Comparative efficacy of inhaled albuterol between two handheld delivery devices in horses with recurrent airway obstruction
EQUINE VETERINARY JOURNAL
Equine vet. J. (2011)
•• (••) ••-••
doi: 10.1111/j.2042-3306.2010.00313.x
Comparative efficacy of inhaled albuterol between twohand-held delivery devices in horses with recurrentairway obstruction
F. R. BERTIN,
K. M. IVESTER and
L. L. COUËTIL*
Department of Veterinary Clinical Sciences, School of Veterinary Medicine, Purdue University, Indiana, USA.
Keywords: horse; recurrent airway obstruction; heaves; aerosol; delivery device; b2 agonist; bronchodilator; AeroHippus; Equine Haler
Reasons for performing study: Studies investigating the
Dynamic lung compliance
clinical efficacy of albuterol administered with the same
Maximum change in transpulmonary pressure
propellant and commercially available delivery devices
Pressurised metered-dose inhaler
in horses with recurrent airway obstruction (RAO) are not
Recurrent airway obstruction
Objectives: To determine the efficacy of aerosolised albuterol
administered to horses with RAO by means of 2 commercially
available, hand-held delivery devices.
Methods: Ten horses with RAO were kept in a dusty
Recurrent airway obstruction (RAO) is the most frequent
obstruction. Lung mechanics were measured before and
cause of chronic respiratory tract disease in horses (Hotchkiss
after the procedure. D
Pmax was measured 5 min after
et al.
administration of 180 m
g of albuterol from a pressurised
bronchospasm with phases of remission when a horse's
metered dose inhaler, using an aerosol delivery device chosen
environment is improved (Robinson
et al. 1996). During disease
randomly. This process was repeated every 5 min until
exacerbation, bronchoconstriction, airway wall oedema and
maximal bronchodilation was achieved. After a 24 h washout
accumulation of mucus result in obstruction of the distal airways.
period, lung mechanics data were again collected using the
These mechanisms induce functional changes: maximum change in
other aerosol delivery device.
transpulmonary pressure (DPmax) increases, pulmonary resistance
Results: Aerosolised albuterol induced a significant and rapid
(RL) increases and dynamic lung compliance (Cdyn) decreases
bronchodilation in the horses using both aerosol delivery
(Gillespie
et al. 1966; Couëtil
et al. 2001). Recurrent airway
devices. No statistically significant difference in pulmonary
obstruction is believed to be an allergic reaction to organic dusts
function was observed in response to albuterol therapy
and has many similarities with human asthma (Ghio
et al. 2006;
between the 2 devices. The dose required to achieve 50% of
Marti
et al. 2008).
maximal bronchodilation was not statistically different
Inhaled short-acting b2-receptor agonists are the most effective
between the 2 devices (173.35 ⫾ 78.35 m
g with Device 1 and
medication for relieving acute bronchospasm (Anon 2007).
228.49 ⫾ 144.99 m
g with Device 2, P =
0.26). The decrease in
Albuterol is the most commonly prescribed medication for asthma
lung resistance tended to be more pronounced after albuterol
in man worldwide (Kelly 2005). Short-acting b2-receptor agonists,
administration with Device 1 (P =
0.066).
amongst other actions, mediate vasodilation and bronchodilation
(Weiss
et al. 2006). In human medicine, nebulisers and spacer
bronchodilator in horses with recurrent airway obstruction.
devices are popular means of delivering aerosols. Small volume
There is no statistically significant difference between the
spacers, composed of a mouth piece and holding chamber with
2 commercially available aerosol delivery devices in terms
valves, have been developed for patients such as infants to avoid
of efficacy.
having to precisely coordinate actuation of the pressurised
metered-dose inhaler (pMDI) and inhalation. Spacers have been
delivered using currently available devices leading to
shown to be clinically effective and result in fewer side effects from
maximal bronchodilation in horses with RAO at an average
medication residue in the oral cavity (Clarke
et al. 1993). Aerosol
dose of 540 m
g.
delivery to infants is more efficient from a pMDI via a small
*Corresponding author email:
[email protected][Paper received for publication 19.06.10; Accepted 16.08.10]
Comparative efficacy of inhaled albuterol between two hand-held delivery devices
volume spacer than from a nebuliser (Wildhaber
et al. 1997).
of ⱖ12 was considered sufficient to warrant lung function testing.
