Doi:10.1016/j.fct.2007.05.017

Food and Chemical Toxicology 45 (2007) 2219–2227
Antioxidant activities of the flaxseed lignan secoisolariciresinol
diglucoside, its aglycone secoisolariciresinol and the mammalian
lignans enterodiol and enterolactone in vitro
Chun Hu a, Yvonne V. Yuan b,*, David D. Kitts a
a Food, Nutrition and Health, University of British Columbia, 2205 East Mall, Vancouver, BC, Canada V6T 1Z4
b School of Nutrition, Ryerson University, 350 Victoria Street, Toronto, ON, Canada M5B 2K3
Received 11 October 2006; accepted 16 May 2007
The flaxseed lignan secoisolariciresinol diglucoside (SDG) and mammalian lignans enterodiol (ED) and enterolactone (EL) were
previously shown to be effective antioxidants against DNA damage and lipid peroxidation. Others reported inhibition of activated cellchemiluminescence by supra-physiological concentrations of secoisolariciresinol (SECO), ED and EL. Thus, we evaluated the antioxi-dant efficacy of potential physiological concentrations of SDG, SECO, ED and EL against 1,1-diphenyl-2-picrylhydrazyl (DPPH�),and 2,20-azo-bis(2-amidinopropane) dihydrochloride (AAPH)-initiated peroxyl radical plasmid DNA damage and phosphatidylcholineliposome lipid peroxidation. SDG and SECO were effective (p < 0.01) antioxidants against DPPH� at 25–200 lM; whereas, ED and ELwere inactive. Efficacy of lignans and controls against AAPH peroxyl radical-induced DNA damage was: SDG > SECO = 17a-estra-diol > ED = EL > genistein > daidzein. Lignan efficacy against AAPH-induced liposome lipid peroxidation was: SDG > SECO =ED = EL. Plant lignan antioxidant activity was attributed to the 3-methoxy-4-hydroxyl substituents of SDG and SECO, versus the metamono-phenol structures of ED and EL. Benzylic hydrogen abstraction and potential resonance stabilization of phenoxyl radicals in anaqueous environment likely contributed to the antioxidant activity of the mammalian lignans. These represent likely extra- and intracel-lular antioxidant activities of flax-derived lignans at concentrations potentially achievable in vivo.
� 2007 Elsevier Ltd. All rights reserved.
Keywords: Antioxidant activity; Flaxseed lignan; Secoisolariciresinol diglucoside (SDG); Secoisolariciresinol (SECO); Enterodiol (ED); Enterolactone(EL)
ingredients and adjuncts to a healthful diet continues togrow, as a result of the increasing body of evidence over
Interest in the use of whole flaxseed (Linum usitatissimum)
the past 20 years, investigating the protective effects of flax-
and its derivatives (ground flax, flax oil, defatted flax, flax
seed against a variety of chronic diseases and risk factors
fibre and lignan extracts) as functional food or nutraceutical
including breast and colon carcinogenesis, atherosclerosis,insulin dependent diabetes mellitus (IDDM) and hyperlipo-proteinemias As a functional food, flax is noted to be an excellent
Abbreviations: AAPH, 2,20-azo-bis(2-amidinopropane) dihydrochlo-
ride; CD, conjugated dienes; DPPH�, 1,1-diphenyl-2-picrylhydrazyl; ED,
source of insoluble and particularly, soluble dietary fibre due
enterodiol; EL, enterolactone; �OH, hydroxyl radical; PBS, phosphate
to its polysaccharide gum and mucilage content associated
buffered saline; ROS, reactive oxygen species; SDG, secoisolariciresinol
with the seed hull, highly polyunsaturated oil rich in a-
diglucoside; SECO, secoisolariciresinol.
linolenic acid, and as the richest food source of the plant
Corresponding author. Tel.: +1 416 979 5000x6827; fax: +1 416 979
lignan secoisolariciresinol diglucoside (SDG, MW 686.7;
E-mail address: (Y.V. Yuan).
). This plant lignan is a precursor of
0278-6915/$ - see front matter � 2007 Elsevier Ltd. All rights reserved.
doi:10.1016/j.fct.2007.05.017
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
the mammalian lignans, enterodiol [ED; 2,3-bis[(3-hydroxy-
between 10 and 1000 lM (i.e. concentrations achievable
phenyl)methyl]-1,4-Butanediol; MW 302] and enterolactone
in the colonic lumen), were equally effective against non-
site-specific (in the presence of EDTA) Fenton-reaction-
Furanone; MW 298] via the activity of colonic facultative
mediated DNA nicking; however, the mammalian lignans
aerobes (Clostridia sp.). During this conversion, SDG first
exhibited greater effectiveness against �OH radical scaveng-
undergoes hydrolysis to yield the aglycone plant lignan seco-
ing and site-specific (in the absence of EDTA) Fenton-reac-
tion-mediated DNA nicking compared to the plant lignan
methoxy phenyl)methyl]-1,4-Butanediol; MW 362], which
SDG (The relevance of the antioxidant
is then dehydroxylated and demethylated to yield ED; ED
activity of SDG is limited however, to the colonic contents,
can then be oxidized to EL (). The mammalian lignans
as the diglucoside is not absorbed into the enterohepatic
differ from the plant precursors due to the presence of pheno-
circulation as are the aglycone and mammalian lignan
lic hydroxyl group moieties only in the meta position on the
metabolites ). In studies with
aromatic rings, compared to the 3-methoxy-4-hydroxyl sub-
activated polymorphonuclear leukocytes,
stituents on the A and B rings of the parent molecules SDG
reported that millimolar concentrations of SECO, ED
and EL (1–33 mM) inhibited reactive oxygen species
The flaxseed lignan and its mammalian metabolites have
(ROS)-initiated chemiluminescence following an in vitro
been reported to exert protective effects against diet-related
incubation of venous blood. However, these concentrations
chronic diseases through a variety of mechanisms including
of lignans are far in excess of those which have been
phytoestrogenic and antioxidant effects from in vitro and
observed in plasma from either rodent model or clinical
studies (rats dosed
with 1.5 mg SDG/day were estimated to have plasma lig-
ter effect is of particular interest as many chronic disease
nan levels of approximately 1 lM; adult female volunteers
states are characterized by an oxidative stress component
fed 25 g flaxseed exhibited plasma lignan concentrations
in the disease aetiology such as the initiation of carcinogen-
ranging from 17 to 519 nM; and healthy postmenopausal
esis (damage to pancreatic islet
women fed a lignan complex containing 500 mg/day
cells in IDDM ); and LDL lipid peroxida-
SDG exhibited plasma EL levels of 385 nM. Moreover,
tion in atherosclerosis (). Previously,
the potential concentration of SDG within the colon of
we reported that SDG, ED and EL, at concentrations
subjects consuming 50 g flaxseed, would be approximately
(GI tract)
(GI tract)
(GI tract)
Fig. 1. Colonic microflora metabolism of the flaxseed plant lignan diglucoside SDG, into the aglycone SECO, and mammalian lignan metabolites EDand EL.
