Prothrombinex-vf pi
Hong Kong
NAME OF THE MEDICINE
Human prothrombin complex, powder for injection.
DESCRIPTION
Prothrombinex®-VF is a sterile freeze-dried powder containing purified human coagulation
factors II, IX and X and low levels of factors V and VII. It is prepared from blood collected
from voluntary donors.
The concentrate is prepared by adsorption of coagulation factors from plasma onto an
ion-exchange medium followed by selective elution. The manufacturing process of
Prothrombinex®-VF contains dedicated and complementary steps to reduce the possibility of
viral transmission including dry heat treatment (80°C for 72 hours) for viral inactivation and
nanofiltration for virus removal.
When reconstituted as recommended, each vial of Prothrombinex®-VF contains the ingredients
listed in
Table 1.
Human plasma proteins
(including low levels of factors V and VII)
Antithrombin III
+ present as sodium citrate, sodium phosphate and sodium chloride.
The factors II, IX, X, the antithrombin III and the plasma proteins are all of human origin. The
heparin sodium is of porcine origin.
Prothrombinex-VF HK PI 2.00
The coagulation factors II, VII, IX and X, which are synthesised in the liver with the help of
vitamin K, are commonly called the prothrombin complex.
Factor VII is the zymogen of the active serine protease factor VIIa by which the extrinsic
pathway of blood coagulation is initiated. The tissue factor-factor VIIa complex activates
coagulation factors X and IX, whereby factor IXa and Xa are formed. With further activation
of the coagulation cascade prothrombin (factor II) is activated and transformed to thrombin.
By the action of thrombin, fibrinogen is converted to fibrin, which results in clot formation.
The normal generation of thrombin is also of vital importance for platelet function as a part of
the primary haemostasis.
Isolated deficiency of factor IX is one of the classical haemophilias (haemophilia B). Isolated
deficiency of factor II or factor X is very rare but in severe form they cause a bleeding
tendency similar to that seen in classical haemophilia. Isolated severe deficiency of factor VII
leads to reduced thrombin formation and a bleeding tendency due to impaired fibrin formation
and impaired primary haemostasis.
Acquired deficiency of the vitamin K dependent coagulation factors occurs during treatment
with vitamin K antagonists (such as warfarin and phenindione). It may also result from
vitamin K deficiency (malabsorption syndrome, antibiotic therapy, cholestasis, prolonged
parenteral alimentation). If the deficiency becomes severe, a severe bleeding tendency results,
characterised typically by retroperitoneal or cerebral bleeds rather than muscle and joint
Severe hepatic insufficiency also results in markedly reduced levels of the vitamin K dependent
coagulation factors and a clinical bleeding tendency which, however, is often complex due to a
simultaneous ongoing low-grade intravascular coagulation, low platelet levels, deficiency of
coagulation inhibitors and disturbed fibrinolysis.
The administration of human prothrombin complex provides an increase in plasma levels of the
vitamin K dependent coagulation factors, and can temporarily correct the coagulation defect
of patients with deficiency of one or several of these factors.
Prothrombinex-VF HK PI 2.00
Prothrombin complex concentrates (PCCs) are distributed and metabolised in the same way as
endogenous coagulation factors. Intravenous administration means that the preparation is
available immediately; bioavailability is 100%.
A pharmacokinetic study with a PCC containing coagulation factors II, VII, IX and X in
healthy volunteers, who received a 50 IU/kg intravenous dose, showed that peak plasma
concentrations of the coagulation factors occur within 5 minutes of infusion.
Table 2 lists PCC
coagulation factor elimination half-lives as median and (inter-quartile range).
Factor II
Factor VII
Factor IX
Elimination t1/2
There are no data for Prothrombinex®-VF, a PCC containing factors II, IX and X. The
European Core Summary of Product Characteristics (SPC) for PCCs cites the following
recoveries for the coagulation factors: Factor II 0.02 IU/mL, Factor VII 0.01 IU/mL,
Factor IX 0.01 IU/mL and Factor X 0.017 IU/mL.