In equine medicine, various aerosolised drugs have been used
For inclusion in the study, a maximum change in transpulmonary
successfully for the treatment of RAO (Rush
et al. 1998; Derksen
pressure (DPLmax) ⱖ15 cmH2O was required after the induction
et al. 1999; Couëtil
et al. 2005). Aerosolised bronchodilators
period. If that pressure was achieved, lung mechanics were
administered with pMDI are effective and associated with minimal
measured at baseline and the horse was enrolled in the treatment trial
side effects. In particular, aerosolised albuterol has been shown to
using one of 2 aerosol delivery devices chosen at random. The horse
be a valuable bronchodilator with rapid onset in the treatment of
then returned to its stall in the dusty environment for a minimum
RAO (Derksen
et al. 1999; Rush
et al. 1999). This type of therapy
of 24 h washout period. The following day, if the horse met the
requires specialised devices to optimise drug delivery in the equine
inclusion criteria, lung mechanics data were again collected using
lung. Convenient delivery devices have been described in horses
the other aerosol delivery device. If the criteria were not met, the
(Tesarowski
et al. 1994; Derksen
et al. 1996). Some of these
horse was maintained in the dusty environment until inclusion
devices use a nose piece, which fits inside the horse's nose, instead
criteria were met. This protocol was approved by the Purdue Animal
of a face mask to deliver a known dose of any given drug from a
Care and Use Committee. Horses were to be removed from the
pMDI into the equine lung (Derksen
et al. 1996). The relative
study if they became anorectic for >24 h.
percentage of a drug deposited in the lung varies based onthe device used and the type of propellant. However, data on the
Aerosol delivery devices
efficacy of inhaled albuterol using available delivery devices arenot available. Currently, 2 aerosol delivery devices that do not
Two aerosol delivery devices, both commercially available,
require a face mask are commercially available. A study using
referred to as
Devices 1 and
2 (Fig 1), were used for albuterol
delivery
Device 1 (Aerohippus)1 found that 18.2 ⫾ 9.3% of
administration using a pMDI and HFA propellant. Both devices are
administered beclomethasone dipropionate with hydrofluoroalkane
hand-held chambers connected to a nose mask which is placed over
(HFA) propellant is deposited in the lung (Hoffman
et al. 2008).
one nostril. The pMDI was inserted into the back piece of the
Another study, using delivery
Device 2 (Equine Haler)2, reports
chamber where the breathable particles were suspended until the
that 8.2 ⫾ 5.2% of administered fluticasone propionate with
horse breathed them through a one-way valve. The device was held
chlorofluorocarbons (CFC) propellant is deposited in the equine
on the nostril for 3 respiratory cycles to ensure complete inhalation
lung (Funch-Nielsen
et al. 2001). These 2 studies focused on lung
of the dose. The breathing chamber of
Device 1 was a cylinder
deposition exploring 2 different drugs administered with 2 different
whereas the breathing chamber of
Device 2 was an ellipsoid.
propellants. In human medicine, it has been demonstrated that thechoice of a propellant considerably influences lung deposition
Lung mechanics
(Leach
et al. 1998; Harrison 2002). However, CFCs have beenbanned from albuterol pMDI since 2008 in most countries
Oesophageal pressure was measured using a balloon catheter
and replaced by HFA. Studies investigating the clinical efficacy
(internal diameter 4.8 mm; outside diameter 6.4 mm; 240 cm in
of albuterol administered with the same propellant and the
length), which was advanced to the mid-thoracic region and
commercially available delivery devices in horses with RAO are
connected to a pressure transducer. The balloon was a condom
not currently available.
taped around the catheter tip and inflated with 3 ml of air. The
The purpose of this study was to evaluate if aerosolised
position of the balloon was recorded for each horse at the time
albuterol administered to horses with RAO using aerosol delivery
of baseline testing prior to induction of RAO exacerbation and
devices currently commercially available have comparable efficacy
used subsequently for all lung mechanics tests. Transpulmonary
on lung function and to provide clinicians with guidelines for the
pressure was defined as the difference between oesophageal
selection of an aerosol delivery device.
pressure and atmospheric or mask pressure, depending on whetherthe horse was fitted with a facemask or not. When measuring
Materials and methods
Device 2 (Equine Haler)
Ten horses (5 mares and 5 geldings), age 7–29 years, with inducibleand reversible airway obstruction that are part of the RAO-affectedherd belonging to Purdue University were used in the study. Allhorses had been housed on pasture and fed a pelleted diet for atleast 3 months to ensure remission from disease. At the beginningof the study, abnormalities were not detected during physicalexamination of the horses. The horses were then exposed to a dustyenvironment by housing them in a barn and placing mouldy hay andstraw in their stall. In addition, mouldy hay was shaken twice a day
for 5 min next to the horses' nose in order to increase dust exposure.