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
diphenyl-2-picrylhydrazyl (DPPH�), 17a-estradiol, pBR322 DNA (from
Escherichia coli, strain RR1), Chelex-100 ion chelating resin, L-a-phos-
phatidylcholine (from soybean) and other reagents were purchased from
Sigma–Aldrich Chemical Co. (St Louis, MO).
Phosphate buffers used throughout this study were passed through a
Chelex-100 ion chelating resin column to minimize the occurrence oftransition metal ions. Water (H2O) used in all assays was purified using an
E-pure Barnstead system (VWR Canlab, Mississauga, ON). For all assays,SDG was solubilized in 0.1% (v/v) ethanol; SECO, ED and EL wereprepared in 100% ethanol and diluted with H2O as necessary.
2.2. DPPH� stable free radical scavenging assay
The DPPH� stable free radical scavenging assay was performed as
previously described by with modifications. Briefly,
lignans and lignan metabolites were incubated with 0.1 mM DPPH� in100% ethanol in a final volume of 1.0 mL. Sample absorbance readings at
Fig. 2. Structures of 17a-estradiol, daidzein, genistein and Trolox used as
519 nm were recorded after 30 min incubation at room temperature.
Inhibition of the DPPH� stable free radical was calculated as follows:
Abscontrol � Abssample
Abscontrol � Absblank
666 lM (). Thus, there is currently a pau-
city of data evaluating the antioxidant activity of the flax-
control = absorbance of 0.1 mM DPPH� alone in ethanol,
Abssample = absorbance of 0.1 mM DPPH� + lignan or lignan metabolite
seed lignan SDG, its aglycone SECO and the mammalian
in ethanol and Absblank = absorbance of ethanol solvent control in
lignan metabolites ED and EL at potentially physiologi-
absence of DPPH� or lignans.
cally relevant concentrations.
The objective of the present study was to evaluate the
2.3. DNA scission induced by AAPH peroxyl radicals
antioxidant efficacy of the flaxseed lignan SDG, its agly-cone SECO, and the mammalian lignans ED and EL using
Supercoiled plasmid pBR322 DNA was used as the substrate for
studying AAPH peroxyl radical-induced DNA nicking at 37 �C as previ-
a variety of in vitro methodologies (e.g. DPPH� stable free
ously described by Briefly, pBR322 DNA was sol-
radical scavenging; AAPH peroxyl radical-induced DNA
ubilized in 10 mM phosphate buffered saline (PBS; 150 mM NaCl, pH 7.4)
nicking; and peroxyl radical-induced liposome oxidation)
at a concentration of 17 ng/mL. Plasmid DNA was preincubated with
at physiologically relevant lignan concentrations. We also
lignans and control compounds prior to the addition of the peroxyl radical
evaluated the efficacy of the aglycone soy isoflavones,
oxidizing agent. Following the addition of AAPH at a concentration of5 mM, samples were incubated at 37 �C for 2 h in the dark and then
daidzein and genistein (
subjected to DNA horizontal gel electrophoresis (0.7% w/v agarose with
) as well as 17a-estradiol
0.5 lg/mL ethidium bromide) using a 40 mM Tris-acetate, 2 mM EDTA,
as antioxidant controls in the aqueous DNA nicking
pH 8.5 buffer (E-C Apparatus Corp., St. Petersburg, FL) at 4 v/cm for 1 h
assay with AAPH due to their structural similarities to the
Intact and nicked pBR322 plasmid DNA were
flaxseed mammalian lignans ). This latter in vitro test
visualized under ultraviolet light and the relative band intensities deter-mined by densitometry. The inhibition of DNA nicking was calculated as
can be viewed as a closer approximation of intracellular
conditions versus the 100% ethanol solvent medium of
the DPPH� assay. Thus, the present study focuses more
so on the antioxidant activity of the flaxseed lignans and
metabolites against AAPH-induced oxidation, due to the
where Asample = amount of supercoiled plasmid DNA in the presence
greater number of studies, particularly in the recent litera-
of AAPH + lignan or control antioxidant; and Acontrol = amount of
ture (), evaluating the efficacy
supercoiled DNA in the absence of AAPH and lignan or control
of these plant and mammalian lignans in protecting against
AAPH peroxyl radical-induced damage, and fewer studieswith DPPH� (
2.4. Liposome oxidation induced by AAPH peroxyl radicals
2. Materials and methods
(0.1 mg/mL) were formulated according to in 10 mMPBS, pH 7.4 with sonication. Lignans, lignan metabolites (6 and 60 lM
concentrations) or Trolox (16 lM) were added to emulsions prior to theaddition of the AAPH peroxyl radical oxidizing agent. Liposomal oxi-
Isolation of secoisolariciresinol diglucoside (SDG) and chemical syn-
dation was initiated with 2 mM AAPH and incubation at 37 �C. Forma-
thesis of enterodiol (ED) and enterolactone (EL) have been previously
tion of conjugated dienes (CDs) was monitored continuously at 234 nm
reported elsewhere ). Genistein and daidzein were
using an Uni-Cam UV2 spectrophotometer equipped with a multi-cell
purchased from K & K Laboratories Inc. (Plainsville, NY). 2,20-azo-bis(2-
holder and temperature control ). Quantitation of
amidinopropane) dihydrochloride (AAPH) was purchased from Wako
molar extinction coefficient
Chemicals USA Inc. (Richmond, VA). Secoisolariciresinol (SECO), 6-
29,500 M�1 cm�1 as described by .
