Implementation of methods translation between liquid chromatography instrumentation
Implementation of Methods Translation between Liquid
Chromatography Instrumentation
Michael D. Jones, Peter Alden, Kenneth J. Fountain, and Andrew Aubin
Waters Corporation, Milford, MA, U.S.
AP PLICATION BENEFITS
Future proof your laboratory
Pharmaceutical research and development (R&D) organizations were early adopters who recognized the many benefits of UltraPerformance LC® (UPLC®) Technology
"Equivalent" vs. "Equal" column
including resolution, sensitivity, throughput, and productivity as compared to
HPLC. Today, the number of projects involving new drug entities are increasingly
Increase productivity while
performed utilizing UPLC.
Adopting UPLC for R&D activities is less complex than for laboratories involved
Maximize asset utilization
with routine analysis, where its use requires consideration about the need to
Understand the importance of L/dp
re-file methods for existing products. Routine analysis areas such as Quality
Discover software tools to facilitate
Control (QC) laboratories own a vast supply and variety of HPLC instrumentation.
method translation
Asset procurement regarding new technologies within these groups often requires convincing financial as well as scientific justification.
Although information illustrating UPLC's return on investment (ROI) for solvent consumption and analysis per unit time can be convincing for R&D, the QC environment requires key practical-use considerations. Managers and end users within QC laboratories require new instrumentation to provide dual purposes: first, the ability to perform both legacy methods and, second, the ability to use sub-2-µm particle columns and methodology in a routine analytical environment without complications. UPLC's adoption must also strategically provide seamless integration within current laboratory practices and decrease learning curves of the end users.
WAT ERS SOLUTIONS ACQUITY UPLC® and
In this application, various U.S. Pharmacopeia (USP) compendial methods are
ACQUITY UPLC H-Class
used as examples to highlight a new method translation strategy to facilitate
Waters Method Transfer Kits
the transfer of methods to and from any LC-based instrument with ease.
ACQUITY UPLC Columns Calculator Reversed Phase Selectivity Chart
K EY WORDS Method Transfer, Compendial Methods, USP, Method Translation, UPLC, HPLC
RESULTS AND DISCUSSIONSuccessful method translation requires understanding three key chromato-
United States Pharmacopeia
graphic attributes before implementation. The analyst must consider the
reference standards
differences between LC instrumentation, column selectivity, and the resolving
USP Monograph Galantamine Hydrobromide
capability of the original methodology versus the target methodology. By
USP Galantamine Hydrobromide RS and
understanding these three essential aspects of method translation, the
USP Galantamine Hydrobromide Related
benefits of increasing productivity and decreasing costs while maximizing
Compounds Mixture RS
asset utilization of present and future instrumentation can be realized.
USP Dietary Supplement: Powdered Soy Isoflavone Extract Method
Future-proofing your laboratory:
USP Apigenin RS, USP Diadzein RS,
Translating HPLC methodology between LC instrumentation
USP Diadzin RS, USP Genistein RS,
The QC laboratory frequently utilizes a variety of LC instruments for API and
USP Genistin RS, USP Glycitein RS,
drug product analysis. Therefore, instrumentation flexibility is essential.
USP Glycitin RS, and USP Defatted
Direct transfer of methods to newer technology may result in retention time and
selectivity differences that may be related to decreases in instrument
USP Monograph Loratadine
dwell volume.
USP Loratadine RS, USP Loratadine
To illustrate the flexibility provided by the Waters ACQUITY UPLC H-Class
Related Compound A RS, and USP
System, the USP method for galantamine hydrobromide and related substances
Loratadine Compound B RS, Claritin
was performed on an HPLC instrument (Figure 1). USP system suitability requirements for the related substances assay specify USP tailing of galantamine NMT 2.0 and a resolution of galantamine and 6-alphagalantamine NLT 4.5.
MET HOD CONDITIONS
When utilizing the same HPLC column on each instrument, the ACQUITY
UPLC Columns Calculator (Figure 2) can be used to calculate the differences
References to LC conditions are addressed
in the instrument dwell volume. The resulting data yielded no compromise in
as per USP Monographs, whereas specific
chromatographic integrity during the translation of the method for use on a
utilization of LC instrumentation for each
UPLC instrument of less dwell volume (Figure 3).
application is discussed in the figure captions.
Galantamine 15.51
Tetrahydro-- 31.87
Figure 1. USP Method for galantamine and related substances performed on an Alliance® HPLC 2695 with measured dwell volume of 1.1 mL. An XBridge™ C18 (L1) column with dimensions 4.6 x 100 mm, 3.5 µm was used.


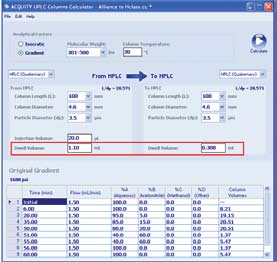
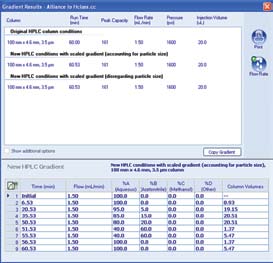
Figure 3. USP method for galantamine related substances performed on the ACQUITY UPLC H-Class with a dwell volume of 280 µL. The relative retention times (RRT), USP Rs, and USP tailing compare to those reported in Figure 1. It should be noted that the decreased extra-column volume of ACQUITY UPLC family of instruments will sharpen the chromatographic peaks, hence slight increases in resolution can result in minimal variations of the calculated RRT when compared to the chromatography of the originating instrumentation.
Figure 2. Example using the ACQUITY UPLC Columns Calculator for HPLC methodology translation, compensating for differences in dwell volume between two different LC system configurations. In red, the dwell volumes of the original and target instrumentation are entered. Once calculated, the gradient table is adjusted to compensate for the instrument differences. The same HPLC column was used for the new HPLC gradient performed on an ACQUITY UPLC H-Class System.
Implementation of Methods Translation between Liquid Chromatography Instrumentation
Implementation of Methods Translation between Liquid Chromatography Instrumentation

Maintaining selectivityDifferences between instrumentation dwell volumes can be easily accounted for with calculated adjustments to the gradient table with the ACQUITY UPLC Columns Calculator. However, the challenging method translations of original methodology to a target methodology reside with differences in column stationary phase selectivity.
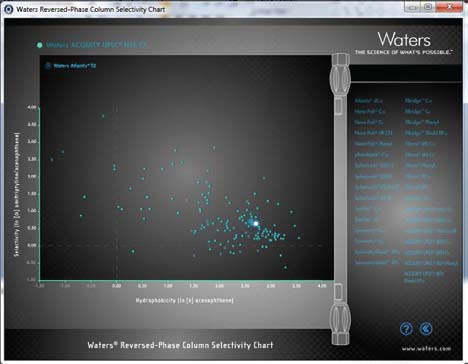
Ideally, when scaling from an HPLC column to a UPLC column, the stationary phase should remain constant (i.e., "equal" stationary phase) to maintain the selectivity of the separation. Unfortunately, many original HPLC columns are not available in the same chemistry in sub-2-µm particle sizes. Therefore, an equivalent stationary phase that is available in sub-2-µm particle size must be determined. This task is facilitated using the Waters Reversed Phase Column Selectivity Chart (www.waters.com/selectivitychart) (Figure 4).
Waters Atlantis T3
Figure 4. In this example,
the original HPLC column used is an Atlantis® T3 column chemistry, however a sub-2-µm particle size utilizing this stationary phase is not available. Using the Reversed Phase Column Selectivity Chart, the ACQUITY UPLC HSS T3 column chemistry was identified as an equivalent sub-2-µm particle size col-umn of similar selectivity.
Increasing productivity while decreasing costs: Translation between HPLC and UPLC methodologyNew pressures in the pharmaceutical industry have created a need for QC laboratories to become more productive. It is important to reduce costs in QC, but not at the expense of R&D or any other part of the organization. Additionally, the reduction in cost cannot come at the expense of chromatographic accuracy, robustness, or reliability.
Dietary supplement manufacturers routinely use HPLC to analyze soy extracts for isoflavone content. The current USP compendial method uses a long, shallow gradient that takes 75 minutes per injection. This long run time limits the ability of manufactures to release products quickly. In addition, a sample set run consisting of a blank, five calibration standards, and two retention time check solutions requires more than 10 hours before running the analysis of the first sample. The benefits of analyzing isoflavones using a faster solution that maintains data quality are improved productivity, increased revenues, enhanced efficiency, faster sample turnover, and reduced labor and training costs.


Using the ACQUITY UPLC Columns Calculator, the HPLC USP method for soy isoflavones shown in Figure 5 was transferred to a UPLC method employing an ACQUITY UPLC HSS T3 Column, 2.1 x 100 mm, 1.8 µm. The geometrically scaled method had a runtime of 24.3 minutes. Because the scaled flow rate of 0.319 mL/min is below the optimum linear velocity for the sub-2-μm particle column, the columns calculator was used to recalculate the gradient at 0.60 mL/min, a flow rate that is closer to optimum (Figure 6).
Figure 5. HPLC chromatogram using isoflavone
USP methodology. Instrument system volume
measured 1.3 mL. USP system suitability
criteria were met. R2 for all compounds across
five working standards; concentrations 0.999,
Daidzin tailing = 1.1, and Genistin %RSD = 0.6.
Figure 6. ACQUITY UPLC Columns Calculator illustrating the ease of
transferring HPLC methodology to UPLC methodology.
1. Choose appropriate column length using similar L/dp value
2. Scaled gradient flow rate would overpressure as indicated in red.
3. Enter new flow optimized for particle size and system pressure limits.
4. Calculator adjusts gradient segments as per correct column volumes
from original method.
Implementation of Methods Translation between Liquid Chromatography Instrumentation
Using the columns calculator, the resolving capabilities of the HPLC column was maintained by choosing a UPLC column dimension with the same column length to particle size (L/dp) ratio. The injection volumes and flow rate were scaled appropriately, and the gradient was corrected to keep the number of column volumes consistent for each time segment. The resulting chromatogram is displayed in Figure 7. We can see that the analysis time has been reduced to 16 minutes. Using this approach, the method was successfully transferred to UPLC with both improved throughput and assay performance. The quality of the analytical results using this new and significantly faster UPLC method were not compromised, and thus met the specified USP criteria.
Calibration curves for each
isoflavone component
Figure 7. UPLC separation of isoflavones. ACQUITY UPLC
instrument system volume
measured 82 µL. USP system
suitability criteria were met.
R2 for all compounds across
five working standards;
concentrations > 0.999,
Daidzin tailing = 0.99 and
Genistin %RSD = 0.12
Maximizing asset utilization: Translating UPLC methodology to HPLC methodologyAnalytical development organizations have decreased their method development time by implementing UPLC, however their customers in many situations across the globe have not yet implemented UPLC technology. Maximizing the utilization of the current instrumentation is key to their productivity until appropriate justifications and budgeting is available to adopt the new technology.
In such cases, the method innovator must adapt the UPLC methodology for HPLC use. Implementing the method translation strategy combining the ACQUITY UPLC Columns Calculator and the appropriate column Method Transfer Kit can facilitate the translation of UPLC methodology to HPLC methodology.
A UPLC method developed for loratadine and its related substances separated nine impurities and the API to meet a set of system suitability criteria as specified in the USP within 10 minutes (Figure 8). The methodology was translated utilizing the ACQUITY UPLC Columns Calculator and ACQUITY UPLC BEH Method Transfer Kit. The key aspects in allowing the transferability to HPLC from UPLC are similar to those stated in the previous example, such that the target column dimensions must have equivalent L/dp values and the column stationary phase selection is equivalent to the originating methodology. The resulting HPLC chromatogram (Figure 9) was compared to the UPLC chromatogram in terms of relative retention time ratios of the related substances.
Figure 8. Example chromatogram of the
ACQUITY UPLC method for loratadine
related substances assay.
Figure 9. Example chromatogram
showing the method translation strategy
successfully converting the original UPLC methodology to the HPLC methodology for
loratadine related substances analysis.
In addition to the UPLC to HPLC transfer of loratadine, the UPLC and HPLC methods were compared on three different instruments (Alliance HPLC 2695, ACQUITY UPLC, and ACQUITY UPLC H-Class) in order to evaluate the accuracy of the entire method transfer process (Table 1).
Relative retention time ratios
ACQUITY UPLC →
Alliance 2695 →
Table 1. Relative retention time ratio comparisons of the loratadine related substances using HPLC and UPLC instrumentation, the ACQUITY UPLC Columns Calculator and the method transfer kit for XBridge C .
Implementation of Methods Translation between Liquid Chromatography Instrumentation
The compendia methods translation experiments were facilitated using a
Successful methods translation is achievable
method translation strategy comprised of software tools, column Method
with a strategy comprised of software tools,
Transfer Kits, and thorough knowledge of the instrumentation used. In
Method Transfer Kits, and an understanding of
each example, the chromatographic attributes and integrity of the original
the basic characteristics of the instrumentation
methodology were maintained.
Choosing a compatible column stationary phase exhibiting "equivalent" or
Three USP compendial methods were
"equal" selectivity and resolution characteristics was key when transferring
successfully transferred to various LC
from legacy HPLC methodology to UPLC methodology. The process of
configurations without compromising the
translating from HPLC to UPLC can be difficult due to the availability of
integrity of the originating method.
a sub-2-µm particle size equivalent columns with the same originating
Techniques were demonstrated to maximize
HPLC stationary phase, especially if the originating HPLC stationary phase
global asset utilization and maintain lab
was introduced many years prior. The reversed-phase selectivity chart can
facilitate proper stationary phase selection in many of these instances,
Methods were successfully translated to take
however, some selectivity differences may be observed.
benefit of sub-2-µm stationary phases.
The process of translating methodology from UPLC to HPLC is made easier
Software tools are available to facilitate the
with columns that are available in both UPLC and HPLC particle sizes, as in
scaling and column selection
the case of ACQUITY UPLC BEH and XBridge, ACQUITY UPLC CSH, and
The AC QUITY UPLC Columns Calculator
XSelect™ CSH, and HSS UPLC and HPLC columns.
accounts for differences within system dwell volumes. Flow rates and injection
In an effort to streamline method translation, QC organizations should open
volumes are scaled while compensating for
communications with R&D organizations presently implementing UPLC
appropriate column volumes per gradient
for methods development. Discussions should focus on the intricacies of
time segment.
maintaining column selectivity for UPLC and HPLC, as well as the importance of L/dp values for maintaining resolving capabilities of a column. These
The Reversed Phase Column Selectivity
discussions would help devise a cohesive implementation strategy that
Chart facilitates the selection of equivalent
can supplement the method translation strategic approach earlier within
column selectivity when an equal
selectivity column is unavailable.
References1. Galatamine Hydrobromide: USP32-NF27 Supplement:No.2,
2. Powdered Soy Isoflavones Extract: USP32-NF27, page 1074.
3. Loratadine: USP32-NF27, page 2805.
Waters, UltraPerformance LC, UPLC, ACQUITY UPLC, and Atlantis are
Waters Corporation
registered trademarks of Waters Corporation. XBridge, XSelect, Empower,
and The Science of What's Possible are trademarks of Waters Corporation.
Milford, MA 01757 U.S.A.
All other trademarks are the property of their respective owners.
T: 1 508 478 2000
2010 Waters Corporation. Printed in the U.S.A.
F: 1 508 872 1990
September 2010 720003721EN LL-PDF
Source: http://wwwp1.waters.com/webassets/cms/library/docs/720003721en.pdf
SINER-GI Task 2: WP5 GI Case Studies Case Study Report : INSTITUTION (Country) Affiliation BIENABE Estelle Western Cape Department of Agriculture SINER-GI WP5 Template for Case Study Report - v4 1. Executive summary 2. National context analysis: GIs and the dynamic of country agrifood interests 3. Product Data Card
"Farmacología kinésica deportiva" Cátedra Kinesiología Deportiva Encargado de enseñanza Dr. Mastrángelo, Jorge Lic. Spinetta, Daniel Integrantes Balzi, Brenda Bettini, Florencia Ferraris, Juan Manuel Fortuondo, María Emilce Gómez, Vanina Guisasola, Pablo L'Afflitto, Mariana Micó, Gustavo Vazquez, Lorena Vignolo, Florencia