CLINICAL TRIALS
There is limited clinical data available on Prothrombinex®-VF. However, clinical trials have
been published using PCCs similar to Prothrombinex®-VF.
Acquired Deficiencies (Warfarin Reversal)
In a retrospective study, 105 patients on warfarin received Prothrombinex®-HT, an earlier form
with similar properties to Prothrombinex®-VF. Fifty-one patients received Prothrombinex®-HT
for bleeding, 8 for suspected bleeding, 11 for high INR and 35 before a procedure.
Prothrombinex®-HT was given alone (n = 74), or co-administered with fresh frozen plasma
(FFP) (n = 31), with the majority of patients also receiving vitamin K (71% and 67.7%
respectively). The mean Prothrombinex®-HT dose was lower than that recommended by the
Australasian Society of Thrombosis and Haemostasis Warfarin Reversal Consensus Group
(WRCG) (see
DOSAGE AND ADMINISTRATION): 13.1 IU/kg without FFP and
12.3 IU/kg with FFP. The vitamin K dose was 1–10 mg and FFP 1–10 units. Reduction in INR
varied considerably from 0–90% in the 74 patients receiving Prothrombinex®-HT without FFP.
Prothrombinex-VF HK PI 2.00
There were no INR data for patients receiving Prothrombinex®-HT with FFP. All patients with
bleeding achieved haemostasis. Achievement of haemostasis did not require normalisation of
INR in patients treated with Prothrombinex®-HT without FFP – 13 patients (18%) had an INR
remaining above 3.0.
In trials of PCCs similar to Prothrombinex®-VF, the onset of effect on INR was rapid (within
15 minutes). When given concurrently with vitamin K, the duration of effect in one trial was
shown to be up to 48 hours.
The optimum dose of Prothrombinex®-VF for warfarin reversal remains to be determined.
There is a need to balance adequate efficacy against the risk of thrombosis. The dose
recommended by the WRCG is 25–50 IU/kg (see
DOSAGE AND ADMINISTRATION).
Congenital Deficiencies
There are two published reports on the efficacy of PCCs in the treatment of eleven
haemophilia B (factor IX deficient) patients undergoing bleeding or surgery. In a separate
study eight haemophilia B patients who received prophylactic treatment with PCC at doses up
to 25–40 IU/kg twice weekly showed reduced joint damage compared to age matched
historical controls who only received on demand therapy. However, as there have been no
dose ranging studies performed with PCCs the doses recommended are based on accumulated
clinical experience (see
DOSAGE AND ADMINISTRATION).
There are very few published case reports on the efficacy of PCCs in the treatment of bleeds in
patients with congenital factor II or X deficiency.
INDICATIONS
Prothrombinex®-VF is indicated in:
• Treatment and perioperative prophylaxis of bleeding in acquired deficiency of prothrombin
complex factors, such as deficiency caused by treatment with vitamin K antagonists, or in
case of overdose of vitamin K antagonists, when rapid correction of the deficiency is
• Treatment and prophylaxis of bleeding in patients with single or multiple congenital
deficiency of factor IX, II or X when purified specific coagulation factor product is not
available (see
PRECAUTIONS).
Prothrombinex-VF HK PI 2.00
Hypersensitivity to the active substances or to any of the excipients including known allergy to
heparin or history of heparin-induced thrombocytopenia (HIT).
Prothrombinex®-VF is also contraindicated in patients who have evidence of active thrombosis
or disseminated intravascular coagulation (DIC).
PRECAUTIONS
The advice of a specialist experienced in the management of coagulation disorders should be
In patients with acquired deficiency of vitamin K dependent coagulation factors (e.g. induced
by treatment with vitamin K antagonists such as warfarin or phenindione), Prothrombinex®-VF
should only be used when rapid correction of the prothrombin complex factor levels is
necessary. In other cases, reduction of the vitamin K antagonist dose or omission of the next
dose and/or administration of vitamin K is usually sufficient.