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
against AAPH-induced DNA damage at 10 (p < 0.01), 50and 100 lM (p < 0.001) concentrations; while 17a-estradiol
All data are expressed as means ± SD of triplicate experiments. Stu-
and the aglycone SECO were less effective (p < 0.05) at
dent's t-test for independent samples was used to test for differences at a
10 lM, but exhibited strong (p < 0.001) protection against
significance level of p 6 0.05 where appropriate (SPSS 10.0 for Windows;SPSS Inc., Chicago, IL). Linear regression analyses of the propagation
AAPH-induced DNA damage at 50 and 100 lM concen-
phases of liposome lipid oxidation curves were performed to determine the
trations. ED and EL both exhibited little effect at 10 lM,
slopes of the control, Trolox control antioxidant and lignan samples
but were strong (p < 0.001) in protecting against AAPH-
induced DNA damage at 50 and 100 lM concentrations(In comparison, the phytoestrogen control anti-
oxidant genistein exhibited little effect against AAPH-induced DNA damage at 10 lM compared to weak
The flaxseed lignan SDG and its aglycone SECO both
(p < 0.05) and strong effects (p < 0.001) at 50 and 100 lM
exhibited strong (p < 0.01) free radical scavenging activity
concentrations, respectively; whereas daidzein exhibited lit-
against the stable free radical DPPH�, whereas the mamma-
tle protective effect against AAPH-induced DNA damage
lian lignan metabolites ED and EL were ineffective in scav-
at 10 and 50 lM, and only a weak protective effect
enging this stable free radical (). The DPPH� stable
(p < 0.05) at the highest concentration of 100 lM tested
free radical scavenging activities of SDG and SECO both
(). The protective effects of SDG, SECO, ED and
exhibited concentration dependence across the range
EL as well as the antioxidant controls, 17a-estradiol, daidz-
tested; in contrast, ED and EL were ineffective in scaveng-
ein and genistein all exhibited concentration dependence
ing DPPH� even at the highest concentrations tested.
across the concentrations studied herein.
is a representative densitometry image scan illus-
In the phosphatidylcholine liposome model system, the
trating the efficacy of the flaxseed lignan SDG, its aglycone
flaxseed lignan SDG exhibited the strongest protection
SECO, mammalian lignan metabolites ED and EL as well
against AAPH-induced lipid oxidation at both 6 and
as control antioxidant compounds (17a-estradiol, daidzein
60 lM concentrations, providing similar inhibition against
and genistein) in protecting supercoiled plasmid pBR322
CD formation as the control antioxidant Trolox (
DNA against AAPH peroxyl radical-induced damage.
In contrast, the aglycone SECO, and mammalian lignan
The flaxseed lignan SDG exhibited the strongest protection
metabolites ED and EL provided little protection againstAAPH-induced lipid oxidation at 6 lM with CD produc-tion similar to the positive control sample d), but
strong protection at 60 lM concentrations with CD forma-
DPPH� stable free radical scavenging activity of the flaxseed lignan SDG,
tion similar to the control antioxidant Trolox. Linear
its aglycone SECO and the mammalian lignan metabolites ED and EL (%
regression analyses of the slopes of the propagation phases
of the liposome emulsions revealed that the slopes of the
control emulsion and those of SECO, ED and EL at the
6 lM concentration were very similar, ranging between
1.94 and 2.56 lmol conjugated dienes/g phosphatidylcho-
line/min; whereas the slopes of SDG, SECO, ED and EL
at 60 lM and the Trolox control at 16 lM ranged between
Indicates a significant difference (p < 0.01) between the sample versus
the control, containing DPPH� alone, by Student's t-test.
a Values represent means ± SD, n = 3.
Table 2Protective effect of the flaxseed lignan SDG, its aglycone SECO, themammalian lignan metabolites ED and EL and control antioxidantcompounds against AAPH-induced plasmid pBR322 DNA strand scission(% Inhibit
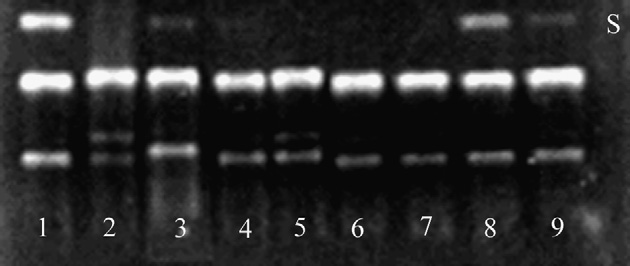
Fig. 3. Effect of the flaxseed lignan SDG, its aglycone SECO, themammalian lignan metabolites ED and EL as well as control antioxidants
* Indicates a significant difference (p < 0.05) between the sample versus
on AAPH peroxyl radical-induced pBR322 plasmid DNA scission. Lane
the plasmid pBR322 DNA control.