People shaking hay were protected from inhalation of dust by
Device 1 (AeroHippus)
wearing an N95 face mask. The horses remained in the confinedenvironment until they developed clinical signs of RAO. Clinicalscores were assigned to each horse once daily by use of a scaleadapted from Tesarowski
et al. (1996) to screen for the onset of
Fig 1: Delivery Devices 1
and 2
and albuterol metered-dose inhaler used in
airway obstruction. The scale ranges from 0–21 and a clinical score
the study.
F. R. Bertin et al.
airflow, a mask was fitted around the horse's nose with a
TABLE 1: Mean ⫾ s.d. maximal changes in pleural pressure (DPLmax),
pneumotachometer coupled to a pressure transducer that generated
dynamic compliance (Cdyn) and pulmonary resistance (RL) recorded
with the mask before (pre) and at the maximum effect after (post)
a signal proportional to airflow. Output signals were recorded by
administration of aerosolised albuterol
computer software as previously reported (Couëtil et al. 2001). Atleast 10 respiratory cycles from breaths devoid of artefacts were
selected for analysis.
For each trial, baseline measurements of lung mechanics
DPLmax post (cmH2O)
including airflow, change in transpulmonary pressure (DPLmax) were
Cdyn pre (l/cmH2O)
recorded. Values for resistance (R
L) and compliance (Cdyn) were
dyn post (l/cmH2O)
computed in accordance with the method described by Amdur and
RL pre (cmH2O/l/s)
Mead (1958). The mask was then removed and another baseline
L post (cmH2O/l/s)
DPLmax measured. The horse was then given 2 puffs (180 mg) ofalbuterol (Ventolin)3 using one of the 2 delivery devices and DPLmaxwas measured again 5 min later. This process was repeated until
maximal bronchodilation was achieved (ⱕ10% difference between
2 consecutive doses) or a maximum of 10 puffs had been
administered. After the last measurement, the mask was replaced
and lung mechanics measured again. A dose-response-curve was
constructed by plotting DPLmax vs. albuterol dose.
Calibration of flow and pressure transducers was performed
once a day before each experiment using a 3 l calibrated syringe
and a water manometer, respectively.
Mean ⫾ s.d. were calculated for data that followed normal
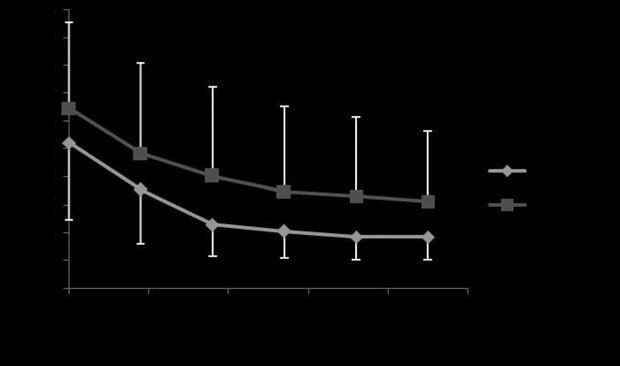
Fig 2: Mean ⫾ s.d. maximal changes in pleural pressure (DP
distribution and median (range) for data with non-normal
Lmax) recorded
without the mask before (0) and after subsequent administrations of
distribution. Comparison of normally distributed data between
aerosolised albuterol with Devices 1 and 2 (P = 0.26).
treatment groups (Device 1 vs. Device 2) was made using a pairedt test. Other data were compared using a Wilcoxon signed ranktest. In particular, the albuterol dose that resulted in a 50% and
maximum decrease in DPLmax from baseline and absolute and
relative difference in RL before and after the last dose of albuterolwere compared between the 2 aerosol delivery devices. Changes in
lung function variables (DPLmax, RL, Cdyn) between baseline and at
the time of maximal bronchodilation were compared between
treatment groups (Device 1 vs. Device 2) using repeated measuresANOVA. Post hoc tests were used when appropriate. Significance
was defined as P<0.05.