In congenital deficiency of any of the vitamin K dependent factors, specific coagulation factor
product should be used when available because of the incremental risk of thrombosis with
Prothrombinex®-VF.
Prothrombinex®-VF is not recommended for the management of patients with isolated
factor V or factor VII deficiency because of the low levels of factors V and VII in the product.
Prothrombinex®-VF should be used with caution in patients with a known allergy to
constituents of the preparation. Allergic or anaphylactic-type reactions (e.g. angioedema,
injection site reactions, chills, flushing, generalised urticaria, headache, pruritus, hypotension,
lethargy, nausea, vomiting, restlessness, tachycardia, tingling, swelling, wheezing or shortness
of breath) have been rarely observed in patients receiving PCCs such as Prothrombinex®-VF.
In some cases, these reactions have progressed to severe anaphylaxis, particularly in patients
with factor IX inhibitors. If allergic or anaphylactic-type reactions occur, Prothrombinex®-VF
administration should be stopped immediately and appropriate measures implemented.
Prothrombinex-VF HK PI 2.00
Prothrombinex®-VF contains heparin sodium which may cause HIT. The possibility of HIT
developing during treatment should be considered if high doses of Prothrombinex®-VF are
required (see
CONTRAINDICATIONS).
Inhibitors
The use of Prothrombinex®-VF in patients with congenital deficiency of any of the vitamin K
dependent factors may lead to the formation of circulating antibodies known as ‘inhibitors' to
one or more of the factors in the product and manifest as a poor clinical response.
Thrombosis
Patients receiving a vitamin K antagonist may have an underlying hypercoagulable state and
infusion of a PCC may exacerbate this.
There is a risk of thrombosis, embolism, DIC or myocardial infarction when patients are
treated with PCCs such as Prothrombinex®-VF. Such events may be fatal. The risk may be
increased with repeated or high doses (especially at dose levels greater than 50 IU/kg of
factor IX). Therefore, patients treated with PCCs should be observed closely for symptoms or
signs of thrombosis, embolism, DIC or myocardial infarction.
In peri- or post-operative patients, patients at risk of thromboembolic events or DIC, patients
with a history of coronary artery disease or patients with liver disease, the potential benefit of
Prothrombinex®-VF should be weighed against the risk of thromboembolism.
Pathogen Safety
This product is made from human plasma. Products made from human plasma may contain
infectious agents, such as viruses and theoretically Creutzfeldt-Jakob Disease (CJD) agents,
that can cause disease. The risk that such products will transmit an infectious agent has been
reduced by screening plasma donors for prior exposure to certain infectious agents and by
testing for the presence of certain viral markers.
In addition, virus removal and inactivation procedures are included in the manufacturing
process. The procedures applied in the manufacture of this product are effective against
enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B and hepatitis C
viruses (HBV and HCV), and non-enveloped viruses, such as hepatitis A (HAV). These
procedures may have some effect against non-enveloped viruses such as human
Prothrombinex-VF HK PI 2.00
Despite these measures, such products may still potentially transmit disease. There is also the
possibility that other known or unknown infectious agents may be present in such products.
Vaccination for patients in receipt of medicinal products from human plasma should be
considered where appropriate.
Effects on fertility
The effects of Prothrombinex®-VF on fertility are unknown.
Use in pregnancy
The safe use of Prothrombinex®-VF during pregnancy has not been established in clinical
Use in lactation
The safe use of Prothrombinex®-VF during lactation has not been established in clinical
Paediatric use
The safe use of Prothrombinex®-VF in the paediatric population has not been established in
clinical studies.
Prothrombinex®-VF should be used with caution in neonates, in whom immature hepatic
function may lead to delayed clearance of activated coagulation factors and an increased risk
of thrombotic complications.
Use in the elderly
The safe use of Prothrombinex®-VF in the elderly has not been established in clinical studies.