1 = DNA control in the absence of AAPH; lane 2 = DNA + AAPH; lanes
** Indicates a (p < 0.01) difference between the sample versus the control.
3–9 = DNA + AAPH + antioxidant: 17a-estradiol (lane 3), daidzein (lane
*** Indicates a (p < 0.001) difference between the sample and the control
4), genistein (lane 5), ED (lane 6), EL (lane 7), SDG (lane 8) and SECO
by Student's t-test.
(lane 9). S = supercoiled DNA.
a Values represent means ± SD, n = 3.
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
Time (min)
Time (min)
Fig. 4. Effects of the flaxseed lignan SDG, its aglycone SECO and the mammalian lignan metabolites ED and EL on L-a-phosphatidylcholine liposomeoxidation induced by AAPH peroxyl radical. Panel a = SDG; b = SECO; c = ED; d = EL. d = control liposome emulsion + AAPH in absence ofantioxidant; 4 = liposome emulsion + AAPH + 6 lM antioxidant; m = liposome emulsion + AAPH + 60 lM antioxidant; s = liposome emul-sion + 16 lM Trolox positive control. Data represent the mean value of triplicate experiments.
However, the antioxidant activity of
Linear regression analyses of the slopes of the propagation phases of the
SDG is likely only relevant to processed food products
control liposome emulsion and emulsions incubated with flaxseed lignan
containing SDG isolates, or within the intestinal tract, par-
SDG, its aglycone SECO, the mammalian lignan metabolites ED and EL
ticularly the colon, since the diglucoside is not absorbed
and Trolox as the control antioxidant
intact; rather, the aglycone SECO and the mammalian lign-
Regression equation
ans ED and EL occur in the portal circulation, plasma and
y = 2.46x � 37.58
urine as glucuronides and sulfates following conjugation by
y = 0.541x + 11.87
colon epithelial cells or within the liver (
y = 0.618x + 10.96
y = 0.733x + 12.46
). Studies with HT29 human colon
y = 2.56x � 59.23
epithelial cells in vitro indicate that measurable amounts of
y = 0.546x + 12.60
intracellular free ED can be detected up to 4 h incubation,
y = 1.94x � 32.76
whereas EL appears to be rapidly conjugated by HT29 cells
y = 0.828x + 1.309
). The free radical scavenging activity of
y = 2.08x � 42.54
y = 0.768x � 0.8499
plant and mammalian lignans can therefore potentiallyconfer protection against oxidative stress or lipid peroxi-dation intra- or extracellularly. The present study is the
0.541 and 0.828, and that of SDG at 6 lM was 0.618
first to report the efficacy of the flaxseed lignan SDG, its
lmol conjugated dienes/g phosphatidylcholine/min
aglycone SECO, and mammalian lignan metabolites EDand EL in protecting supercoiled plasmid DNA as well as
L-a-phosphatidylcholine liposomes against 2,20-azo-bis(2-amidinopropane) dihydrochloride (AAPH) peroxyl radi-
Previously, we reported that SDG, ED and EL were
cal-induced damage. We further demonstrated striking
effective inhibitors against lipid peroxidation of a linoleic
differences between the efficacy of the plant lignans, SDG
acid emulsion model system at 10 and 100 lM concentra-
and SECO, versus the mammalian lignans, ED and EL,
tions, albeit SDG and EL were more effective than ED
in quenching the 1,1-diphenyl-2-picrylhydrazyl stable free
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
radical (DPPH�). These differences in antioxidant activity
oxy group) lignans such as SDG and SECO, as well as
between lignans likely reflect differences in not only the
the reference molecule 2-methoxy-p-cresol, were much
degree of hydroxylation, but also the position of hydroxyl
more powerful antioxidants than ED, EL or the reference
groups and neighbouring substituents such as methoxy
molecule m-cresol. These workers concluded that the meth-
oxy group adjacent to the para hydroxyl on the A and B
rings of SDG and SECO was key to the strong FRAP anti-
example, it is known that the antioxidant activity of poly-
oxidant activity for the plant lignans compared to the
phenols is greater than that of mono-phenols, particularly
mammalian lignans Our
with hydroxyl groups in ortho- or para-arrangements
results indicate that the 3-methoxy-4-hydroxy substituted
rings of SDG and SECO have greater DPPH� quenching
over, methoxy substituents will increase the antioxidant
than the 3-hydroxy substituted rings of ED and EL, which
activity of a mono-phenol (
may be associated with the 3-methoxy substituents on the
former molecules. In order to confirm the effect of the 3-
hand, the effects of glycosylation on the antioxidant activ-
methoxy-substitution on the antioxidant activity of plant
ity of parent molecules is often much less clear with con-
lignans however, it would be valuable to perform future
flicting results reported depending upon the test system
experiments with 4-hydroxy substituted compounds such
used, i.e. DPPH� versus b-carotene bleaching, etc. (
as the lignan conocarpol derived from Conocarpus erectus
This latter point highlights the
(Button mangrove; ).
