiles and summary of
• Inclusion in PubMed, CAS, Scopus and Google Scholar
findings tables. J Clin Epidemiol. 2011;64(4):383–94.
• Research which is freely available for redistribution
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al.
GRADE guidelines: 8. Rating the quality of evidence—indirectness. J ClinEpidemiol. 2011;64(12):1303–10.
Submit your manuscript at www.biomedcentral.com/submit
Source: http://old.systematicreviewsjournal.com/content/pdf/s13643-015-0105-4.pdf
Prevention of Cardiovascular Disease See separate articlesand The revised Joint British Societies' (JBS 3) guidelines on prevention of cardiovascular disease (CVD) in clinicalpractice recommend that CVD prevention should focus equally on the following three groups of patients who areat high risk of CVD: [1] Apparently healthy individuals with 20% or greater risk over 10 years of developing symptomaticatherosclerotic disease.People with diabetes mellitus (type 1 or 2).People with established atherosclerotic CVD.
CASE REPORT Intracerebral haemorrhage and We report on a 65-year-old woman who presented with acute right-sided weakness because of an intracerebral (thalamic) haemorrhage.As a Qigong enthusiast with a long-standing history of hypertension,she developed a stroke syndrome soon after practising Qigong onemorning. Following neurological recovery, the patient exhibited erraticblood pressure responses while practising Qigong, despite the factthat resting blood pressure was normal. The haemodynamic responsesto exercise are discussed and a review of the therapeutic implicationsof practising Qigong is presented.