The effects of Prothrombinex®-VF on carcinogenicity are unknown.
The effects of Prothrombinex®-VF on genotoxicity are unknown.
Prothrombinex-VF HK PI 2.00
Interactions with other medicines
The interaction of Prothrombinex®-VF with other drugs has not been established in specific
The use of Prothrombinex®-VF with tranexamic acid is not recommended since only limited
data are available on the concomitant administration of prothrombin complex products and
antifibrinolytic agents.
Effects on laboratory tests
Prothrombinex®-VF is formulated with heparin sodium and antithrombin III. Therefore, the
results of coagulation tests should be interpreted with care.
ADVERSE EFFECTS
Allergic or anaphylactic-type reactions can occur in PCCs such as Prothrombinex®-VF (see
Although low, there is a potential risk of thromboembolic episodes (including myocardial
infarction) following the administration of a PCC such as Prothrombinex®-VF. This risk is
increased in patients predisposed to thrombosis, or in patients receiving repeated or high
doses. Thrombotic events, particularly pulmonary embolism, may result in a fatal outcome (see
In the post marketing period from 2001 spontaneous reporting of adverse events has been
rare. Post-marketing reporting of adverse reactions is voluntary and from a population of
uncertain size, and consequently it is not always possible to reliably estimate the frequency of
these reactions or establish a causal relationship to product exposure.
The adverse reactions in
Table 3 are based on post-marketing experience of
Prothrombinex®-VF and the previous generation product, Prothrombinex®-HT.
Prothrombinex-VF HK PI 2.00
System Organ Class
Adverse Drug Reaction
Blood & Lymphatic Disorders
Hypercoagulability,
Disseminated Intravascular Coagulation (DIC)
Immune System Disorders
Anaphylactic reaction
Vascular Disorders
Thrombosis (potentially including deep vein thrombosis, myocardial infarction and cerebral infarction)
Respiratory, Thoracic & Mediastinal
Pulmonary Embolism
Disorders
Skin & Subcutaneous Tissue Disorders
General Disorders & Administration Site
Injection site reaction
Conditions
Other reactions may include somnolence, phlebitis, vasodilation, dyspnoea, vomiting, pain,
fever, feeling cold and peripheral oedema.
DOSAGE AND ADMINISTRATION
It is recommended that specialist guidelines are referred to when administering
Prothrombinex®-VF. The recommended dosages of Prothrombinex®-VF are expressed in units
(IU) of factor IX per kg body weight (bw).
Acquired PCC Deficiency – Warfarin Reversal
Elevated INR with or without bleeding
Clinical strategies concerning the management of warfarin reversal are based on the WRCG.
There is a close relationship between INR and risk of bleeding. The risk of bleeding increases
noticeably once INR exceeds 4. Management options will depend on the INR level and
whether bleeding is present. The choice of strategy should be based on clinical judgement, and
may include: stopping warfarin therapy, treating with vitamin K and replacing coagulation
factors with a PCC and/or FFP. It should be noted that no randomised clinical trials have
compared strategies in terms of clinical outcomes.
Management of elevated INR in adult patients with or without bleeding is detailed in
Table 4.
Prothrombinex-VF HK PI 2.00
Clinical Setting
Action / Strategy
Cease warfarin therapy, give 5–10 mg vitamin K1 intravenously, as well as
significant bleeding
Prothrombinex®-VF 25–50 IU/kg and FFP 150–300 mL.
where warfarin-induced
Assess patient continuously until INR <5 and bleeding stops.†
coagulopathy is considered a
Prothrombinex®-VF can be used alone if FFP is unavailable; management
contributing factor
should otherwise continue as described.§
INR >9; bleeding
Cease warfarin therapy, give 1 mg vitamin K1 intravenously.
absent, at high risk of
Consider Prothrombinex®-VF 25–50 IU/kg and FFP 150–300 mL.
Measure INR in 6–12 hours, resume warfarin therapy at a reduced dose once INR <5.