importance of the polarity of the solvent phase between dif-
Azo compounds (diazenes) such as AAPH, are useful
ferent antioxidant test systems; e.g. ethanol used to solubi-
free radical initiators in the study of antioxidant activity
lize the DPPH� stable free radical compared to the PBS
in vitro, due to the predictable thermal decomposition of
used in the liposome model system herein.
these compounds, yielding N2 and two carbon radicals, R�
In the present study, both SDG and the aglycone SECO
(). These radicals may then potentially react with
exhibited strong DPPH� scavenging activity, whereas the
each other to yield stable non-radical termination products,
mammalian lignans ED and EL were relatively inactive
or react with molecular O2 to yield peroxyl radicals, ROO�,
against this stable free radical. The DPPH� scavenging
which can then participate in nicking plasmid DNA or the
activity of SDG and SECO was highly efficient, ranging
peroxidation of phospholipid liposome model systems. As a
between 78% and 83% at 100 lM and increasing only
hydrophilic radical initiator, AAPH would readily generate
between 8.3% and 12.6% points at 200 lM of these plant
peroxyl radicals in the PBS used to solubilize the plasmid
lignans. The loss of linearity in the DPPH� dose response
DNA in the present study. Since AAPH has a T1/2 of
curve at the highest concentrations of SDG and SECO
approx. 175 h (), the rate of generation of radicals
was a reflection of not only the free radical scavenging effi-
(Ri) would have been constant over the 2 h incubation per-
ciency of these plant lignans, but also the concentrations of
iod with the plasmid DNA. The rate of AAPH radical gen-
the reactants used herein. For example, at 100 lM SDG or
eration at 37 �C at a neutral pH can be calculated as
SECO, the reaction consisted of a 1:1 ratio of stable free
follows: Ri (mol/l/s) = 1.36 · 10�6 [AAPH] ();
radical:antioxidant (the assay contained 100 lM DPPH�);
therefore, the total amount of AAPH radicals formed in
this ratio changed to 1:2 at 200 lM SDG or SECO. At
the plasmid DNA preparations over the 2 h incubation per-
200 lM SDG or SECO, there was an excess of antioxidant
iod was 49 lM in the present study. The ratio of AAPH per-
available to scavenge DPPH� radicals and therefore, a lev-
oxyl radicals:antioxidant molecules ranged from 1:0.20 at
elling off in the dose–response observed herein.
10 lM, 1:1 at 50 lM and 1:2 at 100 lM concentrations of
It is noteworthy that SDG and SECO are both charac-
lignans and controls herein. Thus, the strong protective
terized by 3-methoxy-4-hydroxyl substituents on the A and
effects of plant and mammalian lignans against AAPH per-
B rings, whereas the mammalian lignans possess only a
oxyl-induced plasmid DNA damage at 100 lM concentra-
single hydroxyl group in the meta position on each of the
tions may be related to the excess of available antioxidant
A and B rings ). Moreover,
equivalents compared to peroxyl radicals. Interestingly,
reported that the butanediol structure of SECO enabled a
SDG had the greatest efficacy against peroxyl radical plas-
greater antioxidant activity compared to the butyrolactone
mid DNA damage even at the lowest concentration used,
structure characteristic of the related plant lignan mataires-
10 lM. The relative efficacies of the plant and mammalian
inol. These same workers demonstrated that one molecule
lignans and reference antioxidant compounds to inhibit
of SECO is capable of quenching up to 4.5 molecules of
AAPH peroxyl radical-induced plasmid DNA damage in
DPPH�; whereas the inactivity of EL, and thereby ED,
the present study were as follows: SDG > SECO = 17a-
against DPPH�, could be attributed to the lack of reso-
estradiol > ED = EL > genistein > daidzein. Thus, in the
nance stabilization of the phenoxyl radical generated in
aqueous plasmid DNA model system, SDG exhibited
the meta position of the mammalian lignans. Similarly,
greater protection against peroxyl radical-induced damage
using a ferric ion reducing antioxidant power assay
compared to the aglycone SECO, mammalian lignans and
(FRAP), demonstrated that
controls. The greater efficacy of SDG to protect the plasmid
guaiacyl (para hydroxyl group with a neighbouring meth-
DNA was likely associated with the greater water solubility
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
of SDG versus SECO, ED and EL, associated with its two
mammalian lignans against peroxyl radical-induced DNA
glucose moieties, and thereby ease of interaction with the
damage in the present study. Moreover,
hydrophilic AAPH-derived peroxyl radicals. Moreover,
demonstrated weak reducing activity, an absence
the neutral pH of the PBS used in the plasmid DNA model
of pro-oxidant activity and weak inhibition of lipid perox-
system (pH 7.4) would not have likely facilitated the hydro-
idation in rat brain homogenate to confirm the antioxidant
lysis of the glucose moieties from SDG. Other workers eval-
efficacy of estradiol in vitro.
uating the effect of glycosylation on the antioxidant activity
Interestingly, the mammalian lignans exhibited strong
of anthocyanidins demonstrated both enhancing and nega-
antioxidant activity in the PBS medium of the peroxyl
tive effects for the same compounds depending on the model
radical-induced plasmid DNA damage model, at the
system evaluated, attributing the variability in results to sol-
50 and 100 lM concentrations of ED and EL, in contrast
ubility and stability effects, and steric hindrance, respec-
to the lack of effects with DPPH� above. One major vari-
tively Thus, the effect of
able between the DPPH� and AAPH antioxidant assays
glycosylation on the antioxidant activity of polyphenols,
in the present study was the solvent medium used: 100%
such as the plant lignans, is likely dependent in part, on
ethanol for DPPH� versus PBS for AAPH. It is conceivable
the solubility characteristics of the molecule in the model
that the polarity of the solvent medium may have contrib-
system under study.
uted to differences in the antioxidant efficacy of the mam-
SDG exhibited greater efficacy for protecting plasmid
malian lignans through intermolecular interactions. There
DNA against AAPH peroxyl radicals than its aglycone
is also the possibility for the abstraction of a benzylic
SECO at all three peroxyl radical:antioxidant ratios tested
hydrogen from the mammalian lignans and intramolecular
herein, albeit the difference between the two at 50 and
regeneration of the mono-phenol structure via a primary
100 lM was only 14.9% and 6.9% points, respectively.
hydroxyl group from the butanediol structure reducing
These results are in contrast to recent work by
an oxidized benzylic position, particularly with ED (
reporting that SECO was slightly more efficient
For example, in studies with SECO and
at inhibiting liposomal peroxidation than SDG: the stoichi-
AAPH, proposed a mechanism
ometric values for SECO and SDG against AAPH peroxyl
whereby a SECO alkoxy radical could be formed via intra-
radical damage were 1.5 and 1.2, respectively, in a liposo-
molecular or intermolecular hydrogen atom transfer from
the aliphatic butanediol to a phenoxyl radical. Thus, a sim-
200 lM AAPH. More recently, in model studies with
ilar phenomenon may be possible with the mammalian
AAPH incubated with SDG or SECO, these same workers
lignans at the higher concentrations used herein in an aque-
reported that one of the reaction products with SDG was a
ous environment.
labile C5–C5 dimer which decomposes to yield an amidino
The aglycone isoflavone antioxidants, genistein and
propane substitution product and a recycled molecule of
daidzein were the least effective against AAPH peroxyl
SDG (Interestingly, the reaction
radical-induced plasmid DNA damage; albeit, genistein
of AAPH with SECO also yielded a C5–C5 dimer, but in
exhibited greater efficacy than daidzein in the present study.
contrast to SDG, the SECO dimer was stable and did
Similarly, reported that genistein
not decompose to recycle a molecule of SECO back into
exhibited greater antioxidant activity than daidzein in both
the pool of reactants. The ability for AAPH peroxyl radi-
a ferric ion reducing ability of plasma model system as well
cal-induced SDG dimers to decompose and recycle SDG
as in a Trolox equivalent antioxidant capacity (TEAC)
antioxidant equivalents back into the reaction likely played
assay. The greater efficacy of genistein over daidzein can
a role in the greater efficacy of SDG versus SECO against
be attributed to the diphenolic structure of the A ring of
peroxyl radical-induced plasmid DNA damage herein, par-
the former, compared to the mono-phenol A ring of the lat-
ticularly at 10 lM, since the SDG dimers were observed to
ter. Thus, the 5,7-diphenolic structure of the A ring of gen-
form early on in the oxidation reactions by
istein confers greater hydrogen atom donating potential,
As early products of the AAPH peroxyl rad-
phenoxyl radical resonance stabilization and thereby anti-
ical-SDG reaction, recycling of SDG from dimer products
oxidant activity than a mono-phenol such as with the A
would have been particularly important to the efficacy of
ring of daidzein (
SDG at the 10 lM concentration.
The similar protective effects of SECO and 17a-estradiol
The antioxidant activity of polyphenols such as the plant
against the peroxyl radical-induced plasmid DNA damage
and mammalian lignans studied herein can also been seen to
can be attributed to the guaiacyl lignan structure of the for-
play a role in the inhibition of lipid peroxidation attributed
mer, and resonance delocalization of the A ring phenoxyl
to chain-breaking behaviour during the propagation phase
radical to a position para to the A ring hydroxyl group
of autoxidation of the phosphatidylcholine liposomes in the
to stabilize the latter. Thus, while 17a-estradiol is charac-
present study. The generation of CD in the phosphatidyl-
terized by a mono-phenolic A ring, similar to the mono-
choline liposome model systems was inhibited to the great-
phenolic A and B rings of ED and EL, the steroid hormone
est extent by the glycosylated plant lignan SDG even at the
structure enabled resonance delocalization of the phenoxyl
lowest concentration tested (6 lM), while the aglycone
radical and thereby, greater antioxidant activity than the
SECO, and mammalian lignans ED and EL were effective
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
only at the higher concentration tested (60 lM). The effica-
nescence (PMNL-CL), slightly higher concentrations of
cies of both concentrations of SDG and the highest concen-
SECO, ED and EL of 1–2 mM reduced PMNL-CL by
trations of SECO, ED and EL were equivalent to that of the
between 25% and 75%, indicating strong efficacy of the
control antioxidant Trolox in inhibiting CD formation
aglycone plant lignan and mammalian lignans to scavenge
induced by AAPH peroxyl radicals. Increasing UV-absor-
ROS intracellularly It should be noted
bance due to CD formation within the phosphatidylcholine
that the antioxidant efficacy and bioactivity of glucuronide
liposome model system was associated with the resonance
and sulphate conjugates of SECO, ED and EL, the princi-
stabilization and shift in double bond position upon the for-
ple components present in the circulation and urine, is still
mation of isomeric hydroperoxides during autoxidation of
unknown. It is important to investigate the efficacy of these
the polyunsaturated fatty acid moieties of the phospholipid
metabolites since there is evidence that hypercholesterol-
molecules, such as C18:2,x-6 (59.8 ± 1.6% of total fatty
emic rabbits fed on 15 mg SDG/kg body wt/day exhibited
acids by GC) in the early stages of lipid peroxidation initi-
a reduction in aortic intimal surface atherosclerotic plaque
ated by AAPH peroxyl radicals. Using the same calculation
coverage from 80% down to approx. 20% in treated ani-
as above, the total amount of AAPH radicals formed in the
mals Moreover, the antioxidant efficacy
of the flaxseed and mammalian lignans may be a function
100 min incubation period was 16 lM. Thus, the ratios of
of oxidative stress in vivo, since an SDG-rich lignan com-
peroxyl radical:antioxidant in the liposome emulsion sys-
plex fed to healthy postmenopausal women did not affect
tem were 1:0.375 at 6 lM lignans, 1:1 for Trolox and
serum lipoprotein oxidation lag time, FRAP or TEAC
1:3.75 at 60 lM lignans. Similar to the results with the plas-
parameters despite an 8-fold increase in serum EL concen-
mid DNA model, the lowest concentration of SDG was as
tration, albeit these subjects were only mildly hypercholes-
efficacious as 60 lM SDG and Trolox in reducing the slope,
terolemic ().
