Lc/ms/ms analysis of the endogenous dimethyltryptamine hallucinogens, their precursors, and major metabolites in rat pineal gland microdialysate
Received: 20 March 2013,
Revised: 20 May 2013,
Accepted: 23 May 2013
Published online in Wiley Online Library
(wileyonlinelibrary.com) DOI 10.1002/bmc.2981
LC/MS/MS analysis of the endogenousdimethyltryptamine hallucinogens, theirprecursors, and major metabolites in ratpineal gland microdialysate
Steven A. Barkera*, Jimo Borjiginb, Izabela Lomnickaa and Rick Strassmanc
ABSTRACT: We report a qualitative liquid chromatography–tandem mass spectrometry (LC/MS/MS) method for the simulta-neous analysis of the three known N,N-dimethyltryptamine endogenous hallucinogens, their precursors and metabolites,as well as melatonin and its metabolic precursors. The method was characterized using artificial cerebrospinal fluid (aCSF)as the matrix and was subsequently applied to the analysis of rat brain pineal gland-aCSF microdialysate. The method de-scribes the simultaneous analysis of 23 chemically diverse compounds plus a deuterated internal standard by direct injection,requiring no dilution or extraction of the samples. The results demonstrate that this is a simple, sensitive, specific and directapproach to the qualitative analysis of these compounds in this matrix. The protocol also employs stringent MS confirmatorycriteria for the detection and confirmation of the compounds examined, including exact mass measurements. The excellentlimits of detection and broad scope make it a valuable research tool for examining the endogenous hallucinogen pathways inthe central nervous system. We report here, for the first time, the presence of N,N-dimethyltryptamine in pineal glandmicrodialysate obtained from the rat. Copyright 2013 John Wiley & Sons, Ltd.
Keywords: N,N-dimethyltryptamines; pineal gland; microdialysis; rat brain; LC/MS/MS
1999), studies have now shown its presence in the centralnervous system, including the pineal gland (Cozzi et al., 2011),
In a recent review of 69 published studies reporting the detection
motor neurons in the spinal cord (Mavlyutov et al., 2012; Cozzi
of purported endogenous hallucinogens [N,N-dimethyltryptamine
et al., 2011) and in the retina (Cozzi et al., 2011). While the en-
(DMT); 5-hydroxy-DMT (HDMT, bufotenine); 5-methoxy-DMT
zyme is present in these tissues, there has yet to be a definitive
(MDMT)] in humans (Barker et al., 2012), it was concluded that
determination of whether DMT is actually synthesized in these
compelling mass spectral evidence exists for the confirmation of
tissues and, if so, how it is utilized or released from the tissues
their presence in certain human biological fluids [cerebrospinal
under normal or altered physiological conditions.
fluid (CSF; DMT and MDMT), blood (DMT and HDMT) and urine(DMT and HDMT)]. There is as yet no definitive information as tothe possible normal or pathophysiological roles of DMT, HDMT
* Correspondence to: S. A. Barker, Department of Comparative Biomedical
or MDMT in humans or other species owing, in part, to the lack
Sciences, School of Veterinary Medicine, Louisiana State University, Baton
of comprehensive methods to detect and unequivocally confirm
Rouge, LA 70803, USA. E-mail:
[email protected]
the presence of these compounds in biological tissues and fluids
a Department of Comparative Biomedical Sciences, School of Veterinary
(Barker et al., 2012). Methodology to adequately assess their
Medicine, Louisiana State University, Baton Rouge, LA, 70803, USA
synthesis and turnover, simultaneously monitoring their precur-sors and metabolites, is also lacking.
Departments of Molecular & Integrative Physiology
Original interest in endogenous hallucinogens, and DMT in
University of Michigan Medical School, Ann Arbor, MI, 48109, USA
particular, was motivated by the hypothesis that these com-
c Department of Psychiatry, University of New Mexico School of Medicine,
pounds had a biochemical role in the heterogeneous disease
Albuquerque, and Cottonwood Research Foundation, Gallup, New
state of psychosis, especially schizophrenia (for a review see
Barker et al., 1981a, Barker et al., 2012). More recently, interestin DMT has been renewed owing to its characterization as a
Abbreviations used: 2MTHBC, 2-methyl-1,2,3,4-THBC; CSF, cerebrospinalfluid; d
ligand for the sigma-1 (Fontanilla et al., 2009; Su et al., 2009)
DMT, N,N-dimethyltryptamine; DMTNO, DMT-N-oxide; HDMT, 5-hydroxy-
and trace amine receptors (Su et al., 2009). Recent studies
DMT; HIAA, 5-hydroxy-IAA; HNATA, 5-hydroxy-N-acetyl-TA; HNMT,
concerning the enzyme responsible for the biosynthesis of these
5-hydroxy-N-methyl-TA; HTA, 5-hydroxy-TA; HTHBC, 6-hydroxy-THBC;
compounds have also drawn further attention. Although the en-
HTRP, 5-hydroxy-tryptophan; IAA, indol-3-acetic acid; INMT, indole-N-methyltransferase; MAO, monoamine oxidase; MDMT, 5-methoxy-DMT;
zyme for the synthesis of the DMTs, indole-N-methyltransferase
MIAA, 5-methoxy-IAA; MNMT, 5-methoxy-N-methyl-TA; MTA, 5-methoxy-
(INMT), was not thought to occur to any significant extent in
TA; MTHBC, 6-methoxy-THBC; NMT, N-methyl-TA; TA, tryptamine; THBC,
brain (Thompson and Weinshilboum, 1998; Thompson et al.,
Biomed. Chromatogr. 2013
Copyright 2013 John Wiley & Sons, Ltd.
S. A. Barker et al.
Given the reported expression of INMT in the mammalian
provided ad libitum. All animal procedures were approved by
pineal gland, the binding of DMT to the sigma-1 and trace amine
the University Committee on Use and Care of Animals at the
receptors, and the necessity for more comprehensive analytical
University of Michigan.