All the horses met the inclusion criteria within 3 weeks of exposure
to the dusty environment. The median clinical score before lung
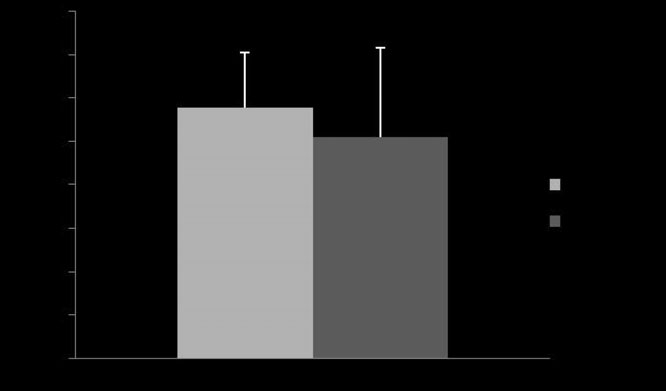
Fig 3: Mean ⫾ s.d. percentage of reduction of initial maximal changes in
function test was 16/21 (9–19). Lung mechanics (DPLmax, RL and
pleural pressure (DPLmax) after subsequent administrations of aerosolised
Cdyn) before administration of albuterol were not statistically
albuterol with Devices 1 and 2 (P = 0.39).
different between the 2 treatment trials (P = 0.51, P = 0.88, P = 0.79,respectively). Lung mechanics (DPLmax, RL and Cdyn), after the
significant further reduction of mean DPLmax was observed by
maximum effect on DPLmax was reached by administration of
increasing the dose beyond 540 mg. However, the response was
albuterol with either spacer, were not statistically different
very variable from one horse to another. Among the 20 tests
(P = 0.27, P = 0.20, P = 0.46, respectively; Table 1).
performed, maximum reduction of DPLmax was achieved after 2
Albuterol administered by both delivery devices induced a
puffs in 4 tests (3 with Device 1 and one with Device 2), after 4
significant decrease in DPLmax (P<0.001), a significant increase
puffs in 2 tests (one with each device), after 6 puffs in 8 tests
in Cdyn (P<0.001) and a significant decrease in RL (P<0.001).
(2 with Device 1 and 6 with Device 2), after 8 puffs in 4 tests
Albuterol administered by both delivery devices induced a dose-
(all with Device 1) and after 10 puffs in 2 tests (all with Device 2).
dependent response and the responses were not statistically
The absolute and relative reduction in RL following
different between devices (P = 0.26; Fig 2). There was no statistical
administration of the last albuterol dose tended to be higher
difference between the maximal reduction of DPLmax observed with
with Device 1 (1.10 [-0.07–3.31] cmH2O/l/s; 65.1 [7.9–89.0]%;
albuterol administered with either mask, either in absolute value (P
P = 0.066) than with Device 2 (0.68 [0.61–1.99] cmH2O/l/s;
= 0.44) or in percentage of reduction (P = 0.39; Fig 3). The mean
53.7 [20.0–79.1]%). The absolute decrease in RL post albuterol
dose required to reach the plateau effect was 540 mg (6 puffs). No
challenge was greater with Device 1 in 6 horses but greater with
Comparative efficacy of inhaled albuterol between two hand-held delivery devices
administration was higher with Device 2 than with Device 1especially for DPLmax (Table 1). The large standard deviation
in DPLmax post albuterol for Device 2 is mainly due to one horse,
which responded poorly to treatment with Device 2 (DPLmax =
65.7 cmH2O at baseline and DPLmax = 47.5 cmH2O after 10 puffs)
but responded well to treatment administered with Device 1 (DPLmax
= 45.9 cmH2O at baseline and DPLmax = 7.1 cmH2O after 10 puffs).