and thereby rate of propagation during AAPH-induced
In conclusion, the flaxseed plant lignans SDG and
lipid peroxidation via similar mechanisms as discussed
SECO exhibited strong antioxidant and protective effects
above. The strong inhibitory effects of SECO, ED and EL
in quenching the DPPH� stable free radical and inhibiting
at 60 lM can be attributed to the excess antioxidant equiv-
alents compared to peroxyl radicals in the liposomal model
DNA and phosphatidylcholine liposomes at potentially
system. Moreover, the higher concentrations of SECO, ED
feasible physiological concentrations; whereas, the mam-
and EL may have increased the likelihood of intermolecular
malian lignans ED and EL were ineffective against the
associations and hydrogen atom transfer as discussed
former, but effective against the latter free radicals in the
above, and thereby, an enhanced antioxidant effect.
present study when present at peroxyl radical:antioxidant
The dose response in the efficacy of the plant lignans to
ratios P 1:1. Taken together, these results indicate that
quench DPPH� stable free radicals as well as the efficacy of
glycosylated and aglycone lignans are likely efficacious
the plant and mammalian lignans to inhibit AAPH peroxyl
against oxidative stress that may occur in the colonic milieu
radical-induced damage in the present study indicate that
as well as intracellularly in colonic epithelial cells exposed
SDG, the aglycone SECO and the mammalian lignans
to these compounds during the microflora metabolism of
ED and EL could potentially be protective against oxida-
plant lignans into the mammalian lignans. However, it will
tive stress within the colonic contents, where it has been
be important in future work to determine whether the glu-
estimated that a dose of 50 g flaxseed would provide a max-
curonide and sulphate conjugates of these compounds also
imal concentration of 666 lM SDG (
possess antioxidant activity, since it is these forms which
However, the lowest concentration of lignans tested in
are present in the portal circulation, plasma and urine
the present study was 6 lM, which is still in excess of the
and thereby most relevant in vivo.
peak intracellular free ED concentration of 30 nM detectedin HT29 colon epithelial cells incubated in the presence of
10 lM ED and EL (). On the other hand,reported that incubation of Chinese
The authors are grateful for the gifts of SDG, ED and
hamster V79 fibroblasts in the presence of up to 100 lM
EL from Dr. L.U. Thompson, Department of Nutritional
SECO, ED or EL resulted in only a slight inhibition of cell
Sciences, University of Toronto, Toronto, ON. This work
growth without cytotoxicity after 48 h. Depending on the
was supported by Discovery Grants from the Natural Sci-
rate of SDG metabolism by colonic microflora, colon epi-
ences and Engineering Research Council of Canada
thelial cells may be exposed to relatively high concentra-
(NSERC) to DDK and YVY, as well as AFMnet to DDK.
tions of SECO, ED and EL approaching 100 lM (thelimit of solubility in cell culture), and thereby, intracellular
levels of free mammalian lignans that are protective againstoxidative stress and DNA damage. For example, while
Ayres, D.C., Loike, J.D., 1990. A registry of the natural lignans. Lignans:
728 lM SDG resulted in only a small reduction of zymo-
Chemical, Biological and Clinical Properties. Cambridge University
san-stimulated polymorphonuclear leukocyte chemilumi-
Press, Cambridge, UK, p. 17.
C. Hu et al. / Food and Chemical Toxicology 45 (2007) 2219–2227
Bannwart, C., Adlercreutz, H., Wa¨ha¨la¨, K., Brunow, G., Hase, T., 1989.
and their metabolic precursors at various endpoints in vitro. Mut. Res.
Detection and identification of the plant lignans lariciresinol, isolaric-
416, 115–124.
iresinol and secoisolariciresinol in human urine. Clin. Chim. Acta 180,
Mitchell, J.H., Gardner, P.T., McPhail, D.B., Morrice, P.C., Collins,
A.R., Duthie, G.G., 1998. Antioxidant efficacy of phytoestrogens in
Daun, J.K., Barthet, V.J., Chornick, T.L., Duguid, S., 2003. Structure,
chemical and biological model systems. Arch. Biochem. Biophys. 360,
composition and variety development of flaxseed. In: Thompson, L.U.,
Cunnane, S.C. (Eds.), Flaxseed in Human Nutrition, second ed. AOCS
Niemeyer, H.B., Metzler, M., 2003. Differences in the antioxidant activity
Press, Champaign, IL, pp. 1–40.
of plant and mammalian lignans. J. Food Eng. 56, 255–256.