Important points:
Bleeding risk increases exponentially from INR 5 to 9, therefore INR ≥6 should be monitored closely.
Vitamin K1 effect on INR can be expected within 6–12 hours.
† In all situations carefully reassess the need for ongoing warfarin therapy. § FFP (10–15 mL/kg) can be used alone if Prothrombinex®-VF is unavailable; management should otherwise continue as described. ‡ Bleeding risk is high in patients with active GI disorders, receiving concomitant antiplatelet therapy, who have undergone major surgery in the two weeks prior, and those with a low platelet count.
Invasive procedures
The individual patient's risk of thromboembolism should be considered prior to any invasive
procedure. PCC use is not recommended among high thromboembolic risk patients, such as
those with prosthetic heart valves, or those who have suffered an acute thrombosis within the
preceding three months (i.e. a recent pulmonary embolism or extensive venous thrombosis).
Such patients should receive intravenous or subcutaneous bridging anticoagulation in the peri-
and post-operative period. Prolonged immobility during surgery and afterwards also increases
the risk of venous thromboembolism.
Patients at low thromboembolic risk such as those receiving warfarin because of atrial
fibrillation, or in whom the index event requiring anticoagulation occurred more than three
months ago, can be managed without bridging anticoagulation.
Management of oral anticoagulation during invasive procedures among patients with a low
risk of thromboembolism is detailed in
Table 5.
Prothrombinex-VF HK PI 2.00
Patients at relatively low risk of thromboembolism
Withhold warfarin therapy 4–5 days before surgery.
Night before surgery: If INR >2, give 1–5 mg vitamin K1 intravenously.
Day of surgery: If INR ≤1.5 surgery can proceed. If INR >1.5, defer surgery, or if surgery is urgent, give Prothrombinex®-VF (25–50 IU/kg) plus 150–300 mL FFP§
Start warfarin therapy on the day of surgery, at the previous maintenance dose.
Employ thromboprophylaxis as per usual practice.
§ or give 10–15 mL/kg of FFP if Prothrombinex®-VF is not used
Whether for elevated INR with or without bleeding or invasive procedures, it is essential that
clinical signs of bleeding and laboratory results (INR) are monitored.
Congenital Deficiency of Factors II, IX and X
The dosage and duration of the substitution therapy depend on the severity of the coagulation
disorder, on the location and extent of the haemorrhage and on the clinical condition of the
The initial dose of a specific coagulation factor may be estimated from the recovery of that
factor. In the absence of recovery data for Prothrombinex®-VF, it is recommended that the
recovery data in the SPC be used:
1 IU of factor II per kg bw (IU/kg) raises the plasma factor II activity by 0.02 IU/mL,
1 IU/kg of factor VII raises the plasma factor VII activity by 0.01 IU/mL, 1 IU/kg of
factor IX raises the plasma factor IX activity by 0.01 IU/mL and 1 IU/kg of factor X raises
the plasma factor X activity by 0.017 IU/mL.
The calculation is as follows:
Dose (IU) = Body Weight (kg) x Desired Factor Rise (IU/mL) x the reciprocal of the
estimated recovery
For example, for a factor X deficiency:
Dose (IU) = Body Weight (kg) x Desired Factor Rise (IU/mL) x 60
Prothrombinex-VF HK PI 2.00
The exact loading and maintenance doses and dosing intervals should be based on the patient's
clinical condition, response to therapy and plasma factor concentration. Maintenance doses
should gradually reduce over the period of treatment (from the higher end of the range to the
lower). Laboratory tests should be performed to ensure that the desired factor levels are
Congenital Deficiency of Factor IX (Haemophilia B)
The recommendations for doses in
Table 6 are provided only as a general guideline for
therapy. Treatment may need to be repeated at varying intervals to maintain the required
concentration of factor IX in the plasma. Thrombotic problems may occur if the suggested
maximum dose is exceeded, however in some circumstances larger amounts than those
calculated may be required (in terms of an initial loading dose).