Data analysis was repeated after excluding data from this horse andresults indicated that post albuterol DPLmax = 14.0 ⫾ 6.5 cmH2O
with Device 2. Previous studies reported lung deposition of 18.2 ⫾
9.3% and 8.2 ⫾ 5.2% using Devices 1 and 2, respectively (Funch-Nielsen et al. 2001; Hoffman et al. 2008). These results should be
interpreted with caution because the studies were not peer-reviewed. Nevertheless, based on these data we would expect the
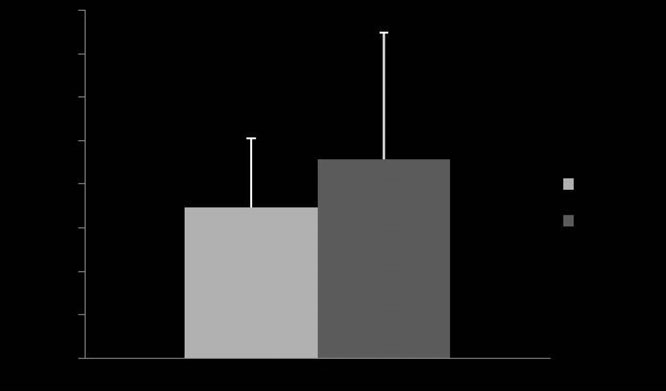
Fig 4: Mean ⫾ s.d. dose of aerosolised albuterol required to observe 50%
dose of albuterol required to achieve 50% of the maximum effect
of reduction of maximal changes in pleural pressure (DPLmax) with Devices
on DPLmax with Device 1 to be approximately half that required with
1 and 2 (P = 0.31).
Device 2. In fact, the dose required with Device 1 was only 24%
Device 2 in 2 horses. In one horse RL increased after albuterol
lower and that difference was not statistically significant. The
administration with both devices and RL measurement was
higher lung deposition reported with Device 1 was obtained with
unavailable prealbuterol in one horse.
beclomethasone dipropionate and an HFA propellant while the
The dose of albuterol required to reach 50% of the maximum
lower deposition was with fluticasone propionate and a CFC
effect on DPLmax was not statistically different between Device 1
propellant (Funch-Nielsen et al. 2001; Hoffman et al. 2008).
(173.35 ⫾ 78.35 mg) and Device 2 (228.49 ⫾ 144.99 mg, P = 0.31;
Human clinical trials indicate that relative drug deposition in the
lungs is approximately 2-fold higher with HFA than with CFCpropellant for drugs such as beclomethasone and flunisolide
(Richards et al. 2001; Harrison 2002). Therefore, lung depositionof beclomethasone-HFA in horse's lungs using Device 2 would be
The 10 horses completed the study protocol and they inhaled the
expected to be around 16.4% which is similar to the 18.2% reported
medication with ease. They did not exhibit any adverse effects of
for Device 1 delivering the same drug formulation. This
b2-agonist therapy and no horse exhibited anorexia throughout
extrapolation is consistent with the present study findings.
the study duration.
The study revealed that albuterol delivered with Device 1
During exposure, the 10 horses exhibited clinical signs of RAO
resulted in a 34% greater improvement in RL than with Device 2 but
and experienced altered DPLmax, RL and Cdyn as previously reported
this difference did not reach statistical significance. Conducting
(Tesarowski et al. 1996; Rush et al. 1998; Derksen et al. 1999).
additional studies with a larger number of horses would be helpful
Administration of albuterol significantly improved pulmonary
to confirm if the 2 devices achieve significantly different drug
function parameters in all the tested horses. These findings are
delivery levels and require different dose recommendation for the
consistent with published studies (Derksen et al. 1999; Rush
treatment of RAO.
et al. 1999).
Pulmonary function is traditionally quantified using DPLmax,
No statistically significant difference was noted in DPLmax, RL
RL and Cdyn. In this study, only DPLmax has been measured between
and Cdyn before the treatment trial using delivery device. These
individual administrations of albuterol. In other reports, DPLmax
results indicate that a 24 h washout period between the 2 trials was
and RL appeared to be the most sensitive markers of improved
adequate and are consistent with the fact that during exacerbation
airway obstruction in horses with RAO (Robinson et al. 1993;
of the disease, a horse's pulmonary function remains relatively
Tesarowski et al. 1994; Derksen et al. 1996; Rush et al. 1998).
stable (Jean et al. 1999).
Measurement of DPLmax does not require a mask fitted around
All doses of albuterol induced a significant decrease in
the horse's nose with a pneumotachometer coupled to a pressure
DPLmax within 5 min of administration, which indicates a rapid
transducer. However, DPLmax is also influenced by voluntary
improvement of airway obstruction. These results are similar to
breathing efforts and may vary with excitement or tachypnoea.
those reported using fenoterol (Tesarowski et al. 1994) and albuterol
In this study, RL was also measured before and after the last dose
(Derksen et al. 1999; Rush et al. 1999) delivered by pMDIs
of albuterol confirming the fact that improvement in lung function
combined with other delivery devices. However, in this study, the
was due to reduced airway obstruction and not just changes in
mean dose required to reach the plateau effect was 540 mg (6 puffs).
breathing strategy.