Eklund, P.C., La˚ngvik, O.K., Wa¨rna˚, J.P., Salmi, T.O., Willfo¨r, S.M.,
Niki, E., 1990. Free radical initiators as source of water- or lipid-soluble
Sjo¨holm, R.E., 2005. Chemical studies on antioxidant mechanisms and
peroxyl radicals. Methods Enzymol. 86, 100–108.
free radical scavenging properties of lignans. Org. Biomol. Chem. 3,
Pool-Zobel, B.L., Adlercreutz, H., Glei, M., Liegibel, U.M., Sittlingon, J.,
Rowland, I., Wa¨ha¨la¨, K., Rechkemmer, G., 2000. Isoflavonoids and
Fukumoto, L.R., Mazza, G., 2000. Assessing antioxidant and prooxidant
lignans have different potentials to modulate oxidative genetic damage
activities of phenolic compounds. J. Agric. Food Chem. 48, 3597–
in human colon cells. Carcinogenesis 21, 1247–1252.
Prasad, K., 1999. Reduction of serum cholesterol and hypercholesterol-
Hallund, J., Ravn-Haren, G., Bu¨gel, S., Tholstrup, T., Tetens, I., 2006. A
emic atherosclerosis in rabbits by secoisolariciresinol diglucoside
lignan complex isolated from flaxseed does not affect plasma lipid
isolated from flaxseed. Circulation 99, 1355–1362.
concentrations or antioxidant capacity in healthy postmenopausal
Prasad, K., 2000a. Oxidative stress as a mechanism of diabetes in diabetic
women. J. Nutr. 136, 112–116.
BB prone rats: effect of secoisolariciresinol diglucoside (SDG). Mol.
Hosseinian, F.S., Muir, A.D., Westcott, N.D., Krol, E.S., 2006. Antiox-
Cell. Biochem. 209, 89–96.
idant capacity of flaxseed lignans in two model systems. J. Am. Oil
Prasad, K., 2000b. Antioxidant activity of secoisolariciresinol diglucoside-
Chem. Soc. 83, 835–840.
derived metabolites, secoisolariciresinol, enterodiol, and enterolactone.
Hosseinian, F.S., Muir, A.D., Westcott, N.D., Krol, E.S., 2007. AAPH-
Int. J. Angiol. 9, 220–225.
mediated antioxidant reactions of secoisolariciresinol and SDG. Org.
Thibodeau, P.A., Kachadourian, R., Lemay, R., Bisson, M., Day, B.J.,
Biomol. Chem. 5, 644–654.
Paquette, B., 2002. In vitro pro- and antioxidant properties of
Hu, C., Kitts, D.D., 2000. Studies on the antioxidant activity of Echinacea
estrogens. J. Steroid Biochem. Mol. Biol. 81, 227–236.
root extract. J. Agric. Food Chem. 48, 1466–1472.
Thompson, L.U., 2003a. Flaxseed, lignans and cancer. In: Thompson,
Hu, C., Kitts, D.D., 2001. Evaluation of antioxidant activity of epigal-
L.U., Cunnane, S.C. (Eds.), Flaxseed in Human Nutrition, second ed.
locatechin gallate in biphasic model systems in vitro. Mol. Cell.
AOCS Press, Champaign, IL, pp. 194–222.
Biochem. 218, 147–155.
Thompson, L.U., 2003b. Analysis and bioavailability of lignans. In:
Jansen, G.H.E., Arts, I.C.W., Nielen, M.W.F., Mu¨ller, M., Hollman,
Thompson, L.U., Cunnane, S.C. (Eds.), Flaxseed in Human Nutrition,
P.C.H., Keijer, J., 2005. Uptake and metabolism of enterolactone and
second ed. AOCS Press, Champaign, IL, pp. 92–116.
enterodiol by human colon epithelial cells. Arch. Biochem. Biophys.
Thompson, L.U., Robb, P., Serraino, M., Cheung, F., 1991. Mammalian
435, 74–82.
lignan production from various foods. Nutr. Cancer 16, 43–52.
Kitts, D.D., Yuan, Y.V., Wijewickreme, A.N., Thompson, L.U., 1999.
White, P.J., Xing, Y., 1997. Antioxidants from cereals and legumes. In:
Antioxidant activity of the flaxseed lignan secoisolariciresinol digly-
Shahidi, F. (Ed.), . In: Natural Antioxidants: Chemistry, Health Effects
coside and its mammalian lignan metabolites enterodiol and entero-
and Applications. AOCS Press, Champaign, IL, pp. 25–36.
lactone. Mol. Cell. Biochem. 202, 91–100.
Yuan, Y.V., Rickard, S.E., Thompson, L.U., 1999. Short-term feeding of
Kulling, S.E., Jacobs, E., Pfeiffer, E., Metzler, M., 1998. Studies on the
flaxseed or its lignan has minor influence on in vivo hepatic antioxidant
genotoxicity of the mammalian lignans enterolactone and enterodiol
status in young rats. Nutr. Res. 19, 1233–1243.
Source: http://ryersonu.info/content/dam/nutritionandfood/contact/research/Hu,_Yuan_%26_Kitts_2007_FCT.pdf
C A S E R E P O R T Hellenic Journal of Atherosclerosis 1(1):65–67 Case report of rhabdomyolysis possibly associated to the interaction of ciprofloxacin with simvastatin N. Fountoulakis, L. Khafizova, M. Logothetis, G. Fanti, J.A. Papadakis Department of Internal Medicine, University Hospital of Heraklion, Heraklion Crete, Greece,
A Journal of Rhetoric in Society Research Update: Pain Medication and the Figure of the Pain Patient University of Birtish Columbia Present Tense, Vol. 2, Issue 2, 2012. Research Update: Pain Medication and the Figure of the Pain PatientJudy Z. Segal Coalition, pain patients are "treated as complainers, malingerers, and drug seekers" (Walton). Clearly, there is some