methodology to begin to assess the possible function of theDMTs in vivo, we undertook to develop a protocol to screen for
Pineal microdialysis
the presence of the DMTs, their precursors and metabolites inmammalian body fluids and tissues. We describe here the appli-
Rats were implanted with linear microdialysis probes with a mo-
cation of our method to rat pineal gland microdialysates using
lecular weight cut-off of 13 kDa and a membrane length of
liquid chromatography–tandem mass spectrometry (LC/MS/MS)
12–15 mm, manufactured in-house, as described previously
for the qualitative analysis of the three known endogenous hal-
(see Borjigin and Liu, 2008, for surgical techniques and probe
lucinogens and of 20 compounds that constitute most of their
preparation). The linear probe traversed both the pineal gland
known precursors and major metabolites (Fig. 1), as well as
as well as superficial layers of occipital cortex on either side of
melatonin and its biochemical precursors. The method was
the gland. The pineal location was ascertained in each rat by
developed in artificial CSF (aCSF), which was used as the pineal
the presence of melatonin in the dialysates (Borjigin and Liu,
gland dialysate. The method described uses positive ion
2008). Following a recovery period of 2–3 days, animals were
electrospray ionization, monitoring the protonated molecular
placed in microdialysis chambers. The chambers consisted of
ion (M + 1+) of each targeted analyte and corresponding frag-
enclosed animal housing units equipped with their own lighting,
ment ions (multiple reaction monitoring). It also applies strin-
which was controlled by an on–off timer, and fitted with a venting
gent analytical criteria for the detection and confirmation of
fan. Sample collection was accomplished with the aid of a liquid
these compounds in this matrix, including exact mass determi-
swivel. Pineal microdialysis was performed with aCSF solution
nation using high-resolution MS. The developed protocol was
flowing continuously through the pineal gland at 2 μL/min for 2 h.
applied to pineal gland-aCSF-microdialysates obtained from
All sample collections were performed during daylight hours. Two
freely moving rats. These studies revealed, for the first time,
tubes of dialysate were collected, each of which contained 120 μL
the presence of DMT in pineal gland microdialysate obtained
collected over 1 h (2 μL/min). The pineal dialysates were collected
from the rat.
in microcentrifuge tubes on ice, capped and stored at
2–4 weeks prior to being shipped on dry-ice for further analysis.
Samples experienced a single freeze–thaw cycle prior to analysis.
Materials and methods
Analytical standards
Adult (12 weeks of age), male, Wistar rats, weighing 300–350 g
The following compounds were obtained from a commercial
each, were obtained from Harlan Laboratories (Indianapolis, IN,
source (Sigma Aldrich, St Louis, MO, USA), and were of the highest
USA) and were housed in light–dark conditions of 12:12 h for
available purity (>98%): DMT, MDMT, HDMT, tryptamine (TA), N-
at least one week before experiments. Food and water was
methyl-TA (NMT), 5-hydroxy-TA (serotonin; HTA), 5-hydroxy-N-
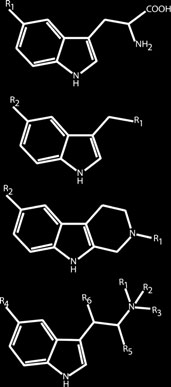
Figure 1. Compounds examined.
Copyright 2013 John Wiley & Sons, Ltd.
Biomed. Chromatogr. 2013
LC/MS/MS of endogenous DMTs in rat pineal gland microdialysate
methyl-TA (HNMT), 5-methoxy-TA (MTA), 5-methoxy-N-methyl-TA
(MNMT), melatonin, tryptophan (TRP), 5-hydroxy-tryptophan
The chromatography system was coupled to an ambient tem-
(HTRP), 5-hydroxy-N-acetyl-TA (HNATA), indol-3-acetic acid
perature electrospray ionization probe on the Quantum Access
(IAA), 5-hydroxy-IAA (HIAA) and 5-methoxy-IAA (MIAA). The
system. For analytes of interest, precursor-to-product ion transi-
following compounds were synthesized (as noted) and their
tions were established through direct infusion of neat standards
structures and purity confirmed (>98%) by LC/MS/MS: DMT-N-
of each compound into the ion source. Analytical standards
oxide (DMTNO), HDMTNO, MDMTNO (N-oxides were prepared
(1 μg/mL) were dissolved in water or methanol and co-infused
as described by Fish et al., 1955), 1,2,3,4-tetrahydro-β-carboline
into the mass spectrometer with mobile phase to obtain optimal
signal and fragmentation patterns/information (see Table 1). The
(HTHBC) and 6-methoxy-THBC (MTHBC) (β-carbolines were
sensitivity was optimized for each compound by manipulating
prepared by Pictet–Spengler reactions of the corresponding
collision energy to achieve the best signals.
amines with formaldehyde according to the methods described
Relevant MS settings for the analysis are shown in Table 1. The
by Ho and Walker, 1964). The internal standard was α,α,β,β-
source conditions were set as follows: 4.0 kV ion spray voltage,
350 °C capillary temperature, sheath gas (N
and was kindly provided by Dr David Nichols (Purdue Univer-
2) pressure of 50 psi.
The resolutions of Q1 and Q3 were set at unit mass. The dwell
sity). The structures of the target analytes are shown in Fig. 1.
time was 10 ms for each multiple reaction monitoring transition.
The Quantum Access system used a tune file, established priorto the analyses being initiated, for DMT as the tune compound.
Solvents and reagents
Solvents for liquid chromatography (LC) were obtained from
Limits of detection and confirmation
Fisher Scientific (Fairlawn, NJ, USA) and were Optima grade(0.1% formic acid in water, 0.1% formic acid in acetonitrile).
Serial dilutions of standards in aCSF (n = 4) were analyzed usingthe Quantum Access LC/MS/MS system to determine the lowestlimit of detection (LOD) and confirmation. All data were basedon a 30 μL injection volume. Detection of the analyte was not
considered positive unless all necessary confirmation criteria
Artificial CSF was prepared in Dr Borjigin's laboratory as
were met: retention time match vs in-run standard and relative
defined by Alzet
retention time to internal standard (±1%), presence of
) and contained NaCl (148 mM), KCl (3 mM),
predetermined fragment ions (two to four for each compound,
CaCl2 · 2H2O (1.4 mM), MgCl2 · 6H2O (0.8 mM), Na2HPO4 · 7H2O
not including the protonated molecular ion) and the agreement
(0.8 mM) and NaH2PO4 · H2O (0.2 mM). This solution was used in
of ion ratios for the analyte vs in-run reference standards, within
the analyses for preparation of standards, method blanks and
±25% relative. Signals for each ion were not considered as
spiked controls. The aCSF used for the mass spectral analyses
detected unless they also exceeded 3 times baseline noise.
was from the same batch as used to perform the microdialyses.
Both blank aCSF and pineal dialysates from rats were examinedfor the presence of interferences. The target analytes were also
Routine analyses were conducted using a Thermo (Thermo
examined to determine if any cross-talk would occur between
Electron North America, West Palm Beach, FL, USA) Quantum
the different compounds, since several of the analytes have
Access LC/MS/MS (triple quadrupole) system equipped with
the same molecular formula.
Accela 600 pumps and a multiplexed Accela open autosampler/injection system. Analyses were conducted using Thermo Scientificsoftware (LC Quan 2.7.0 20, Xcalibur 2.2 SP1).
Sample preparation
No further preparation of the collected pineal dialysate, otherthan thawing, was required. The internal standard (d4-MDMT),
dissolved in 90:10 mobile phase, was added (10 μL) to each sam-
Samples (aCSF dialysate obtained from rats, aCSF blanks, spikes
ple aliquot (50 μL) to give a final concentration of 10 ng/mL.
and controls) were injected (30 μL) onto an Agilent (Santa Clara,
Samples were placed into injection vials, capped, mixed and
CA, USA) Zorbax Eclipse Plus C
held at 4 °C in the dark in the injector tray during the entire
18, 3.0× 100 mm, 3.5 μm particle
size column fitted with a 2.0 μm pre-filter (Grace Davison,
period of the analysis (less than 8 h).
Deerfield, IL, USA). Chromatography of the components was ac-complished using a gradient LC program: solvent A = water–
Additional confirmation methods
0.1% formic acid (Fisher Optima); solvent B = acetonitrile–0.1%formic acid (Fisher Optima); 0–2 min hold at 98% A–2% B,
Analyses for confirmation of DMT employed different fragmen-
2–6 min changing to 50% A–50% B with a 1 min hold, 7–8 min
tation conditions than previously reported, generating four
changing to 2% A–98% B with a 9 min hold, 17–18 min changing
fragment ions (58, 115, 143, 144 m/z) rather than the typical
to 98% A–2% B and holding for 6 min before the next injection.
two ions (58 and 144 m/z) for DMT (Kärkkäinen et al., 2005;
The flow rate was 300 μL/min throughout the analysis. The waste
McIlhenny et al., 2011, 2012).
divert valve was initiated from 0.0 to 1.3 min post injection and
Additional confirmation data for DMT, as well as other com-
again at 15–24 min.
pounds detected, were also obtained using a Thermo LC/
Biomed. Chromatogr. 2013
Copyright 2013 John Wiley & Sons, Ltd.
S. A. Barker et al.
Table 1. MS parameters for analytes and internal standard, average retention times and limits of detection
CE, Collision energy.
Exactive OrbiTrap high-resolution mass spectrometer equipped
Results and discussion
with Accela 1250 pumps and a multiplexed Accela openautosampler/injection system. These analyses were performed on
Compounds selected for analysis
selected samples chosen on the basis of analyte response (inten-
The 23 compounds chosen for analysis represent a significant
sity). Data were collected and processed using Thermo Xcalibur
number of the known metabolites of tryptophan in biological
2.2.0.48 and LC Quan 2.7.0 software. The OrbiTrap system was
species. They also represent the compounds in the specific
tuned using the exact mass of caffeine (M + 1+; 195.08037) as the
pathways related to the potential formation and subsequent
reference/lock mass. Spray voltage was 3.8 kV, capillary tempera-
metabolism of the three known endogenous DMTs. The ability
ture was 300 °C, sheath gas (N2) was set to 25 psi, and the source
to monitor their precursors, metabolites, and related central
heater temperature was 350 °C. The collision-induced-dissociation
nervous system indoleamines, as well as the three DMTs,
function was disabled. The same type of LC column was used for
simultaneously offers a significantly broader opportunity to
LC separation of the samples on the Thermo Exactive instrument
assess the possible presence and role of these compounds
experiments as was used for the experiments conducted on the
individually and as a group than has ever been conducted
Quantum Access, but a different LC program was utilized to further
before (Barker et al., 2012).
assist in confirmation: solvent A = water–0.1% formic acid (Fisher
Each of the DMTs (DMT, HDMT, MDMT) is the biosynthetic
Optima); solvent B = acetonitrile–0.1% formic acid (Fisher Optima);
product of INMT acting on their respective precursors, TA, HTA
0–1 min hold at 90% A–10% B, 1–4 min changing to 50% A–50% B
and MTA, yielding as intermediates NMT, HNMT and MNMT
with a 1 min hold, 6–7 min changing to 2% A–98% B with a 9 min
(for reviews see Barker et al., 1981a, 2012). The DMTs may also
hold, 16–17 min changing to 90% A–10% B and holding for 6 min
be enzymatically demethylated to yield the same mono-
before the next injection. The flow rate was 300 μL/min through-
N-methylated compounds. Several studies have also shown the
out the analysis.
conversion of these precursors and metabolites to the correspond-ing β-carbolines (THBC, 2MTHBC, HTHBC and MTHBC), occurringeither through condensation with formaldehyde or through a
Analyte stability
common intermediate, also occurring during demethylation
All of the compounds examined were tested for stability in solu-
(Barker et al., 1980, 1981a). THBC and MTHBC have been reported
tion (artificial CSF) at
80 °C and 4 °C for up to 6 months and at
as endogenous substances appearing in adrenal and pineal
ambient temperatures for at least 1 week in the dark. Absolute
glands, as well as other tissues (Shoemaker et al., 1978; Barker,
responses vs time were compared to determine changes in
1982; Barker et al., 1984, 1979, 1981b; Kari et al., 1983; Beaton
and Morris, 1984). All of these precursors and products, except
Copyright 2013 John Wiley & Sons, Ltd.
Biomed. Chromatogr. 2013
LC/MS/MS of endogenous DMTs in rat pineal gland microdialysate
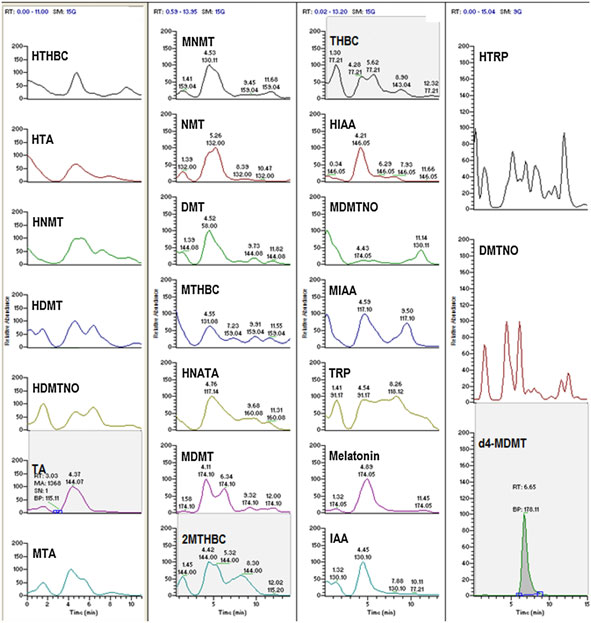
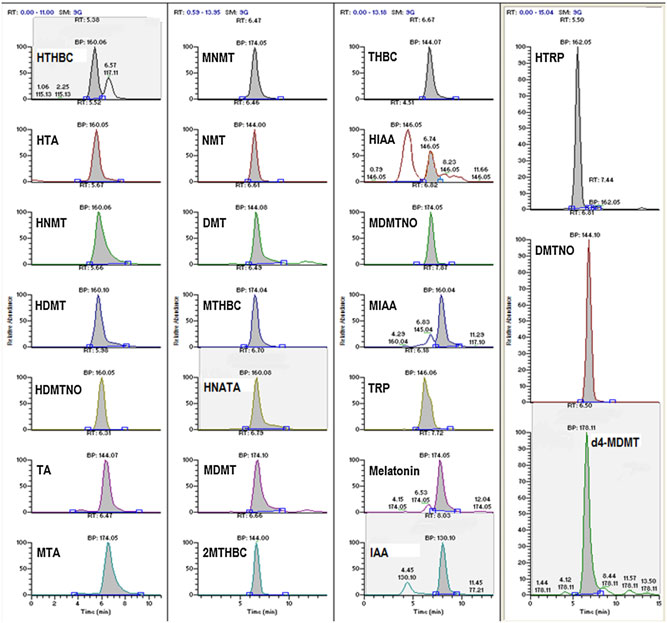
Figure 2A. (A) Chromatogram of artificial CSF (aCSF) fortified with internal standard only (d4-MDMT). (B) Chromatogram of analytes fortified into aCSF.
(C) Multi-reaction monitoring ions selected for the detection and confirmation of compounds found in pineal gland CSF microdialysate illustratingretention times and ion ratios compared with reference standards.
the β-carbolines, are substrates for monoamine oxidase (MAO),
dialysate obtained from the pineal gland, we also monitored
yielding as a product the respective indoleacetic acids, IAA,
the presence of melatonin, HTRP and HNATA.
HIAA and MIAA. Metabolism of the corresponding primary, sec-ondary or tertiary amines by MAO is the predominant pathway
Ion profiles and monitoring
for their degradation. However, the β-carbolines have beenshown to possess MAO inhibition activity.
The mass spectrometer (Quantum Access) settings required to at-
Another major pathway for metabolism of the DMTs is forma-
tain the best response and fragment ion information for each com-
tion of the corresponding N-oxides (DMTNO, HDMTNO and
pound analyzed are given in Table 1 and are typical for such
MDMTNO). Inhibition of MAO in vivo greatly elevates the con-
compounds on this type of instrument. Three compounds gave
centration of the N-oxides relative to the IAAs in the metabolism
at least two diagnostic ions while most of the compounds pro-
of the DMTs, often making them the major metabolites (Sitaram
duced at least three ions for monitoring. Three of the compounds
et al., 1987a–c; Sitaram and McLeod, 1990; Kärkkäinen et al.,
analyzed gave four ions, in addition to the acknowledged source
2005; McIlhenny et al., 2011, 2012; Riba et al., 2012). The N-oxides
of the ions being the monitored protonated molecular weight
are not substrates for monoamine oxidase (Barker et al., 1980,
ion [(M + 1)+]. These protonated molecular ions, their product ions
1981a) and are excreted unchanged in urine (Sitaram et al.,
and their ratios were used to assist in the identification and confir-
mation of the analytes. It should be noted that DMT typically gives
Kynurenine metabolites of tryptophan or the corresponding
only two ions, 58+ and 144+ (Table 1) under previously reported
metabolites of the other indolamines were not included in this
conditions, as observed by us and others (Kärkkäinen et al., 2005;
assay. Recent data generated in vivo suggest that this pathway
McIlhenny et al., 2011, 2012). However, to assist in the confirma-
is not relevant to the metabolism of DMT, and this is also antic-
tion, special conditions for fragmentation were established for
ipated to be the case for the other DMTs (McIlhenny et al., 2011,
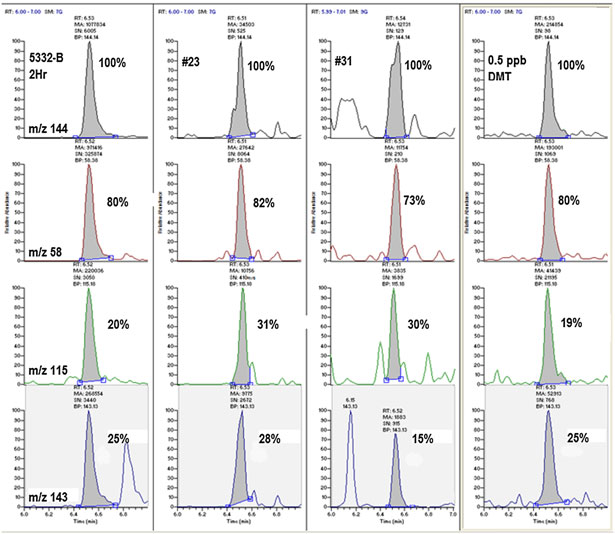
the analysis, with DMT giving four diagnostic ions, 58+, 115+,
2012). Since the analytical study in this case was based on
143+ and 144+ m/z (Table 1 and Fig. 3). The use of more fragment
Biomed. Chromatogr. 2013
Copyright 2013 John Wiley & Sons, Ltd.
S. A. Barker et al.
Figure 2B. (Continued)
ions was deemed necessary since the identification of DMT has
acetonitrile), allowed the target analytes to be sufficiently retained
been controversial (Barker et al., 2012) and low mass ions such as
at the head of the column to permit the elution of salts to occur. As
58+ (m/z) are not always truly diagnostic.
would be expected, the acidic media of the mobile phase lessenedsolution ionization of some of the organic acids being analyzedand produced longer retention times for some of them relative
to that observed for some of the bases. The acids, bases and am-
Average retention time data for the analysis are shown in Table 1.
photerics represented by these 23 compounds were eluted in a
Representative chromatographic data for blank aCSF and ana-
5–9 min window in an order relatively consistent with their polari-
lyte-fortified aCSF (10 ng/mL shown), demonstrating the tempo-
ties in the solvent gradient used. This afforded temporal separation
ral elution of the target analytes, using a representative ion
of most of the components of the analysis. All of the compounds
selected for detection for each compound, are shown in Fig. 2
were resolved by the combination of time and mass.
(A and B), respectively. Representative results for compounds
The mobile phase was varied during the experiments to move
detected in pineal microdialysate samples, illustrating the diag-
the compounds to shorter (more initial acetonitrile) or longer
nostic ions, their ratios and retention times relative to reference
(higher initial water content for a longer period) retention times
standards, are shown in Fig. 2(C).
and to establish optimum conditions. Pineal dialysate samples
The mobile phase gradient developed afforded the ability to al-
shown to be positive for some of the target analytes were treated
low salts, which are in millimolar concentrations in aCSF, to be
to demonstrate whether or not the retention time match persisted.
eluted to waste before actual ion profile monitoring began, and
In every case the shift in retention times was matched by the
they were diverted in the first 1.3 min of the analyses. The use of
detected analyte (data not shown). A more organic initial condi-
the waste diversion valve assists in keeping the cone and ion
tion/mobile phase gradient (90:10, water–acetonitrile) was subse-
source clean. This approach is common practice in LC/MS/MS anal-
quently used for the further confirmation of the analytes using the
ysis but was, in this case, also based on other published studies
Exactive OrbiTrap mass spectrometer, creating different (shorter)
monitoring neurotransmitters and related compounds in rat aCSF
retention times for the analytes as confirmed from reference stan-
microdialysates (Greco et al., 2013; Uutela et al., 2009). The LC
dards. Matching of retention times in varying LC conditions is an
gradient, starting with a high water content (98% water
additional technique to assist in confirmation of unknowns.
Copyright 2013 John Wiley & Sons, Ltd.
Biomed. Chromatogr. 2013
LC/MS/MS of endogenous DMTs in rat pineal gland microdialysate
Figure 2C. (Continued)
Biomed. Chromatogr. 2013
Copyright 2013 John Wiley & Sons, Ltd.
S. A. Barker et al.
Selectivity and specificity
Limits of detection
An examination of aCSF showed no interfering substances that led
Limits of detection (LODs) ranged from 0.02 to 2.0 ng/mL (HIAA;
to misidentification or false detection (as established by the stated
Table 1). Standards were analyzed between the range of
criteria) of any of the target analytes. Pineal dialysate aCSF from
concentrations of 0.01–25 ng/mL to establish the LODs. The
rats did show the presence of several of the target analytes as
limiting factor in each was the ability to observe the weakest
endogenous substances, but did not show any detectable or
of the established diagnostic ions at a concentration giving a
significant interferences with these or other analytes. Cross-talk
response greater than 3 times baseline noise. The resulting data
between targeted analytes was also examined, since (1) HNMT
suggest that the assay is capable of providing reliable analytical
and MTA, (2) TRP, HDMT, DMTNO and MNMT, (3) DMT and HTHBC,
results for these compounds with a high degree of sensitivity in
(4) MDMT and HNATA, and (5) HDMTNO and HTRP have, respec-
this matrix.
tively, the same molecular formula and, thus, the same exact mass
Detection, and especially quantitation, of these compounds
and protonated molecular ion. However, there was adequate tem-
in tissue microdialysates is complicated by the dynamic aspects
poral separation of the compounds and differences in mass and
of the microdialysis process itself (Chefer et al., 2009). Recovery
mass fragment ion formation such that no detectable cross-talk/
of the compounds to be analyzed, often called the extraction
interference for any of the compounds was observed. The method
fraction, relative recovery or probe efficiency, can be affected
as described and performed here suggests that it possesses a high
by the type of probe, probe membrane, flow rate, tissue
degree of selectivity and specificity.
resistance and a number of other factors. For example, theanalyses using the system applied in this case showed thatthe in vitro rate of probe recovery was 14% for 5-HT, 15% forN-acetylserotonin and 12% for melatonin under our experi-
Analyte stability
mental conditions (data not shown). This is in line with other
All of the compounds examined were stable in solution (artificial
published studies. Several studies have also shown that IAA
CSF) when stored at
80 °C for up to 6 months (longest time mea-
and HIAA, two of the other compounds detected, are also
sured) and stable at 4 °C for the same period of time. All of the
recovered in this same percentage range using a variety of
compounds were stable at ambient temperatures for at least
techniques (Kendrick, 1989). It may also be assumed that the
1 week in the dark.
rate of in vitro DMT and TA recovery, the last two compounds
Figure 3. The detection of N,N-dimethyltryptamine (DMT) in samples 5332-B 2Hr, nos 23 and 31 as determined by retention time, occurrence of allfour diagnostic ions and the correct ion ratios (±25% relative) vs a DMT reference standard spiked into aCSF at 0.5 ng/mL (ppb).
Copyright 2013 John Wiley & Sons, Ltd.
Biomed. Chromatogr. 2013
LC/MS/MS of endogenous DMTs in rat pineal gland microdialysate
detected, would be within this range as well. Given the
quantifying the compounds examined here initially screen
chemistry of the remaining compounds examined, it is also
samples using the method described to first determine which
reasonable to assume that their recovery rate is in the same
of the many analytes can be confirmed to be present. A suitable
range, with many being quite water-soluble or closely related
method may then be developed that targets these analytes and,
in physicochemical properties to the compounds detected. As
thus, simplify the analysis. At that juncture, appropriate valida-
with any assay, the combination of recovery rate, inherent in
tion, including determination of probe recovery rates and matrix
microdialysis techniques, and lack of adequate sensitivity may
effects, should be conducted.
combine to give a negative result.
The present protocol was established for the qualitative
analysis or screening of pineal microdialysate and, thus, was
Method performance
not examined for matrix effects. While direct analyses asdescribed here provide a high degree of efficiency, they can also
The analytical procedure presented here is straightforward,
lead to problems arising from the effects of co-eluting matrix
requiring, in our hands, no filtration or dilution of the dialysate
components. Such effects can enhance or suppress detectability.
for analysis. Such direct analysis limits the ability to gain sensitiv-
This is a particular problem for quantitative analyses. Previous
ity through the process of extraction and concentration. None-
quantitative studies analyzing rat aCSF dialysate for neurotrans-
theless, it avoids losses of some of the compounds vs others in
mitters and related compounds have examined their methodol-
attempting to establish a comprehensive extraction protocol
ogy for matrix effects. For example, Cannazza et al. (2012) found
for such a large number of chemically diverse compounds.
no evidence for matrix effects in their assay and concluded that
Further, we are typically dealing with a rather small sample size
this was due to the fact that an initial high water content mobile
(240 μL as performed here), which also complicates the use of
phase and use of a diverter valve effectively eliminated inor-
complex extraction procedures. Such an approach seems ideal
ganic salts that often are the source of ion suppression. Potential
for this matrix. We noted, however, that the use of a heated
interference from proteins is also greatly reduced in microdialy-
electrospray probe caused significant loss of signal and response
sis samples, since the pineal dialysate, in this case specifically,
in the progression of samples. This problem was remedied by
had to pass a dialysis probe with a molecular weight cut-off of
using an ambient temperature setting on the probe and early
13 kDa. We suggest that future efforts directed toward
solvent diversion to waste.
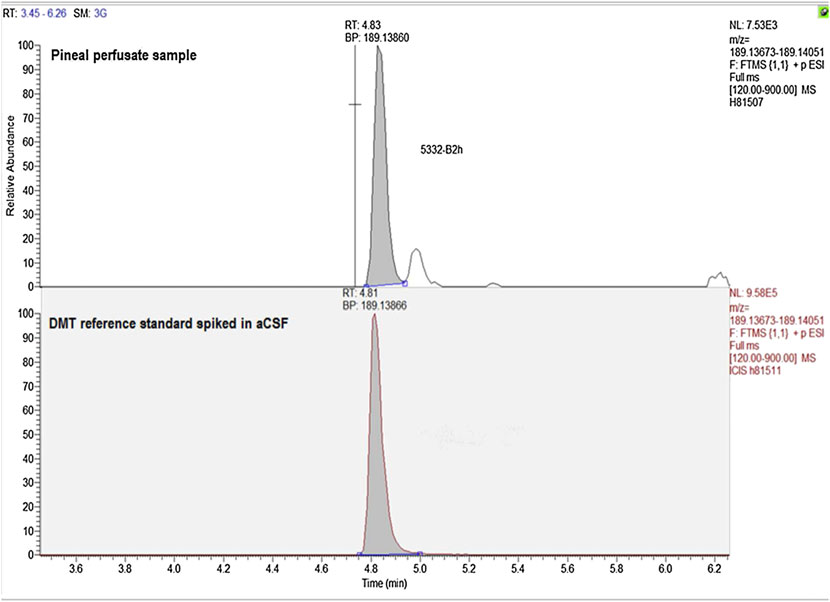
Figure 4. Representative exact mass data for a DMT reference standard and the presumptive DMT peak observed in pineal gland perfusate.
Biomed. Chromatogr. 2013
Copyright 2013 John Wiley & Sons, Ltd.
S. A. Barker et al.
Analytes detected and not detected in rat CSF
obtained for a DMT reference standard, occurring at the matchingretention time for DMT (Fig. 4).
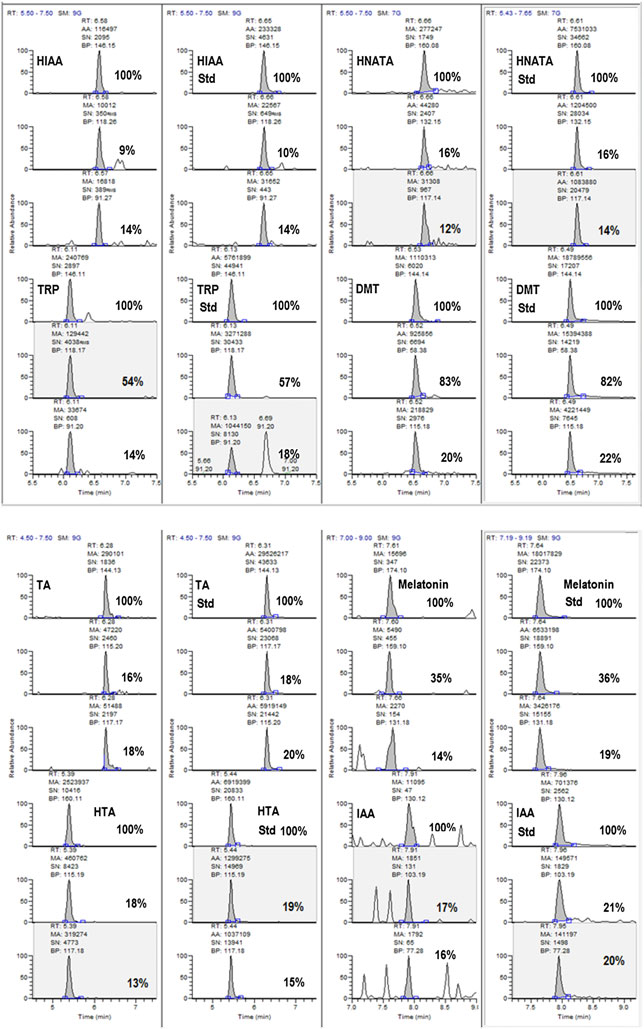
Figure 2(C) is illustrative of chromatograms of pineal microdialysate
Taken together, the retention time matches (using two differ-
samples, showing the major ions used for monitoring and
ent LC methods), the presence of four ions generated by moni-
confirming the analytes. The chromatograms for the analysis
toring and fragmenting a molecule with a nominal protonated
of aCSF collected from pineal perfusion (Fig. 2C) show the
molecular ion (m/z) of 189+ and the high-resolution matching
presence of the compounds HTA, melatonin, TRP, HNATA, HIAA,
of the exact mass for this compound vs a reference standard
IAA, TA and DMT. Not all of the compounds were detected at all
are all scientifically compelling evidence for the presence of
times in all samples. However, as expected, melatonin and HTA
DMT in rat pineal gland microdialysate. As noted, DMT was not
were consistently detected in pineal dialysate samples. It is
detected in all samples, as melatonin and HTA were, and it is
important to note that samples positive for DMT were also
possible that the presence of measurable levels of DMT may
positive for its biosynthetic precursor TA and its terminal
be subject to yet undetermined physiological conditions or
metabolite IAA. This data illustrates the need to monitor
changes as well as time.
precursors and metabolites and the need to follow the affected
DMT has previously been reported in human CSF collected by
pathways of biosynthesis and degradation, especially with
lumbar puncture (Corbett et al., 1978; Smythies et al., 1979). Its
changing conditions in experimentation.
origin in this matrix remains unknown. DMT has also previously
Three additional representative chromatograms and ion
been reported to be present in whole rat brain. In 2005, using a
traces of samples collected by pineal gland perfusion of freely
sensitive LC/MS/MS approach, Kärkkäinen et al. (2005) reported
moving rats confirming the presence of DMT are shown in Fig. 3
the presence of DMT at low pg/g levels (10 and 15 pg/g) in two
and are compared with a reference standard for DMT. Figure 3
whole rat brain samples analyzed from MAO-inhibitor treated
illustrates the matches obtained for the retention times, pres-
animals. In 1984 Beaton and Morris (1984), using GC/MS method-
ence of four diagnostic ions and the matches for their ion ratios,
ology, also reported DMT as being present in whole rat brain in
all meeting stated criteria for confirmation.
low ng/g concentrations. This latter study illustrated changing
Within the limits of the assay as described and for the small
concentrations of DMT in whole brain with age of the animal
number of samples examined here, there was no indication of
and also reported the detection of MDMT, THBC and TA. Thus,
the presence of the other compounds analyzed. However, the
the present research may be seen as corroborating these studies
failure to detect many of these substances should not be taken
with regard to DMT but is the first such study to report the
as evidence that they are not biosynthesized or present in the
presence of DMT in pineal gland and in live animals.
pineal gland of the rat. In the present study, samples were onlycollected from pineal gland for 2 h during day time. Longitudinalstudies or studies altering physiological conditions may show
different data. Nonetheless, despite the presence of the precur-sor for their synthesis (HTA), no MTA, MNMT, MDMT, MDMTNO
The method described is a simple, sensitive, specific and direct
or MIAA nor the corresponding β-carboline (MTHBC; pinoline)
approach to the qualitative analysis of compounds in the trypto-
was detected. Similarly, no HNMT, HDMT, HDMTNO or HTHBC
phan pathway related to the biosynthesis and metabolism of the
was detected, despite the presence of HTA. However, HIAA
endogenous hallucinogens, or DMTs. The method involves the
was detected in most samples. Also, although TA was detected
direct injection of rat pineal gland aCSF microdialysate and anal-ysis by LC/MS/MS, using stringent MS confirmatory criteria,
in some samples as well as DMT, no THBC, NMT, 2MTHBC or
including exact mass measurements. It is anticipated that the
DMTNO was observed, although IAA, the terminal metabolite
same approach would also be viable for application to other
for DMT, TA and NMT, was detected in most samples. Pharmaco-
tissue perfusates, biological fluids or samples with little or no
logical intervention may alter these patterns.
modification. The excellent limits of detection, as well as thecapacity to perform qualitative analyses for a large number of
Exact mass confirmation of DMT and other detected com-
compounds in the tryptophan pathway simultaneously, without
pounds in rat CSF
requiring extraction, make it a valuable research tool, particularlyfor examining the endogenous hallucinogen pathways in the
Additional confirmation of the identity of detected analytes was
CNS. We report here for the first time the presence of DMT in pi-
obtained using the Thermo Exactive mass spectrometer. Thus,
neal gland dialysate obtained from the rat. However, the
233.12828 amu/found
method will permit the conduct of a range of future experi-
233.12849 amu), HTA (177.10224/177.10214 amu), TA (161.10732/
ments, such as measuring possible circadian changes or
161.10735 amu), IAA (176.07061/176.07057 amu), HIAA (192.06552/
performing dialysis studies in other brain tissues or in the ventri-
192.06511 amu), TRP (205.09715/205.09711 amu) and HNATA
cles. Regardless of the sample source, it is also important to be
(219.11280/219.11296 amu) detected in samples were further con-fi
able to assess the effects of pharmacological treatments, such
rmed, with their exact masses agreeing to within ±0.1–0.9 ppm
as MAO inhibition or other physiological interventions, on
and matching the retention time for the corresponding reference
changes in the presence or concentrations of DMT and the other
standards (data not shown) using a modified LC program.
endogenous hallucinogens, their precursors and/or metabolites.
Figure 4 shows representative data obtained for the exact mass
measurement of a DMT standard and DMT peaks in rat pinealmicrodialysate samples using the Thermo Exactive instrument
and a different LC program from that used in the screening of
Barker SA. GC/MS quantification and identification of endogenous
samples using the Thermo Quantum Access instrument. Analysis
tetrahydro-beta-carbolines in rat brain and adrenal. In Usdin E (ed.)
of samples for DMT by exact mass gave 189.13860 amu [(M + 1)+]
The Symposium on Beta-Carbolines and Tetrahydro-isoquinolines. Alan
compared with the calculated exact mass of 189.13866 amu
R. Liss: New York, 1982; pp. 113–124.
Copyright 2013 John Wiley & Sons, Ltd.
Biomed. Chromatogr. 2013
LC/MS/MS of endogenous DMTs in rat pineal gland microdialysate
Barker SA, Harrison RE, Brown GB and Christian ST. Gas chromato-
Kärkkäinen J, Forsstrom T, Tornaeus J, Wahala K, Kiuru P, Honkanen A,
graphic mass spectrometric evidence for the identification of
Stenman UH, Turpeinen U and Hesso A. Potentially hallucinogenic
1,2,3,4-tetrahydro-beta-carboline as an in vivo constituent of rat
5-hydroxytryptamine receptor ligands bufotenine and dimethyltryp-
brain. Biochemical and Biophysical Research Communications 1979;
tamine in blood and tissues. Scandinavian Journal of Clinical Labora-
87: 146–154.
tory Investigation 2005; 65: 189–199.
Barker SA, Monti JA and Christian ST. Metabolism of the hallucinogen N,
Kendrick KM. Use of microdialysis in neuroendocrinology. Methods in
N-dimethyltryptamine in rat brain homogenates. Biochemical Phar-
Enzymology 1989; 168: 182–205.
macology 1980; 29: 1049–1057.
Mavlyutov TA, Epstein ML, Liu P, Verbny YI, Ziskind-Conhaim L and Ruoho
Barker SA, Harrison REW, Monti JA, Brown GB and Christian ST. The
AE. Development of the sigma-1 receptor in C-terminals of motoneu-
identification and quantification of 1,2,3,4-tetrahydro-beta-carboline,
rons and colocalization with the N,N′-dimethyltryptamine forming
enzyme, indole-N-methyl transferase. Neuroscience 2012; 206: 60–68.
tetrahydro-beta-carboline as in vivo constituents of rat brain and adrenal
McIlhenny EH, Riba J, Barbanoj MJ, Strassman R and Barker SA. Method-
gland. Biochemical Pharmacology 1981b; 30: 9–17.
ology for and the determination of the major constituents and me-
Barker SA, Monti, JA and Christian ST. N, N-Dimethyltryptamine: an en-
tabolites of the Amazonian botanical medicine ayahuasca in human
dogenous hallucinogen. International Review of Neurobiology 1981a;
urine. Biomedical Chromatography 2011; 25: 970–984.
22: 83–110.
McIlhenny EH, Riba J, Barbanoj MJ, Strassman R and Barker SA. Method-
Barker SA, Beaton JM, Christian ST, Monti JA and Morris PE. The in vivo me-
ology for determining major constituents of ayahuasca and their me-
tabolism of alpha, alpha, beta, beta-tetradeutero-N, N-dimethyl-
tabolites in blood. Biomedical Chromatography 2012; 26: 301–313.
tryptamine in rat brain. Biochemical Pharmacology 1984; 33: 1395–1400.
Riba J, McIlhenny EH, Valle M, Bouso JC and Barker SA. Metabolism and
Barker SA, McIlhenny EH and Strassman R. A critical review of reports of
disposition of N, N-dimethyltryptamine and harmala alkaloids after
endogenous psychedelic N, N-dimethyltryptamines in humans:
oral administration of ayahuasca. Drug Testing and Analysis 2012; 4:
1955–2010. Drug Testing and Analysis 2012; 4: 617–635.
Beaton JM and Morris PE. Ontogeny of N, N-dimethyltryptamine and
Shoemaker DW, Cummins JT and Bidder TG. β-Carbolines in rat arcuate
related indolealkylamine levels in the neonatal rat. Mechanisms of
nucleus. Neuroscience 1978; 3: 233–239.
Aging and Development 1984; 25: 343–347.
Sitaram BR and McLeod WR. Observations on the metabolism of the
Borjigin J and Liu T. Application of long-term microdialysis in circadian
psychotomimetic indolealkylamines: implications for future clinical
rhythm research. Pharmacology, Biochemistry and Behavior 2008; 90:
studies. Biological Psychiatry 1990; 28: 841–848.
Sitaram BR, Lockett L, Blackman GL and McLeod WR. Urinary excretion
Cannazza G, Carrozzo MM, Cazzato AS, Bretis IM,Troisi L, Parenti C,
Braghiroli D, Guiduccic S, Zoli M. Simultaneous measurement of
and their N-oxides in the rat. Biochemical Pharmacology 1987a; 36:
adenosine, dopamine, acetylcholine and 5-hydroxytryptamine in
cerebral mice microdialysis samples by LC-ESI-MS/MS. Journal of
Sitaram BR, Lockett L, Talomsin R, Blackman GL and McLeod WR. In vivo
Pharmaceutical and Biomedical Analysis 2012; 71: 183–186.
metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimeth-
Chefer VI, Thompson AC, Zapata A and Shippenberg TS. Overview of
yltryptamine in the rat. Biochemical Pharmacology 1987b; 36: 1509–
brain microdialysis. Current Protocols in Neuroscience 2009; 47:
Sitaram BR, Talomsin R, Blackman GL and McLeod WR. Study of metabo-
Corbett L, Christian ST, Morin RD, Benington F and Smythies JR. Halluci-
lism of psychotomimetic indolealkylamines by rat tissue extracts
nogenic N-methylated indolealkylamines in the cerebrospinal fluid
using liquid chromatography. Biochemical Pharmacology 1987c; 36:
of psychiatric control populations. British Journal of Psychiatry 1978;
132: 139–144.
Smythies JR, Morin RD and Brown GB. Identification of dimethyltrypta-
Cozzi NV, Mavlyutov TA, Thompson MA and Ruoho AE. Indolethylamine
mine and O-methyl-bufotenin in human cerebrospinal fluid by com-
N-methyltransferase expression in primate nervous tissue. Society
bined gas chromatography/mass spectrometry. Biological Psychiatry
for Neuroscience Abstracts 2011; 37: 840.19.
1979; 14: 549–556.
Fish MS, Johnson NM, Lawrence EP and Horning EC. Oxidative N-
Su TP, Hayashi T and Vaupel DB. When the endogenous hallucinogenic
dealkylation. Biochemica Biophysica Acta 1955; 18: 564–565.
trace amine N,N-dimethyltryptamine meets the sigma-1 receptor.
Fontanilla D, Johannessen, M, Hajipour AR, Cozzi NV, Jackson MB and
Science Signaling 2009; 2: pe12.
Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an
Thompson MA and Weinshilboum RM. Rabbit lung indolethylamine N-
endogenous sigma-1 receptor regulator. Science 2009; 323: 934–937.
methyltransferase: cDNA and gene cloning and characterization.
Greco S, Danyszb W, Zivkovicc A, Grossb R and Stark H. Microdialysate
Journal of Biological Chemistry 1998; 273: 34502–34510.
analysis of monoamine neurotransmitters – a versatile and sensitive
Thompson MA, Moon E, Kim UJ, Xu J, Siciliano MJ and Weinshilboum RM.
LC–MS/MS method. Analytica Chimica Acta 2013; 771; 65–72.
Human indolethylamine N-methyltransferase: cDNA cloning and
Ho BT and Walker KE. 1,2,3,4-Tetrahydro-β-carboline. Organic Synthesis
expression, gene cloning, and chromosomal localization. Genomics
1964; 51: 136–138.
1999; 61: 285–297.
Kari I, Airaksinen MM, Gynther J and Huhtikangas A. Mass spectrometric
Uutela P, Reinila R , Harju K, Piepponen P, Ketola RA and Kostiainen R.
identification of 6-methoxy-1,2,3,4-tetrahydro-β-carboline in pineal
Analysis of intact glucuronides and sulfates of serotonin, dopamine,
gland. (Analytical Chemistry Symposia Series 12.) Recent Develop-
and their phase I metabolites in rat brain microdialysates by liquid
ments in Mass Spectrometry, Biochemistry. Medicine and Environmental
chromatography–tandem mass spectrometry. Analytical Chemistry
Research 1983; 8: 19–24.
2009; 81: 8417–8425.
Biomed. Chromatogr. 2013
Copyright 2013 John Wiley & Sons, Ltd.
Source: http://www.darta.art.pl/xxx/Pineal%20DMT.pdf
Framework for Continuous Palliative Sedation Therapy in Canada Dean MM1, Cellarius V2, Henry B3, Oneschuk D4, and Librach L5 Preamble Sedation is a commonly used procedure in many medical disciplines including palliative care. It is indicated for a variety of reasons and the type of sedation varies considerably. For example, intentional temporary sedation is sometimes used for procedures (chest-tube insertion, endoscopy, etc.) or insomnia whereas at other times sedation is unintentional (sometimes called secondary or consequential sedation) such as when sedation occurs as a side-effect of a drug being used to control a symptom. Thus the topic of sedation in palliative care practice is vast and complex. To develop this framework the authors reviewed the international literature and palliative sedation policies and protocols from within and without Canada. Recommendations from the first draft were presented at two workshops to full-time and part-time palliative care physicians and to family physicians and a subsequent draft based on feedback from the workshops was then sent to selected inter-professional reviewers across Canada. Their feedback was incorporated into the next draft and this was sent to members of the Canadian Society of Palliative Care Physicians (CSPCP) who were then surveyed for their level of agreement with the recommendations. There was a 29.3% response with over 70% agreement with all but three of the recommendations. This final document addresses planned sedation for management of intolerable and refractory symptoms. It does not address emergency sedation e.g. for an acutely agitated and delirious patient.
Annals of Internal Medicine The Effectiveness of a Primer to Help People Understand RiskTwo Randomized Trials in Distinct Populations Steven Woloshin, MD, MS; Lisa M. Schwartz, MD, MS; and H. Gilbert Welch, MD, MPH Background: People need basic data interpretation skills to under- point validated scores (interest and confidence in interpreting med-