No significant further bronchodilation was observed by increasing
Bronchodilation induced by albuterol lasts for 30–60 min
the dose beyond 540 mg. This dose is higher than the dose of 360 mg
(Derksen et al. 1999). During the study we chose to administer
previously reported (Derksen et al. 1999). Since the same HFA
albuterol 2 puffs at a time in order to reduce the time elapsed
propellant was used in both studies, the difference may be explained
between the first administration of albuterol and the last
by the higher percentage of drug deposited in the lungs using the
measurement. Using this method, the time required to administer
device that is no longer commercially available.
the maximal dose (900 mg or 10 puffs) and perform the
No statistically significant difference was noted in DPLmax, RL
last measurement was 32 min on average. If the mask were
and Cdyn after the treatment trial using either delivery device. These
repositioned to perform a complete measurement of the lung
results suggest that the 2 devices achieved a similar amount of drug
function between each administration, the incremental dose-
response curve would not have been accurate for the last
F. R. Bertin et al.
measurements. Using this protocol, it was considered that the
bronchodilator effect induced by the first administration ofalbuterol was still present when the last measurement of pulmonary
1Trudell Medical International, London, Ontario, Canada.
2
function was performed. This assumption was reinforced by the
Equine Health Care Aps, Horsholm, Denmark.
3GlaxoSmithKline, Brentford, Middlesex, UK.
fact that DPLmax either continued to decrease or reached a plateauas increasing dosages were delivered but it never increased by thetime the last dose was administered.
A large variation was observed between horses. Some horses
reached maximal bronchodilation with as little as 2 puffs (180 mg)
Amdur, M. and Mead, J. (1958) Mechanics of respiration in unanesthetized guinea
while others required 10 (900 mg). Also, most of the improvement
pigs. Am. J. Physiol. 192, 364-368.
in DPLmax was seen within the first few puffs after which the effect
Anon (2007) Expert Panel Report 3: Guidelines for the Diagnosis and Management of
levelled off. As reported in other studies (Derksen et al. 1996),
Asthma. Department of Health and Human Services, National Institutes of Health:National Heart, Lung, and Blood Institute. National Asthma Education Prevention
small airways of horses with RAO are occluded by mucus, airway
Program. Bethesda.
wall thickening and bronchospasm, thus resulting in unpredictable
Clarke, J.R., Aston, H. and Silverman, M. (1993) Delivery of salbutamol by metered
amount of inhaled drug deposition. Furthermore, horses did not
dose inhaler and valved spacer to wheezy infants: Effect on bronchial
reach the same clinical score or degree of airway obstruction based
responsiveness. Arch. Dis. Child. 69, 125-129.
on lung function testing. It is likely that some horses were more
Couëtil, L.L., Rosenthal, F.S., DeNicola, D.B. and Chilcoat, C.D. (2001) Clinical
affected and therefore produced more mucus and experienced
signs, evaluation of bronchoalveolar lavage fluid, and assessment of pulmonary
more severe bronchospasm resulting in decreased lung deposition
function in horses with inflammatory respiratory disease. Am. J. vet. Res. 62,
538-546.
of albuterol. However, all the horses had an improved lung
Couëtil, L.L., Chilcoat, C.D., DeNicola, D.B., Clark, S.P., Glickman, N.W. and
function test after repeated administration, possibly because an
Glickman, L.T. (2005) Randomized, controlled study of inhaled fluticasone
initial bronchodilation aided drug deposition during the subsequent
propionate, oral administration of prednisone, and environmental management of
horses with recurrent airway obstruction. Am. J. vet. Res. 66, 1665-1674.
According to the third Expert Panel Report on Diagnosis and
Derksen, F.J., Olszewski, M.A., Robinson, N.E., Berney, C., Hakala, J.E., Matson, C.J.
