Pharmacological treatment of deep brain stimulation-induced hypomania leads to clinical remission while preserving motor benefits

This article was downloaded by: [Leo Schilbach]On: 15 September 2011, At: 06:26Publisher: Psychology PressInforma Ltd Registered in England and Wales Registered Number: 1072954 Registered office: MortimerHouse, 37-41 Mortimer Street, London W1T 3JH, UK
Neurocase
Publication details, including instructions for authors and subscription information:
Pharmacological treatment of deep brain
stimulation-induced hypomania leads to clinical
remission while preserving motor benefits
L. Schilbach a , P. H. Weiss b c , J. Kuhn a , L. Timmermann c , J. Klosterköötter a &
W. Huff aa Department of Psychiatry, University of Cologne, Cologne, Germanyb Institute of Neuroscience and Medicine, Cognitive Neurology Section (INM-3),Research Center Juelich, Juelich, Germanyc Department of Neurology, University of Cologne, Cologne, Germany
Available online: 15 Sep 2011
To cite this article: L. Schilbach, P. H. Weiss, J. Kuhn, L. Timmermann, J. Klosterköötter & W. Huff (2011):
Pharmacological treatment of deep brain stimulation-induced hypomania leads to clinical remission while preserving
motor benefits, Neurocase, DOI:10.1080/13554794.2011.568502
To link to this article:
PLEASE SCROLL DOWN FOR ARTICLE
This article may be used for research, teaching and private study purposes. Any substantial orsystematic reproduction, re-distribution, re-selling, loan, sub-licensing, systematic supply or distributionin any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that thecontents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drugdoses should be independently verified with primary sources. The publisher shall not be liable for anyloss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arisingdirectly or indirectly in connection with or arising out of the use of this material.
NEUROCASE2011, iFirst, 1–8
Pharmacological treatment of deep brain
stimulation-induced hypomania leads to clinical
remission while preserving motor benefits
L. Schilbach1, P. H. Weiss2,3, J. Kuhn1, L. Timmermann3, J. Klosterkötter1, and W. Huff1
1Department of Psychiatry, University of Cologne, Cologne, Germany2Institute of Neuroscience and Medicine, Cognitive Neurology Section (INM-3), Research CenterJuelich, Juelich, Germany3Department of Neurology, University of Cologne, Cologne, Germany
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is an effective treatment for Parkinson's dis-ease, but can lead to adverse effects including psychiatric disturbance. Little is known about the risk factorsand treatment options for such effects. Here, we describe a patient who reproducibly developed stimulation-in-duced hypomania when using ventrally located electrodes and responded well to pharmacological interventionwhile leaving the stimulation parameters unchanged to preserve motor benefits. In spite of clinical remission,[15O]-positron-emission-tomography (PET) demonstrated activation patterns similar to those reported duringmania. This case, therefore, highlights an important treatment option of adverse effects of DBS, but also pointstoward the need for investigations of its risk factors and their underlying neurobiological mechanisms.
Keywords: Subthalamic nucleus; Deep brain stimulation; Parkinson's disease; Stimulation-induced hypomania;
Pharmacological treatment; Positron-emission-tomography (PET).
Deep brain stimulation (DBS) of the subtha-
of these effects, their underlying mechanisms and
lamic nucleus (STN) is an effective treatment
treatment options (e.g., Troster, 2009).
option for idiopathic Parkinson's disease (IPD)
Here, we describe the case of a patient who
and alleviates motor symptoms (Deuschl, Schade-
reproducibly developed hypomanic episodes when
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
Brittinger, Krack, Volkmann, Schafer, Botzel, et al.,
switching STN-DBS from ‘dorsal' to ‘ventral' stim-
2006). Adverse effects of STN-DBS, however, are
ulation sites (Figure 1a & b; ‘dorsal' stimula-
also known and include severe psychiatric distur-
tion shown in blue, ‘ventral' stimulation shown
bance (Appleby, Duggan, Regenberg, & Rabins,
in red). Due to significant motor impairment
2007; Soulas, Gurruchaga, Palfi, Cesaro, Nguyen,
during the former, we opted for pharmacolog-
& Fenelon, 2008; Voon, Krack, Lang, Lozano,
ical treatment of the stimulation-induced hypo-
Dujardin, Schupbach, et al., 2008). Relatively little
mania. While the hypomanic syndrome could be
is known about the risk factors for the occurrence
well controlled pharmacologically, [15O]-positron-
L.S. gratefully acknowledges Esther Florin's help in anatomically localizing the electrodes by means of image fusion. L.S. is also
grateful to Carolin Urbach and Eun-Hae Kim for their involvement in patient care and to David Sharp for helpful comments on anearlier version of the manuscript. The authors thank the PET group of the Physics of Medical Imaging Section of the Institute ofNeuroscience and Medicine (INM-4, Research Centre Juelich), especially Professor Hans Herzog, for their expert assistance.
The authors have no conflicts of interests to declare.
Address correspondence to Leonhard Schilbach, M.D., Department of Psychiatry, University of Cologne, Kerpener Str. 62, 50924
Cologne, Germany. (E-mail: [email protected]).
� 2011 Psychology Press, an imprint of the Taylor & Francis Group, an Informa business
SCHILBACH ET AL.
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
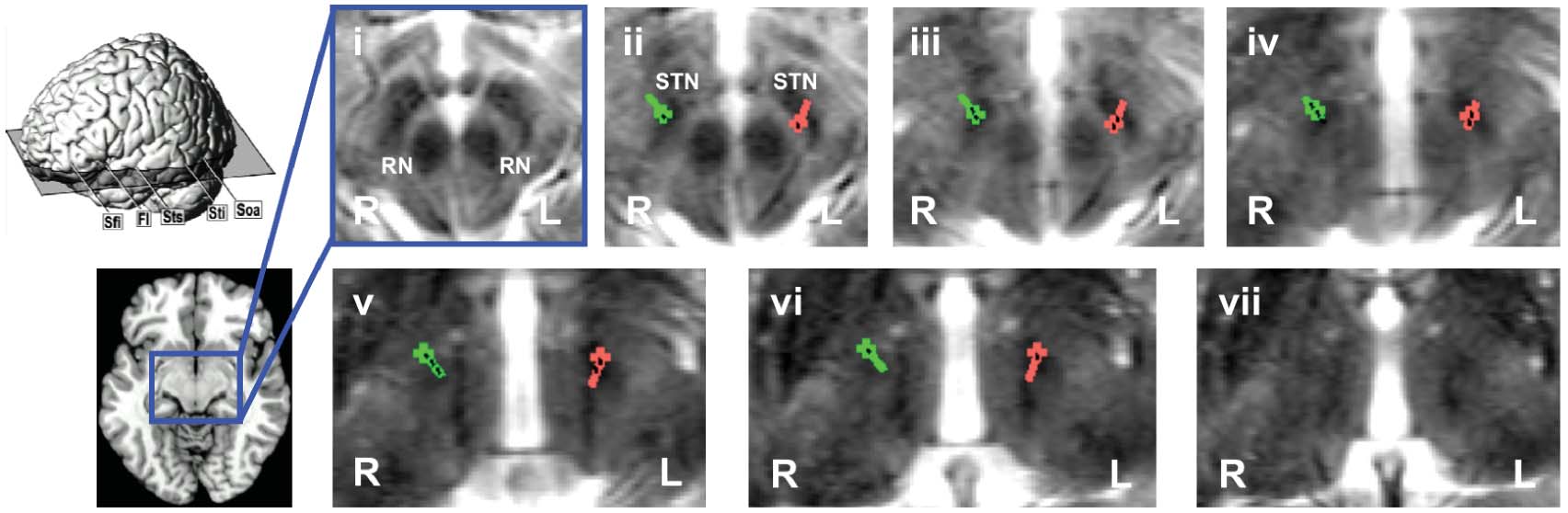
Figure 1. (a) Quadripolar electrodes (Medtronic® model 3387) used for DBS in patient KS illustrating ‘ventral' (red) vs. ‘dorsal' (blue)
stimulation. Numbers denote the different contacts of each electrode that can be used for stimulation. (b) Localization of electrodes in KS
(right electrode shown in green, left electrode shown in red; electrode contacts shown in black) as assessed by postoperative stereotactic
X-ray imaging illustrated by means of overlays onto ascending axial slices (i–vii) of the preoperative T2 weighted MRI. The level of the
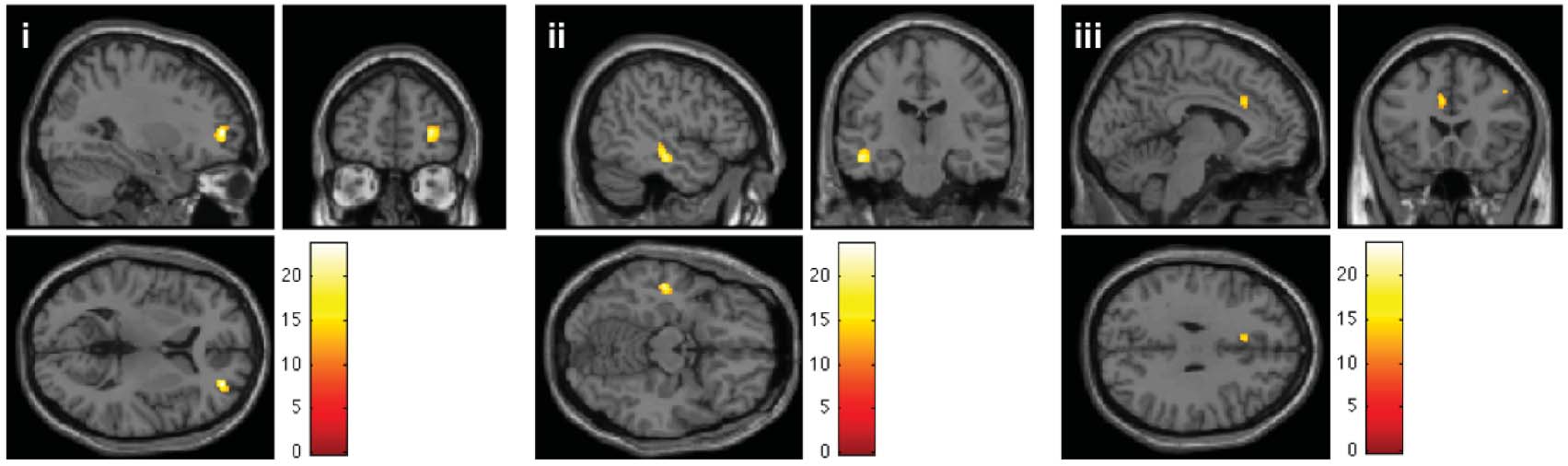
axial slices is shown schematically on the left hand side. RN: Red nucleus; STN: Subthalamic nucleus. (c) Neural correlates of ‘ventral' as
compared to ‘dorsal' stimulation of STN in KS (thresholded at p < .05 FWE corrected for multiple comparisons). Differential increase
of neural activity in (i) DLPFC (Right middle frontal gyrus∗; MNI: 30, 5, 6; k=164; T=23.64 & MNI: 46, 16, 40; k=20; T=15.54),
(ii) left MTG∗ (MNI: −48, −20, −14; k=117; T=21.48) and (iii) dACC (Left anterior cingulate cortex∗; MNI: −6, 22, 30; k=37;
T=15.54). Common activations for ‘dorsal' as compared to ‘ventral' stimulation were observed in left lingual gyrus∗ (not illustrated;
MNI: −22, −72, −2; k=42; T=19.63). ∗Anatomy assigned by using the SPM Anatomy Toolbox (Eickhoff et al., 2005).
HYPOMANIC EPISODES INDUCED BY DEEP BRAIN STIMULATION
emission-tomography (PET) performed during
(50 mg/12.5 mg/day), ropinirole (8 mg/day), aman-
clinical remission several weeks later demonstrated
tadine (2× 50 mg/day), valproate (450 mg/day) and
stimulation-dependent neural activation differences
clozapine (150 mg/day).
similar to those reported during clinically manifest
The past medical history revealed a diagnosis of
idiopathic Parkinson's disease (IPD) at the age of32 which had been made due to a typical clinicalpresentation and in spite of a history of paranoid
CASE REPORT
psychosis. Psychosis had been diagnosed at the ageof 23 and led to three other acute episodes at the age
Patient KS was a 48-year-old man referred for
of 24, 28 and 41. First-generation antipsychotics
admission to our inpatient psychiatric unit for
were used briefly during the acute episodes that
evaluation and management of a manic syndrome
required hospitalization, while long-term treatment
which had developed after the STN-DBS stimu-
consisted of second-generation drugs. After the
lation parameters had been changed from ‘dor-
last episode requiring hospitalization clozapine was
sal' to ‘ventral' stimulation several weeks before
used continuously. After good response to L-DOPA
(Figure 1a). Using a more symmetrical ventral stim-
medication and a stable course of treatment of
ulation (electrodes 1 & 5) had not been effective
IPD for almost 10 years, the patient developed on-
in alleviating motor impairment. After having been
off fluctuations and pronounced dyskinesia. Three
changed to the ‘ventral' stimulation (Figure 1a),
years later, off-states comprised 30% of the day leav-
the patient reported elation and developed symp-
ing the patient immobile during these periods. In
toms of grandiosity, insomnia, racing thoughts and
light of the progressive worsening of Parkinsonian
increased speed of speech. Reports by family mem-
symptoms, a lack of benefit from medication and
bers indicated conflicts resulting from the patient's
no further psychotic episodes since 2002, the rec-
aberrant behavior and lack of proper judgment with
ommendation for DBS was given by a neurolo-
respect to financial activities. Due to these difficul-
gist outside our department in 2007. Thereupon
ties KS was seen by a neurologist, who re-set the
implantation of bilateral quadripolar electrodes
DBS to the dorsally located electrodes (Figure 1)
was performed. Upon preoperative neuropsycho-
and had the patient transferred to the hospital.
logical assessment, executive function, attention
Upon admission – a few hours after STN-
and memory were normal. STN-DBS implantation
DBS had been re-set from the ‘ventral' to ‘dor-
resulted in an improvement of motor impairments.
sal' stimulation the mental status of the patient
Several months after the implantation, the patient
had changed significantly: All symptoms charac-
developed a right-sided subdural hematoma, which
teristic of mania had subsided and the patient
required surgical intervention. After the operation,
was found to be cooperative and calm during the
the patient made a full recovery and lead loca-
evaluation. His speech was found to be of nor-
tion was controlled by means of neuroimaging.
mal speed and fluency. He described his mood
Subsequently, bilateral stimulation of STN resulted
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
as ‘lower than in the past days' and did not
in significant improvement of motor impairment
appear irritable. His affect was euthymic and con-
comparable to the benefit observed prior to the
gruent with his mood. His thought processes were
subdural hematoma. Over the next two years, how-
somewhat circumstantial, but there was no loos-
ever, the DBS had to be adjusted to rely more on
ening of associations. His self-attitude was nor-
the ventral contacts due to the progressive develop-
mal, and he exhibited adequate judgment of the
ment of significant motor impairment (Figure 1a).
current situation. He scored 30 of 30 on the
Switching to these contacts, however, also lead to
Mini-Mental State Examination. Abnormalities
the development of two hypomanic episodes that
upon neurological examination included significant
required hospitalization. During each episode, volt-
motor impairment manifest in a hypokinetic–rigid
age applied to the ventral contacts was reduced
syndrome including a resting tremor of the left
to limit the hypomanic syndrome, but eventually
hand and predominantly left-sided rigidity mak-
STN-DBS had to be re-set to the dorsal contacts.
ing the patient wheelchair-bound. Routine blood
Concomitantly, however, a significant worsening of
work, a toxicology screening, EKG and EEG did
motor symptoms was observed.
not show any significant abnormalities. Medication
Due to the motor impairment apparent upon
upon admission consisted of levodopa & car-
admission and in agreement with the patient,
bidopa (5 × 50 mg/day), levodopa & benserazide
STN-DBS was again switched to the ‘ventral'
SCHILBACH ET AL.
stimulation (Figure 1a) as this configuration had
a cane, began to speak more quickly and appeared
been most successful in treating motor symptoms
more animated and expressive in mimic and ges-
and in spite of the fact that this stimulation setting
ture. He also described a sense of invigoration and
had previously led to stimulation-induced hypoma-
improvement in mood. Within days the changes in
nia. Within minutes, a significant improvement of
cognition and behavior became more pronounced,
motor functions and change in mental status could
finally resulting in a hypomanic syndrome (as mea-
be observed. KS was able to walk with the help of
sured by the Young Mania Rating Scale; Figure 2a).
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
Figure 2. (a) Observed change in motor function (as assessed by the UPDRS-III; higher scores indicating more pronounced motor
impairment) and mental status (as assessed by the YMRS; <5: Clinical remission, >12: Hypomania, >20: Mania) during the first two
weeks of treatment after the change of stimulation (flash) from dorsal to ventral electrodes on the day of admission. (b) Daily dosages
of anti-manic medication (VPT: Valproate; CLZ: Clozapine) used to treat stimulation-induced change of mental status during the first
two weeks of treatment. (c) Blood serum levels of anti-manic and antipsychotic drugs (VPT: Valproate; CLZ: Clozapine) measured at
different time points. Therapeutic range of VPT: 50–100 mg/l. Therapeutic range of CLZ: 350–600 µg/l.
HYPOMANIC EPISODES INDUCED BY DEEP BRAIN STIMULATION
In order to preserve the motor benefits (as mea-
LEFTcontact6: −11.2, −0.5, −1.7; LEFTcontact7:
sured by the Unified Parkinson Disease Rating
−11.8, 0.9, −0.2).
Scale-III; Figure 2a), we decided to leave the stimu-lation parameters unchanged this time and optedfor pharmacological treatment. The dosage of
PET scanning
valproate and clozapine were increased while leav-ing the anti-parkinsonian medication unchanged
The patient was accompanied to the scanning site
(Figure 2b & c). This adjustment resulted in a
by the first author (LS). Upon arrival at about
remission of the hypomanic syndrome within the
08.00 h the patient was familiarized with the details
next week while preserving most of the motor ben-
of the scanning procedure by the second author
efits (Figure 2a).
(PW), YMRS and UPDRS ratings were carried
In spite of the clinical remission, psychopatho-
out (YMRS: 5 UPDRS-III: 14) and informed con-
logical differences in KS continued to be observ-
sent was obtained. Approval for the procedure had
able depending upon the stimulation site: This was
been obtained from the University ethics commit-
assessed eight weeks later and reproducibly demon-
tee and the regulatory authorities (Bundesamt fuer
strated ‘dorsal' stimulation to result in a decrease
Strahlenschutz) gave permission to administer the
of psychomotor function and lowering of his mood,
radioactive substances. The first six PET measure-
while switching back to ‘ventral' stimulation would
ments were carried out starting at 09.00 h during
increase his rate of speech and his subjective sense
which DBS continued making use of the ventral
of well-being immediately. Based upon this clin-
stimulation settings. At 12:30 h, the stimulation
ical insight and in light of studies, which have
parameters were changed to the dorsal settings. At
demonstrated wide-spread activation differences
14:30 h, YMRS and UPDRS ratings were again
as a result of STN stimulation during clinically
carried out (YMRS: 0 UPDRS-III: 25). The second
manifest mania (e.g., Mallet, Schupbach, N'Diaye,
six PET measurements were carried out starting
Remy, Bardinet, Czernecki, et al., 2007), we utilized
at 15:00 h. rCBF was measured by recording the
PET to investigate regional cerebral blood flow
regional distribution of cerebral radioactivity after
(rCBF) during ventral vs. dorsal STN-DBS. This
the intravenous injection of [15O]-water. The PET
study demonstrated a differential increase of rCBF
measurements were carried out using an ECAT
in right dorsolateral prefrontal cortex (DLPFC),
EXACT HR+ scanner (CTI Siemens, Knoxville,
right middle temporal gyrus (MTG) and dorsal
TN), with a total axial field of view of 155 mm
anterior cingulate cortex (dACC) (Figure 1c).
covering the whole brain. Data were acquired inthree-dimensional mode with interdetector colli-mating septa removed and a Neuro-Insert installed
to limit the acceptance of events originating fromout-of-field-of-view activity (i.e., the whole body).
Anatomical localization of electrodes
For each measurement of rCBF, 555 MBq of [15O]-
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
and stimulation sites
water were given intravenously as a bolus injection.
The patient was subjected to a radiation dose of
In order to assess the precise anatomical local-
7.7 mSv (effective dose) during the entire course
ization of the different stimulation sites, i.e., the
of the PET measurement. Twelve PET scans were
location of the quadripolar electrodes within STN
collected, each beginning when the brain activity
bilaterally, the stereotactic coordinates of the elec-
exceeded a threshold of 5 kilo counts per second
trodes as assessed by post-operative imaging were
(kcps) above the background level. Emission data
used to generate an overlay onto the individual
were thereafter collected sequentially over 40 s.
preoperative MRI scan that had been coregistered
This process was repeated for each emission scan,
to the stereotactic cerebral computed tomography
with 10 min between scans to allow for the ade-
(CCT) (Figure 1b). Schaltenbrand–Wahren atlas
quate decay of radioactivity. All emission scan data
(SWA) coordinates (x,y,z) were determined for all
were corrected for scattered events and for radiation
4 contact sites of the left and right electrode
attenuation by means of a transmission scan taken
(RIGHTcontact0: 11.0 −1.4 −2.9; RIGHTcontact1:
prior to the first emission measurement. The cor-
11.7, −0.2, −1.5; RIGHTcontact2: 12.3, 1.0, 0.0;
rected data were FORE rebinned and reconstructed
RIGHTcontact3: 13.1, 2.2, 1.5; LEFTcontact4: −10.0,
into 63 transverse images (separation 2.4 mm) of
−3.2, −4.6; LEFTcontact5: −10.5, −1.9, −3.1;
128 × 128 pixels (size 2.0 × 2.0 mm2) by
SCHILBACH ET AL.
two-dimensional filtered back projection (DIFT)
resulting from other etiologies. Likewise, the
using a Shepp filter with a width of 6 mm. The
syndrome in KS did respond well to pharmaco-
reconstructed PET images had a resolution of 7 mm
logical intervention. We opted for pharmacolog-
and were regarded to represent rCBF qualitatively.
ical treatment to control the symptoms in orderto preserve the motor benefit that resulted from‘ventral' stimulation. Even during clinical remis-
sion occurring as a result of pharmacological treat-ment, however, differential stimulation effects on
All calculations and image manipulations were
psychopathology continued to be noticeable. In
performed on a Transtec Linux cluster using
accordance with these observations, PET imaging
MATLAB version 6.5 (The Mathworks Inc.,
performed months later demonstrated a differen-
Natick, MA). Statistical parametric mapping
tial increase of neural activity in DLPFC, MTG
and dACC for ‘ventral' as compared to ‘dorsal'
Imaging Neuroscience, London, UK; http://www.
STN-DBS. This pattern of activation serves as evi-
fil.ion.ucl.ac.uk/spm/software/spm5) was used for
dence for widespread changes of brain metabolism
image realignment, normalization, and smooth-
as a result of targeting what has been described
ing (low-pass Gaussian filter of 12 mm) and to
as the ‘limbic' subregion of the STN and is highly
create statistical maps of significant relative rCBF
consistent with findings observed during clini-
changes. The resulting voxel size in stereotactic
cally manifest mania (Mallet et al., 2007; Ulla,
space was 2 × 2 × 2 mm. The stereotactic coor-
Thobois, Lemaire, Schmitt, Derost, Broussolle,
dinates of the voxels of local maximum significant
et al., 2006). Here, it has been described as
changes in relative rCBF within areas of significant
an activation of a subcortico-cortical limbic net-
relative rCBF change associated with the different
work whose modulation can alter mood, atten-
factors were determined. The anatomical localiza-
tional and emotional processes (Mayberg, Lozano,
tion of these local maxima was assessed by refer-
Voon, McNeely, Seminowicz, Hamani, et al., 2005),
ence to MNI space as well as by making use of the
but could also reflect compensatory processing of
SPM Anatomy toolbox. Additional validation of
abnormal behavior (Ulla et al., 2006). More specif-
this method of localization was obtained by super-
ically, our findings seem to be in line with evidence,
imposition of the SPMs maps on the single subject
which suggests that the STN forms part of a net-
MRI template (in MNI space) provided by SPM5.
work, which includes medial and lateral frontalcortex and contributes to cognitive control (e.g.,
Aron, Behrens, Smith, Frank, & Poldrack, 2007).
Alterations of this network could, therefore, possi-
This case report adds to the growing body of evi-
bly contribute to mania-related cognitive changes.
dence suggesting that a considerable number of
Recent evidence, indeed, suggests that STN-DBS
patients treated with STN-DBS experiences signif-
may significantly impact impulse control (Frank,
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
icant psychiatric disturbance (e.g. Appleby et al.,
Samanta, Moustafa, & Sherman, 2007; Halbig, Tse,
2007; Soulas et al., 2008; Voon et al., 2008).
Frisina, Baker, Hollander, Shapiro, et al., 2009),
Furthermore, our case demonstrates that pharma-
which could relate to neurofunctional alterations
cological intervention can be an important treat-
of the above described neurocircuits (Ballanger,
ment option for stimulation-induced hypomania.
van Eimeren, Moro, Lozano, Hamani, Boulinguez,
Even though the risk factors for ‘psychiatric'
et al., 2009). In light of the suggestion of a possi-
adverse effects of STN-DBS are not well under-
ble link between a higher risk of suicide attempts
stood, it has been demonstrated that their occur-
after STN-DBS and stimulation-induced changes
rence may depend upon the exact location with
in impulsivity (Rodrigues, Rosas, Gago, Sousa,
anterior and ventrally located contacts being more
Fonseca, Linhares, et al., 2010), systematic inves-
likely to produce them (e.g., Mallet et al., 2007;
tigations thereof and the underlying neural mech-
Okun, Fernandez, Wu, Kirsch-Darrow, Bowers,
anisms as well as interdisciplinary approaches to
Bova, et al., 2009). Accordingly, we found evi-
postoperative patient care seem to be warranted.
dence that ‘ventral' as compared to ‘dorsal' stim-
With respect to the exact localization of the
ulation of the STN reproducibly resulted in hypo-
electrodes in the case of our patient, intra-
manic episodes in our patient. Clinically, the
operative recordings and post-operative anatomical
psychopathological features of hypomania induced
localization are consistent with placement of the
by STN-DBS did not differ from manic syndromes
contacts within the STN. In light of the behavioral
HYPOMANIC EPISODES INDUCED BY DEEP BRAIN STIMULATION
responses to the different stimulation settings and
and ventral stimulation vs OFF states would have
their neural correlates as assessed by PET imag-
been helpful to investigate this issue further.
ing, it seems likely that the most ventral con-
With respect to the putative neural mechanisms
tacts target the anterior-medial or ‘limbic' sub-
that underlie mania-associated change in cogni-
region of the STN (Mallet et al., 2007). It is
tion, it is tempting to speculate that STN-DBS-
noteworthy, however, that the ‘ventral' stimula-
induced differences in cognitive function could be
tion included relatively high voltage on the left-
related to changes in dopaminergic neurotransmis-
sided contact, which is closest to the substantia
sion (Hershey, Revilla, Wernle, Gibson, Dowling,
nigra (SN). In light of recent reports by Ulla
& Perlmutter, 2004). Recently, it has been demon-
and colleagues (Ulla et al., 2006; Ulla, Thobois,
strated that DBS can lead to increased levels of
Llorca, Derost, Lemaire, & Chereau-Boudet, 2010),
dopamine in parts of the subcortico-cortical loops
the possibility must, therefore, be raised that the
that are targeted. Furthermore, this increase of
observed effects of ‘ventral' stimulation could also
dopamine may be accompanied by an increase
be due to current spread to neighbouring regions
of impulsive behavior (Sesia, Bulthuis, Tan, Lim,
such as the SN. In this respect, the resemblance
Vlamings, Blokland, et al., 2010). Here again, the
of the activation pattern resulting from bilateral
previous history of a paranoid psychosis – known
stimulation of the SN described by Ulla et al.
to be associated with alterations of the mesolim-
(2006) and our activation results is informative.
bic dopamine system – appears to be of crucial
Additional neuroimaging data (e.g., high-resolution
importance. In light of this diagnosis, it makes sense
contrast-based and diffusion-based magnetic res-
to assume that KS may have been even more vul-
onance images) could have been helpful to relate
nerable than other Parkinsonian patients to the
individual contact location to the differential stim-
modulatory effects of STN-DBS on dopaminer-
ulation effects observed in other brain regions (e.g.,
gic neurotransmission. Consistent with the view
Gutman, Holtzheimer, Behrens, Johansen-Berg, &
of DBS-induced alterations of dopaminergic trans-
Mayberg, 2009). However, such data was not avail-
mission possibly contributing to mania-related cog-
able in our patient.
nitive changes, effective pharmacological treatment
In the case of KS, it is interesting to note that
of our patient included the dopamine receptor-
the PET activation differences were observed even
binding agent clozapine.
though he was in clinical remission. In the case
Taken together, this case report demonstrates
of KS, we speculate that this might be related to
that hypomanic episodes induced by STN-DBS
his previous psychiatric history (cf. Bejjani, Damier,
can depend upon the exact stimulation site and
Arnulf, Thivard, Bonnet, Dormont, et al., 1999;
that the resulting states can be controlled phar-
Lilleeng & Dietrichs, 2008). In light of this, it
macologically. The latter may be clinically rele-
might seem plausible that KS has a neurobiolog-
vant when motor improvement and hypomanic side
ical predisposition to show an activation pattern
effect result from stimulation of the same elec-
that is also observed in bipolar disorder (Blumberg,
trode contacts. Given that therapeutic adjustments
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
Stern, Martinez, Ricketts, de Asis, White, et al.,
to adverse effects of DBS conventionally involve
2000) and stimulation-induced, clinically manifest
changes of stimulation sites and parameters, our
mania (Ulla et al., 2006), but longitudinal inves-
finding suggests a clear benefit of pharmacologi-
tigations would be necessary to investigate this.
cal intervention. Furthermore, the here-described
Furthermore it must be noted that relatively little
case demonstrates that stimulation-dependent acti-
evidence appears to exist about the neural cor-
vation differences, previously reported as the neural
relates of differential STN stimulation and their
correlate of mania-related behavioral alterations,
impact on cognition (Kalbe, Voges, Weber, Haarer,
can be observed after clinical remission. We suggest
Baudrexel, Klein, et al. 2009; Hirano, Eckert,
that this highlights the need for investigations into
Flanagan, & Eidelberg, 2009). Additionally, similar
the risk factors of adverse effects of DBS and their
activation patterns as those observed in the case of
underlying neurobiological mechanisms.
our patient have been reported for effective STN-DBS without mania (Hilker, Voges, Weisenbach,
Kalbe, Burghaus, Ghaemi, et al. 2004). This sug-gests that caution needs to be exercised with respect
Appleby, B. S., Duggan, P. S., Regenberg, A., &
to interpretations of causality. Additional imaging
Rabins, P. V. (2007). Psychiatric and neuropsychiatric
comparisons between dorsal stimulation vs OFF
adverse events associated with deep brain stimulation:
SCHILBACH ET AL.
A meta-analysis of ten years' experience. Movement
Lilleeng, B. & Dietrichs, E. (2008). Unmasking psychi-
Disorders, 22 (12), 1722–1728.
atric symptoms after STN deep brain stimulation in
Aron, A. R., Behrens, T. E., Smith, S., Frank, M. J., &
Parkinson's disease. Acta Neurologica Scandinavica
Poldrack, R. A. (2007). Triangulating a cognitive con-
Supplement, 188, 41–45.
trol network using diffusion-weighted magnetic reso-
Mallet, L., Schupbach, M., N‘Diaye, K., Remy, P.,
nance imaging (MRI) and functional MRI. Journal of
Bardinet, E., Czernecki, V., et al. (2007). Stimulation
Neuroscience, 27 (14), 3743–3752.
of subterritories of the subthalamic nucleus reveals
Ballanger, B., van Eimeren, T., Moro, E., Lozano,
its role in the integration of the emotional and
A. M., Hamani, C., Boulinguez, P., et al. (2009).
motor aspects of behavior. Proceedings of the
Stimulation of the subthalamic nucleus and impulsiv-
Nationall Academy of Science (USA), 104 (25),
ity: Release your horses. Annals of Neurology, 66 (6),
Mayberg, H. S., Lozano, A. M., Voon, V., McNeely, H.
Bejjani, B. P., Damier, P., Arnulf, I., Thivard, L.,
E., Seminowicz, D., Hamani, C., et al. (2005). Deep
Bonnet, A. M., Dormont, D., et al. (1999). Transient
brain stimulation for treatment-resistant depression.
acute depression induced by high-frequency deep-
Neuron, 45 (5), 651–660.
brain stimulation. New England Journal of Medicine,
Okun, M. S., Fernandez, H. H., Wu, S. S., Kirsch-
340 (19), 1476–1480.
Darrow, L., Bowers, D., Bova, F., et al. (2009).
Blumberg, H. P., Stern, E., Martinez, D., Ricketts, S.,
Cognition and mood in Parkinson's disease in sub-
de Asis, J., White, T., et al. (2000). Increased ante-
thalamic nucleus versus globus pallidus interna deep
rior cingulate and caudate activity in bipolar mania.
brain stimulation: The COMPARE trial. Annals of
Biological Psychiatry, 48 (11), 1045–1052.
Neurology, 65 (5), 586–595.
Deuschl, G., Schade-Brittinger, C., Krack, P., Volkmann,
Reck, C., Florin, E., Wojtecki, L., Krause, H., Groiss,
J., Schafer, H., Botzel, K., et al. (2006). A random-
S., Voges, J., et al. (2009). Characterisation of tremor-
ized trial of deep-brain stimulation for Parkinson's
associated local field potentials in the subthalamic
disease. New England Journal of Medicine, 355 (9),
nucleus in Parkinson's disease. European Journal of
Neuroscience, 29 (3), 599–612.
Eickhoff, S. B., Stephan, K. E., Mohlberg, H., Grefkes,
Rodrigues, A. M., Rosas, M. J., Gago, M. F., Sousa,
C., Fink, G. R., Amunts, K., et al. (2005). A new SPM
C., Fonseca, R., Linhares, P., et al. (2010). Suicide
toolbox for combining probabilistic cytoarchitectonic
attempts after subthalamic nucleus stimulation for
maps and functional imaging data. Neuroimage, 25
Parkinson's disease. European Neurology, 63 (3),
(4), 1325–1335.
Frank, M. J., Samanta, J., Moustafa, A. A., & Sherman,
Sesia, T., Bulthuis, V., Tan, S., Lim, L. W., Vlamings, R.,
S. J. (2007). Hold your horses: Impulsivity, deep brain
Blokland, A., et al. (2010). Deep brain stimulation
stimulation, and medication in parkinsonism. Science,
of the nucleus accumbens shell increases impulsive
behavior and tissue levels of dopamine and serotonin.
Gutman, D. A., Holtzheimer, P. E., Behrens, T. E.,
Johansen-Berg, H., & Mayberg, H. S. (2009). A trac-
Soulas, T., Gurruchaga, J. M., Palfi, S., Cesaro, P.,
tography analysis of two deep brain stimulation white
Nguyen, J. P., & Fenelon, G. (2008). Attempted
matter targets for depression. Biological Psychiatry, 65
and completed suicides after subthalamic nucleus
(4), 276–282.
stimulation for Parkinson's disease. Journal of
Halbig, T. D., Tse, W., Frisina, P. G., Baker, B. R.,
Hollander, E., Shapiro, H., et al. (2009). Subthalamic
deep brain stimulation and impulse control in
Troster, A. I. (2009). Neuropsychology of deep brain
Downloaded by [Leo Schilbach] at 06:26 15 September 2011
Parkinson's disease. European Journal of Neurology,
stimulation in neurology and psychiatry. Frontiers in
16 (4), 493–497.
Bioscience, 14, 1857–1879.
Hershey, T., Revilla, F. J., Wernle, A., Gibson, P. S.,
Ulla, M., Thobois, S., Lemaire, J. J., Schmitt, A., Derost,
Dowling, J. L., & Perlmutter, J. S. (2004). Stimulation
P., Broussolle, E., et al. (2006). Manic behaviour
of STN impairs aspects of cognitive control in PD.
induced by deep-brain stimulation in Parkinson's
Neurology, 62 (7), 1110–1114.
disease: Evidence of substantia nigra implication?
Hilker, R., Voges, J., Weisenbach, S., Kalbe, E.,
Journal of Neurology, Neurosurgery & Psychiatry, 77
Burghaus, L., Ghaemi, M., et al. (2004). Subthalamic
(12), 1363–1366.
nucleus stimulation restores glucose metabolism in
Ulla, M., Thobois, S., Llorca, P.M., Derost, P., Lemaire,
associative and limbic cortices and in cerebellum:
J.J., Chereau-Boudet, I., de Chazeron, I., Schmitt, A.,
Evidence from a FDG-PET study in advanced
Ballanger, B., Broussolle, E., Durif, F. (2010). Contact
Parkinson's disease. Journal of Cerebral Blood Flow &
dependent reproducible hypomania induced by deep
Metabolism, 24 (1), 7–16.
brain stimulation in Parkinson's disease: Clinical,
Hirano, S., Eckert, T., Flanagan, T., & Eidelberg, D.
anatomical and functional imaging study. Journal of
(2009). Metabolic networks for assessment of ther-
Neurology, Neurosurgery & Psychiatry [Epub ahead of
apy and diagnosis in Parkinson's disease. Movement
Disorders, 24 (Suppl 2), S725–731.
Voon, V., Krack, P., Lang, A. E., Lozano, A. M.,
Kalbe, E., Voges, J., Weber, T., Haarer, M., Baudrexel, S.,
Dujardin, K., Schupbach, M., et al. (2008). A mul-
Klein, J. C., et al. (2009). Frontal FDG-PET activity
ticentre study on suicide outcomes following subtha-
correlates with cognitive outcome after STN-DBS in
lamic stimulation for Parkinson's disease. Brain, 131,
Parkinson disease. Neurology, 72 (1), 42–49.
Source: http://www.leonhardschilbach.de/publications_files/Schilbach_Neurocase_2011.pdf
Combined hormonal contraceptives Hans-Joachim Ahrendt, Magdeburg Praxis für Frauenheilkunde, Klinische Forschung und Weiterbildung (Clinical research and further education), Magdeburg, Germany Reviewers: Kai J. Bühling, Hamburg e Medicine and Petra Stute, Bern SummaryIn the past years, hormonal contraception underwent sub-stantial development. The dose of ethinylestradiol (EE) hascontinuously been decreased to reduce the risk of venousthromboembolism. Estradiol valerate (E2V), a "natural"
Ethical considerations in biomedical HIV prevention trials UNAIDS/WHO guidance document Cover photos: L Taylor/UNAIDS, S Noorani/UNAIDS UNAIDS/07.28E / JC1349E (English original, July 2007) © Joint United Nations Programme on HIV/AIDS (UNAIDS) 2007. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of