Indication
Desired plasma
Dose (IU/kg)
Frequency of
Duration of
concentration of
dosing (per day)
treatment (days)
factor IX
Minor haemorrhage
Moderate to severe
For long term prophylaxis against bleeds in patients with congenital factor IX deficiency, doses
of 25 to 40 IU of factor IX per kg bodyweight can be given twice weekly.
It is recommended that plasma factor IX concentrations be monitored during the treatment
Patients requiring more than 4 to 5 days of treatment with Prothrombinex®-VF should be
monitored carefully for signs of thrombosis or DIC.
1. Before reconstitution, allow the vials of Prothrombinex®-VF and Water for Injections to
reach a temperature between 20°C and 30°C.
Prothrombinex-VF HK PI 2.00
2. Remove the caps from the tops of the Prothrombinex®-VF and Water for Injections vials.
3. Apply a suitable antiseptic to the exposed part of the rubber stoppers of both
Prothrombinex®-VF and Water for Injections and allow to dry.
4. Open the outer package of the Mix2Vial™ filter transfer set by peeling away the lid. If the
seal of the lid is not intact or there are any concerns about the integrity of the
Mix2Vial™, do not use it but return it to the appropriate Blood Tranfusion Service.
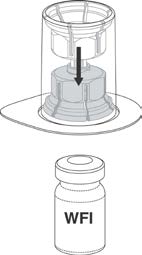
Place the Water for Injections on a level surface and hold the vial firmly. Take the
Mix2Vial™ together with its outer package and invert it. Push the blue plastic cannula of
the Mix2Vial™ firmly through the rubber stopper of the Water for Injections. See
Figure 1. (20 mL Water for Injections is provided for 500 IU vial).
WFI = Water for Injections
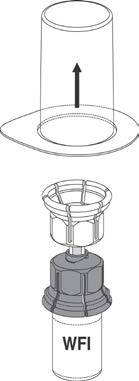
5. While holding onto the vial of Water for Injections, carefully remove the outer package
from the Mix2Vial™, being careful to leave the Mix2Vial™ attached firmly to the Water for
Injections vial. Ensure that only the package and not the Mix2Vial™ is removed. See
Figure 2.
Prothrombinex-VF HK PI 2.00
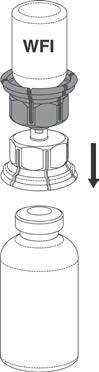
6. With the Prothrombinex®-VF vial held firmly on a level surface, invert the Water for
Injections with the Mix2Vial™ attached and push the transparent plastic cannula end of the
Mix2Vial™ firmly through the Prothrombinex®-VF stopper. See Figure 3. The water will
be drawn into the vial by the vacuum within. In the unlikely event that the vial does not
contain a vacuum, do not use the product, but return it to the appropriate Blood
Tranfusion Service.
7. With the Water for Injections and Prothrombinex®-VF vial still attached, gently swirl the
product vial to ensure the product is fully dissolved. Avoid excessive frothing. A clear or
slightly opalescent solution is usually obtained in 10 minutes or less. The solution should
be used immediately as described under Administration.
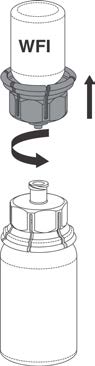
8. Once the contents of the Prothrombinex®-VF vial are completely dissolved, firmly hold
both the transparent and blue parts of the Mix2Vial™. Unscrew the Mix2Vial™ into two
separate pieces (see Figure 4), and discard the empty Water for Injections vial and the
blue part of the Mix2Vial™ in an appropriate waste container.
The Mix2Vial™ is intended to filter the contents of a single vial of
Prothrombinex®-VF only.
If multiple vials of Prothrombinex®-VF are to be administered, a separate Mix2Vial™
must be used for each vial.
Do not refrigerate Prothrombinex®-VF once it has been reconstituted.