Management of Asthma (Anon 2007), short-acting b2-agonists are
and Ruth, D.T. (1999) Aerosolized albuterol sulfate used as a bronchodilator in
horses with recurrent airway obstruction. Am. J. vet. Res. 60, 689-693.
the most effective medication for relieving acute bronchospasm;
Derksen, F.J., Olszewski, M.A., Robinson, N.E., Berney, C., Lloyd, J.W., Hakala, J.,
however, increasing the use of short-acting b2-agonists treatment
Matson, C. and Ruth, D. (1996) Use of a hand-held, metered-dose aerosol delivery
or using short-acting b2-agonists >2 days/week for symptom
device to administer pirbuterol acetate to horses with ‘heaves'. Equine vet. J. 28,
relief indicates an inadequate control of asthma in addition to the
need for initiating or intensifying anti-inflammatory therapy.
Funch-Nielsen, H., Roberts, C., Weekes, J.S., Deaton, C.M. and Marlin, D.J. (2001)
Similarly in horses, the use of short-acting b2-agonists such as
Evaluation of a new spacer device for delivery of drugs into the equine respiratorytract. Proceedings of the WEAS-CRS Symposium, p 56.
albuterol is only recommended as rescue medication, as a
Gillespie, J.R., Tyler, W.S. and Eberly, V.E. (1966) Pulmonary ventilation and
diagnostic test for RAO or as therapy in combination with inhaled
resistance in emphysematous and control horses. J. appl. Physiol. 21, 416-422.
corticosteroids. In addition, the emphasis should be placed on the
Ghio, A.J., Mazan, M.R., Hoffman, A.M. and Robonson, N.E. (2006) Correlates
need to improve the environment of a horse affected by RAO by
between human lung injury after particle exposure and recurrent airway
limiting antigen inhalation, particularly thermophilic moulds and
obstruction in the horse. Equine vet. J. 38, 362-367.
actinomycetes that grow in mouldy hay (Robinson et al. 1996;
Harrison, L.I. (2002) Local versus total systemic bioavailability of beclomethasone
Hotchkiss et al. 2007b).
dipropionate CFC and HFA metered dose inhaler formulations. J. Aerosol Med.
15, 401-406.
In conclusion, aerosolised albuterol from a pMDI administered
Hoffman, A.M., Foley, M. and Spendlove, P.J. (2008) Respiratory medicine: Advances
with either of 2 commercially available delivery devices is an
in inter-species aerosol delivery. Proc. Austr. equine Sci. Symp. 2, 47.
effective bronchodilator in horses experiencing RAO crises.
Hotchkiss, J.W., Reid, S.W. and Christley, R.M. (2007a) A survey of horse owners in
However, the primary therapy for horses affected by RAO should
Great Britain regarding horses in their care. Part 1: Horse demographic
focus on managing and controlling their environment.
characteristics and management. Equine vet. J. 39, 294-300.
Hotchkiss, J.W., Reid, S.W. and Christley, R.M. (2007b) A survey of horse owners in
Great Britain regarding horses in their care. Part 2: Risk factors for recurrent
Conflict of interest
airway obstruction. Equine vet. J. 39, 301-308.
Jean, D., Vrins, A. and Lavoie, J.P. (1999) Monthly, daily, and circadian variations of
The authors declare no conflict of interest.
measurements of pulmonary mechanics in horses with chronic obstructive
pulmonary disease. Am. J. vet. Res. 60, 1341-1346.
Kelly, H.W. (2005) What is new with the b2-agonists: Issues in the management of
Sources of funding
asthma. Ann. Pharmacother. 39, 931-938.
Leach, C.L., Davidson, P.J. and Bourdeau, R.J. (1998) Improved airway targeting with
Supported by Trudell Medical International, London, Ontario,
the CFC-free HFA-beclomethasone metered-dose inhaler compared with CFC-
Canada, the state of Indiana and the Purdue University School
beclomethasone. Eur. Respir. J. 12, 1346-1353.
of Veterinary Medicine Research account funded by the total
Marti, E., Gerber, V., Wilson, A.D., Lavoir, J.P., Horohov, D., Crameri, R., Lunn, D.P.,
Antczak, D., Bjornsdottir, S., Bjornsdottir, T.S., Cunningham, F., Derer, M., Frey,
R., Hamza, E., Horin, P., Heimann, M., Kolm-Stark, G., Olafsdottir, G., Ramery,E., Russel, C., Schaffartzik, A., Svansson, V., Torsteinsdottir, S., Wagner, B.
(2008) Report of the 3rd Havemeyer workshop on allergic diseases of the horse,
Hólar, Iceland, June 2007. Vet. Immunol. Immunopathol. 126, 351-361.
The authors thank Donna Griffey, Jessica Squires, Mitchell
Richards, J., Hirst, P., Pitcairn, G., Mahashabde, S., Abramowitz, W., Nolting, A. and
Neewman, S.P. (2001) Deposition and pharmacokinetics of flunisolide delivered
Jessup, Christina Carey and Caroline Chauché for their technical
from pressurized inhalers containing non-CFC and CFC propellants. J. Aerosol.
Med. 14, 197-208.
Comparative efficacy of inhaled albuterol between two hand-held delivery devices
Robinson, N.E., Derksen, F.J., Olszewski, M.A. and Buechner-Maxwell, V.A. (1996)
Tesarowski, D.B., Viel, L. and McDonnel, W.N. (1996) Pulmonary function
The pathogenesis of chronic obstructive pulmonary disease of horses. Br. vet. J.
measurements during repeated environmental challenge of horses with recurrent
airway obstruction (heaves). Am. J. vet. Res. 57, 1214-1219.
Robinson, N.E., Derksen, F.J., Berney, C. and Goossens, L. (1993) The airway
Tesarowski, D.B., Viel, L., McDonell, W.N. and Newhouse, M.T. (1994) The
response of horses with recurrent airway obstruction (heaves) to aerosol
rapid and effective administration of b2-agonist to horses with heaves using a
administration of ipratropium bromide. Equine vet. J. 25, 299-303.
compact inhalation device and metered-dose inhalers. Can. vet. J. 35, 170-
Rush, B.R., Hoskinson, J.J., Davis, E.G., Matson, C.J., Hakala, J.E. (1999) Pulmonary
distribution of aerosolized technetium TC 99m pentetate after administration of a
Weiss, S.T., Litonjua, A.A., Lange, C., Lazarus, R., Liggett, S.B., Bleecker, E.R. and
single dose of aerosolized albuterol sulfate in horses with recurrent airway
Tantisira, K.G. (2006) Overview of the pharmacogenetics of asthma treatment.
obstruction. Am. J. vet. Res. 60, 764-769.
Pharmacogenomics J. 6, 311-326.
Rush, B.R., Raub, E.S., Rhoads, W.S., Flamino, M.J., Matson, C.J., Hakala, J.E. and
Gillepsie, J.R. (1998) Pulmonary function in horses with recurrent airway
Wildhaber, J.H., Devadason, S.G., Hayden, M.J., Eber, E., Summer, Q.A., and
obstruction after aerosol and parenteral administration of beclomethasone
LeSouerf, P.N. (1997) Aerosol delivery to wheezy infants: A comparison between
dipropionate and dexamethasone respectively. Am. J. vet. Res. 59, 1039-1043.
a nebulizer and two small volume spacers. Pediatr. Pulmonol. 23, 212-216.
Source: http://vetnetinfo.com/vetnetproducts/files/2013/02/couetil_EVJ2011.pdf
praktikum Versuch Nummer F-09/10: Isolierung von Ribosomen und Bestimmung ribosoma- Sven Enterlein 108 097 236 174 Versuch Nummer FF---009 I. Einleitung Die Zentrifugation bildet eine effektive Möglichkeit, Stoffe nach Größe und Gestalt zu trennen. Die Trennung kann entweder einfach zwischen einer festen und einer flüssigen Phase erfolgen, aber auch unterschiedlich große Partikel können (aus einer Lösung) ge-trennt werden. Physikalische Einflüsse dabei sind Reibung, Auftrieb, Viskosität, Dichte und Zentrifugalkraft. Die treibende Kraft ist die letztgenannte Zentrifugalkraft; sie steht senkrecht zur Drehachse und ist nach außen gerichtet. Durch sie erfahren die Teilchen eine Beschleunigung, die als Zentrifugalbeschleunigung bezeichnet wird. Sie hängt vom Radius r und der Winkelgeschwindigkeit ! ab über
MICUSP Version 1.0 - BIO.G0.11.3 - Biology - Final Year Undergraduate - Male - Native Speaker - Report Introduction Numerous types of fungi are able to infect the eye, including Fusarium, Aspergillus, Curvularia, and Candida (4). The fungus responsible for the recent outbreaks of keratitis (infection of the cornea) due to ReNu MoistureLoc use is thought to