The product does not contain an antimicrobial preservative. It must, therefore, be used
immediately after reconstitution. Any unused solution should be discarded
appropriately. Use in one patient on one occasion only. If a clot or gel forms, do not use
the product but return it to the appropriate Blood Tranfusion Service.
1. With the Prothrombinex®-VF vial upright, attach a plastic disposable syringe to the
Mix2Vial™ (transparent plastic part). Invert the system and draw the reconstituted
Prothrombinex®-VF into the syringe by pulling the plunger back slowly. One large syringe
may be used to pool several vials of reconstituted Prothrombinex®-VF.
Prothrombinex-VF HK PI 2.00
2. Once the Prothrombinex®-VF has been transferred into the syringe, firmly hold the barrel
of the syringe (keeping the syringe plunger facing down) and detach the Mix2Vial™ from
the syringe. Discard the Mix2Vial™ (transparent plastic part) and empty
Prothrombinex®-VF vial in an appropriate waste container. Fit the syringe to a suitable
injection needle to administer the reconstituted Prothrombinex®-VF. Do not use the
Mix2Vial™ for injection.
3. Give the dose slowly (approximately 3 mL per minute or as tolerated by the patient) by
the intravenous route. When the contents of more than one vial are to be given, it may be
convenient to pool the total amount prior to administration in a large syringe or sterile
bag. This must be done aseptically.
4. To reduce microbiological hazard, use as soon as practicable after
reconstitution/preparation. The solution must not be stored and infusion should be
completed within three hours of reconstitution. Any unused portion remaining in the vial
must be discarded appropriately.
5. The solution must not be added to or mixed with any other fluids to be given, including
Spillage or breakages
Should a break in the container or spillage occur, due precautions should be taken to avoid
contamination of cuts and abrasions, as well as to avoid inhalation or swallowing of the
spillage. Adequate disinfection can be obtained with the application of 1% sodium
hypochlorite for 15 minutes. Commercial bleaches may be diluted appropriately to obtain this
OVERDOSAGE
The use of high doses of PCCs has been associated with instances of myocardial infarction,
DIC, venous thrombosis and pulmonary embolism. Therefore, in overdose, the risk of
thromboembolic complications or DIC is enhanced.
Prothrombinex-VF HK PI 2.00
PRESENTATION AND STORAGE CONDITIONS
Prothrombinex®-VF is available in vials containing 500 IU of factor IX, 500 IU of factor II
and 500 IU of factor X. Each single pack contains one vial of product, one 20 mL vial of
Water for Injections and one Mix2Vial™ filter transfer set.
Store at 2°C to 8°C. (Refrigerate. Do not freeze). Protect from light.
Do not use after the expiry date.
NAME AND ADDRESS OF THE MANUFACTURER
CSL Behring (Australia) Pty Ltd
189–209 Camp Road
Broadmeadows VIC 3047
NAME AND ADDRESS OF THE DISTRIBUTOR
Hong Kong Red Cross Blood Transfusion Service
15 King's Park Rise
® Registered trademark of CSL Limited
™ Mix2Vial is a trademark of West or one of its subsidiaries
Prothrombinex-VF HK PI 2.00
Source: http://www.cslbehring.com.au/docs/880/875/Prothrombinex-VF%20HK%20PI%20v.2.00.pdf
MICUSP Version 1.0 - BIO.G0.11.3 - Biology - Final Year Undergraduate - Male - Native Speaker - Report Introduction Numerous types of fungi are able to infect the eye, including Fusarium, Aspergillus, Curvularia, and Candida (4). The fungus responsible for the recent outbreaks of keratitis (infection of the cornea) due to ReNu MoistureLoc use is thought to
This article was downloaded by: [Leo Schilbach]On: 15 September 2011, At: 06:26Publisher: Psychology PressInforma Ltd Registered in England and Wales Registered Number: 1072954 Registered office: MortimerHouse, 37-41 Mortimer Street, London W1T 3JH, UK NeurocasePublication details, including instructions for authors and subscription information:




