Medpsych.biz
Journal of Learning Disabilities
Executive Impairment Determines ADHD Medication Response: Implications for Academic Achievement
James B. Hale, Linda A. Reddy, Margaret Semrud-Clikeman, Lisa A. Hain, James Whitaker, Jessica Morley, Kyle
Lawrence, Alex Smith and Nicole Jones
J Learn Disabil
The online version of this article can be found at:
can be found at:
Journal of Learning Disabilities
Additional services and information for
1Hale et al.Journal of Learning Disabilities
Hammill Institute on Disabilities 2011
Reprints and permission:
Journal of Learning Disabilities44(2) 196 –212
Executive Impairment Determines
Hammill Institute on Disabilities 2011Reprints and permission:sagepub.com/journalsPermissions.nav
ADHD Medication Response:
Implications for Academic Achievement
James B. Hale1, Linda A. Reddy2, Margaret Semrud-Clikeman3,
Lisa A. Hain4, James Whitaker5, Jessica Morley4,
Kyle Lawrence4, Alex Smith4, and Nicole Jones4
Abstract
Methylphenidate (MPH) often ameliorates attention-deficit/hyperactivity disorder (ADHD) behavioral dysfunction according
to
indirect informant reports and rating scales. The standard of care behavioral MPH titration approach seldom includes
direct neuropsychological or academic assessment data to determine treatment efficacy. Documenting "cool" executive-
working memory (EWM) and "hot" self-regulation (SR) neuropsychological impairments could aid in differential diagnosis
of ADHD subtypes and determining cognitive and academic MPH response. In this study, children aged 6 to 16 with ADHD
inattentive type (IT;
n = 19) and combined type (
n = 33)/hyperactive-impulsive type (
n = 4) (CT) participated in double-blind
placebo-controlled MPH trials with baseline and randomized placebo, low MPH dose, and high MPH dose conditions. EWM/
SR measures and behavior ratings/classroom observations were rank ordered separately across conditions, with nonpara-
metric randomization tests conducted to determine individual MPH response. Participants were subsequently grouped
according to their level of cool EWM and hot SR circuit dysfunction. Robust cognitive and behavioral MPH response was
achieved for children with significant baseline EWM/SR impairment, yet response was poor for those with adequate EWM/
SR baseline performance. Even for strong MPH responders, the best dose for neuropsychological functioning was typically
lower than the best dose for behavior. Findings offer one possible explanation for why long-term academic MPH treatment
gains in ADHD have not been realized. Implications for academic achievement and medication titration practices for children
with behaviorally diagnosed ADHD will be discussed.
Keywords
ADHD, executive function, methylphenidate, medication response
Children with attention-deficit/hyperactivity disorder (ADHD)
co-occurs with other psychiatric disorders (Spencer, 2006)
exhibit complex and severe neuropsychological and cognitive
and is often accompanied by poor academic achievement when
deficits that profoundly impact behavioral, social, and aca-
executive function deficits are present (Biederman et al., 2004).
demic functioning both at home and in school (DuPaul &
Many children with ADHD are also diagnosed with specific
Stoner, 2004; Reddy & De Thomas, 2006; Willcutt, Doyle,
learning disabilities (SLD) in reading, writing, and/or math-
Nigg, Faraone, & Pennington, 2005). In addition to telltale
ematics (e.g., Capano, Minden, Chen, Schachar, & Ickowicz,
signs of developmentally inappropriate inattention, impulsiv-ity, and hyperactivity, these children experience poor planning, organization, self-monitoring, problem-solving, and social
1University of Victoria, Canada
skills (Hale, Reddy, Wilcox, et al., 2009). Prevalence rates are
Rutgers University, NJ, USA
3Michigan State University, Lansing, USA
fairly consistent across class, culture, and race (Barkley, 2006),
4Philadelphia College of Osteopathic Medicine, PA, USA
with approximately 5% of children affected with ADHD
5Geisinger Medical Center, Danville, PA, USA
worldwide (Polanczyk & Rohde, 2007), making it one of the most common neuropsychiatric childhood disorders (Konrad,
Gunther, Hanisch, & Herpertz-Dahlmann, 2004).
James B. Hale, PhD, Associate Professor, Department of Psychology, P.O. Box 3050, University of Victoria, Victoria, British Columbia,
Considered by many to be a disruptive behavior disorder
(American Psychiatric Association, 2000), ADHD frequently
Email:
[email protected]
Hale et al.
2008; Mayes & Calhoun, 2006; Semrud-Clikeman, 2005).
children and adolescents, while lower doses may be best
It remains unclear whether these learning problems are due
for improving executive control of attention (e.g., Konrad
to behavioral interference with learning, such as on-task
et al., 2004).
behavior (DuPaul & Stoner, 2004), comorbidities separate and distinct from the ADHD (Isles & Humby, 2006), and/or
MPH Effects on Cognitive and
core ADHD neuropsychological deficits (e.g., sustained atten-tion, planning, working memory) that may lead to inadequate
academic achievement (Goldstein & Naglieri, 2008; Hale,
The extant treatment literature suggests that MPH is highly
Reddy, Wilcox, et al., 2009).
effective in reducing noncompliant and disruptive behaviors in children with ADHD (e.g., Abikoff et al., 2004; Pearson
Methylphenidate Treatment in ADHD
et al., 2003; Van der Oord, Prins, Oosterlaan, & Emmelkamp, 2008; Waxmonsky et al., 2008), yet comparatively few inves-
With the ADHD diagnostic focus on informant reports of
tigations have examined the MPH effects on cognition and/
overt behavior problems, it is not surprising that educators
or academic functioning. While some propose MPH improves
and clinicians frequently use child and parent behavior train-
neuropsychological functioning in children with ADHD (e.g.,
ing for affected children (e.g., Fabiano et al., 2009) and
Bedard, Martinussen, Ickowicz, & Tannock, 2004; Hood,
behavioral strategies that improve academic performance
Baird, Rankin, & Isaacs, 2005; Langleben et al., 2006; Wilson,
(e.g., DuPaul & Stoner, 2004). However, the most common
Cox, Merkel, Moore, & Coghill, 2006), others assert that
and efficacious form of ADHD treatment remains psychotro-
MPH does not show beneficial cognitive effects (e.g., Kemner
pic medication, with methylphenidate (MPH) being the most
et al., 2004; Kobel et al., 2008; Lufi, Parish-Plass, & Gai,
researched and prescribed (Barkley, 2006). MPH is a dopa-
1997). Inconsistent findings may be in part due to differential
mine agonist that can impact levels of dopamine and a related
MPH dose-response effects on cognitive and behavioral func-
neurotransmitter norepinephrine availability in the prefrontal
tioning, even within the same child (e.g., Hale, Fiorello, &
cortex (Berridge et al., 2006). By blocking the dopmaine trans-
Brown, 2005; Konrad et al., 2004; Pearson et al., 2004), with
porter, MPH inhibits dopamine reuptake into the presynaptic
some arguing MPH dosages above optimal levels may exac-
membrane and thus increases overall dopamine concentra-
erbate cognitive dysfunction in children with ADHD (e.g.,
tions in the prefrontal and associated subcortical structures
Kuhle et al., 2007).
(Julien, 2005).
One of the earliest MPH studies found positive drug effects
Dopamine is a critical neurotransmitter for prefrontal-
on cognition and behavior but noted deterioration of cognitive
subcortical circuit control of attention and executive function
functioning at higher doses (Sprague & Sleator, 1976). High
(Lichter & Cummings, 2001) circuits that meta-analyses sug-
doses of MPH have been shown to produce "zombie effects"
gest are hypoactive in ADHD (Dickstein, Bannon, Castellanos,
in which children can become quiet, unresponsive, hypoactive,
& Milham, 2006). These frontal-subcortical circuits are highly
and hyperfocused, with poorer cognitive performance as a
interrelated and may be impacted by different medications that
result (Swanson, Cantwell, Lerner, McBurnett, & Hanna, 1991;
target cortical (e.g., prefrontal) or subcortical (e.g., striatum)
Tannock, Shachar, & Logan, 1995). With response curves
structures. MPH appears to influence dopamine and norepi-
inconsistent across variables and children diagnosed with
nephrine availability in both regions, leading to cognitive (e.g.,
ADHD, Hoeppner and colleagues (1997) argued for careful
attention, response inhibition) and behavioral (e.g., on-task
examination of cognitive and behavior MPH dose-response
behavior) improvement as a result (Engert & Pruessner, 2008).
relationships, particularly for children with ADHD-inattentive
Positive effects on hippocampal functioning have been reported
type or those with comorbid internalizing disorders, who have
also, which could account for improved learning and mem-
been found to be poor responders to stimulant treatment (e.g.,
ory in children with ADHD treated with MPH (Dommett,
Barkley, DuPaul, & McMurray, 1991; Tannock, Ickowicz, &
Henderson, Westwell, & Greenfield, 2008).
Schachar, 1995).
Animal research suggests that the prefrontal cortex and
Despite early findings supporting evaluation of cognitive/
associated circuits are highly sensitive to changes in catechol-
neuropsychological and behavioral MPH response in ADHD,
amine modulation, with variations affecting executive con-
it continues to be a behaviorally diagnosed disorder (McKenzie
trol of behavior (Arnsten & Li, 2005). Although MPH clearly
& Wurr, 2004), with little attention given to the potential MPH
enhances executive modulation of cognition and behavior,
effects on cognition. In the 1990s, the movement away from
evidence is emerging that differences among dopamine recep-
examining cognitive/neuropsychological MPH effects was
tors (Floresco & Magyar, 2006) could lead to differential
spurred, in part, by contradictory early evidence that suggested
MPH effects, with low doses improving attention control and
no untoward cognitive effects with increasing MPH dosage
working memory and higher doses impairing these functions
(Berman, Douglas, & Barr, 1999; Douglas, Barr, Desilets,
(Arnsten, 2006b; Berridge et al., 2006). These findings are
& Sherman, 1995; Solanto & Wender, 1989), the absence of
consistent with results that suggest higher MPH doses may
cognitive MPH effects in the presence of robust behavioral
be necessary to reduce behavioral intensity and disruption in
MPH response (Lufi et al., 1997), and the limited utility of
Journal of Learning Disabilities 44(2)
neuropsychological tests of executive functioning in ADHD
et al., 2005), but findings have not been consistent (e.g.,
diagnosis (Brown & LaRosa, 2002). Similar executive deficits
Tucha & Lange, 2005; Van der Oord et al., 2008). Volkow,
are found among other neuropsychiatric disorders (Sergeant,
Fowler, Wang, and Swanson (2004) argued that MPH appears
Geurts, & Oosterlaan, 2002), leading to what many have
to increase reward-related DA availability in the striatum and
called the "discriminant validity problem" in using executive
associated structures (e.g., nucleus accumbens), thereby
function measures for ADHD differential diagnosis (Ozonoff
increasing motivation for academic tasks, suggesting MPH
& Jensen, 1999).
may make classroom reinforcers more salient (Northup, Fusilier, Swanson, Roane, & Borrero, 1997). This increased
Renewed Interest in Assessing
availability for learning likely leads to long-term positive MPH-achievement outcomes as measured by both standard-
Neuropsychological Response to MPH
ized tests and grades (Powers, Marks, Miller, Newcorn, &
Given recent meta-analytic evidence confirming frontal-
Halperin, 2008). Direct positive MPH effects have been
subcortical hypoactivity using MRI/fMRI (Dickstein et al.,
reported for writing legibility (Tucha & Lange, 2001), math
2006) and response inhibition-executive impairments (Willcutt
computation (Gorman, Klorman, Thatcher, & Borgstedt,
et al., 2005) in ADHD, there has been renewed interest in
2006), reading performance (Keulers et al., 2006), and listen-
direct assessment of neuropsychological medication response.
ing comprehension (McInnes et al., 2007), with recent lon-
Considering neuropsychological MPH response may be espe-
gitudinal evidence suggesting both math and reading
cially important given behavioral titration methods alone do
improvement with MPH treatment (Scheffler et al., 2009).
not appear to lead to long-term treatment gains (e.g., Jensen
Several MPH outcome studies on academic functioning
et al., 2007). A recent review found approximately 66% of
have also reported that medication produced no effect on math
studies showed positive cognitive-MPH effects, with improve-
computation accuracy and completion (Benedetto-Nash &
ment in attention, visual tracking, planning, cognitive flexibil-
Tannock, 1999) and led to poorer handwriting fluency (Tucha
ity, vigilance, inhibition, and memory/working memory noted
& Lange, 2005). Frankenberger and Cannon (1999) reported
(Pietrzak, Mollica, Maruff, & Snyder, 2006). Positive MPH
no change in achievement scores for MPH-treated children
effects on sustained attention, visual-spatial working memory,
with ADHD followed longitudinally. Although behavioral
interference control, and response inhibition have also been
gains are common, meta-analyses suggest that both MPH
reported (e.g., Bedard et al., 2004; Hood et al., 2005; Langleben
and psychosocial treatments, even when combined, do not
et al., 2006; McInnes, Bedard, Hogg-Johnson, & Tannock,
lead to better academic achievement in children with ADHD
2007; Tamm & Carlson, 2007; Wilson et al., 2006), with reduc-
(Van der Oord et al., 2008). The nonspecific MPH effects
tions in impulsivity cited as the possible source of positive
found in many studies led some early researchers to conclude
MPH treatment effects (e.g., Huang, Chao, Wu, Chen, & Chen,
that only academic task-related behavior improved on medi-
2007). Such a finding would be consistent with Barkley's
cation (Balthazor et al., 1991).
(1997) ADHD theory, which argues that response inhibition
Inconsistent MPH-achievement findings may be in part due
is a core deficit in ADHD. Although MPH-cognitive effects
to differences in cognitive and behavioral dose-response rela-
are often positive in children with ADHD, such children may
tionships. When differential MPH dose-response relationships
show slower processing speed as a result, a finding often
have been reported, lower doses typically improve academic
termed the "speed-accuracy trade-off" (Lajoie et al., 2005).
behavior, with little or no additional benefit found for higher
The long-term use of MPH on cognitive functioning has
doses. For instance, Chacko et al. (2005) found positive MPH
also been studied. Sustained use of MPH has been found to
effects on academic and social behavior, but few children showed
improve global IQ (Gimpel et al., 2005), executive functioning
significant academic improvement with increased dosage. Simi-
(Vance, Maruff, & Barnett, 2003), and motor timing deficits,
larly, Evans et al. (2001) found improved academic performance
but apparently not time perception (Rubia, Noorloos, Smith,
(e.g., note-taking, quiz performance, written language, on-task
Gunning, & Sergeant, 2003), among ADHD children. A recent
behavior, and homework completion) for low MPH dosing,
study comparing medicated and nonmedicated children with
with high doses improving performance for very few children
ADHD found potential benefits in extended MPH treatment
and deterioration noted for others. This pattern replicates earlier
over time, with normalized or improved attention, working
studies that showed academic gains were associated with low
memory, interference control, and academic performance in
MPH doses, and increasing dosage beyond low to moderate
those treated relative to treatment naïve and control children
levels produced little additional academic benefit (e.g., Greenhill
(Semrud-Clikeman, Pliszka, & Liotti, 2008).
et al., 2001; Pelham & Gnagy, 1999; Smith, Taylor, Brammer, Toone, & Rubia, 1998; Swanson et al., 1995).
MPH Dose-Response Effects on Academic Functioning
Purpose of Current Study
MPH may improve academic performance in children with
The present investigation builds upon previous research docu-
ADHD (e.g., Balthazor, Wagner & Pelham, 1991; Chacko
menting cognitive and behavioral MPH effects at the single
Hale et al.
subject (Hale et al., 1998; Reddy & Hale, 2007) and group
affecting cognitive or neuropsychological performance; or
(Hale, Blaine-Halperin, & Beakley, 2007; Hale et al., 2005;
had missing or different instruments for measuring MPH
Hale, Mulligan, & Simmerman, 2006; Hoeppner et al., 1997)
response (i.e., missing data).
levels of analyses. In this investigation, a double-blind placebo
The final sample consisted of 39 males and 17 females
controlled study of MPH response in children with ADHD
ranging in age from 74 to 200 months (
M = 120.84 months,
was conducted to examine whether executive-working mem-
SD = 30.85). Most participants were in the first through fifth
ory (EWM) and self-regulation (SR) neuropsychological
grades (
n = 43; 77%) and were European American (
n = 46;
impairments affected cognitive and behavioral MPH response.
82%), with the remainder African American. A majority were
There were two predictions. First, it was predicted that level
from middle (
n = 44) or lower (
n = 12) socioeconomic back-
and pattern of baseline data obtained from EWM/SR neuro-
grounds living in urban (
n = 36), suburban (
n = 13), or rural
psychological measures would differentiate MPH responders
(
n = 7) communities. Consistent with epidemiological studies
from nonresponders. Second, it was predicted that the best
(Barkley, 2006), most children were diagnosed with ADHD-
MPH dose for improving EWM/SR neuropsychological func-
CT (
n = 33), with fewer diagnosed with IT (
n = 19) and HIT
tioning would be lower than the best MPH dose for home/
(
n = 4). Comorbid diagnoses included specific learning dis-
classroom behavioral functioning.
ability (
n = 13), oppositional defiant disorder/conduct disorder (ODD/CD;
n = 11), and anxiety/depression (A/D;
n = 6).
Fairly equal numbers of children in the ADHD-IT (
n = 6) and ADHD-CT (
n = 7) groups were diagnosed with comorbid LD.
Most of the children diagnosed with ODD/CD were in the CT group (
n = 9). Consistent with the ADHD and internalizing
The study sample was drawn from a group of 65 elementary
disorders literature (e.g., Biederman, Faraone, & Lapey, 1992),
and high school children diagnosed with ADHD and referred
all children diagnosed with A/D were in the IT group (
n = 6).
by physicians in the northeastern United States for double-
Most participants were receiving regular education or inclu-
blind placebo controlled MPH trials. The participants had to
sion classroom instruction (
n = 44; 79%), with the remainder
meet several inclusion and exclusion criteria. First, physicians
receiving resource or self-contained special education ser-
determined if children met diagnostic criteria for ADHD-
vices. Available intelligence test data suggested the group to
inattentive type (IT), ADHD-hyperactive-impulsive type (HIT),
be relatively high functioning compared to most children with
or ADHD-combined type (CT) based on semi-structured
ADHD (see Barkley, 2006), with global IQ scores in the aver-
diagnostic interview,
Diagnostic and Statistical Manual of
age range (
M = 99.56,
SD = 6.84;
n = 41). All participants
Mental Disorders–Fourth Edition–Text Revision (
DSM-
were either medication naïve or received an appropriate wash-
IV-TR; American Psychiatric Association, 2000) criteria, and
out period of 2 days before the medication trial began.
behavior rating scales. Second,
DSM-IV-TR diagnosis was confirmed independently by a licensed or certified psycholo-
gist using a semi-structured interview of parent, child, and/or teacher, including medical, developmental, social, and academic
After physician evaluation and referral to the principle inves-
histories;
DSM-IV-TR; and objective behavior rating scales.
tigator for an MPH medication trial, parents were sent an
Comorbid diagnoses were also obtained by licensed and/or
information packet addressing the medication, potential side
certified psychologists in the schools or clinic following com-
effects, and medication trial protocol. Those interested were
prehensive evaluation of cognitive, academic, and behavior
evaluated by a licensed psychologist who conducted a semi-
functioning. Third, participants demonstrated significant
structured interview, determined
DSM-IV-TR and parent
attention, hyperactivity, and/or impulse control problems that
behavior rating scale inclusion criteria, and obtained informed
interfered with a major life function in both home and school
consent. A teacher meeting was then held where the treat-
settings. Fourth, participants were rated at least 1.5
SDs above
ment protocol and classroom observation schedule were
the mean (
M = 50,
SD = 10; higher scores more problematic)
discussed. The TRF (Achenbach, 1991) was used for the
on at least one of the following rating subscales:
Attention
classroom behavior baseline assessment only. Four other
Problems of the Achenbach (1991)
Child Behavior Checklist
forms were used for classroom behavior assessment at base-
(CBCL) or
Teacher Report Form (TRF), or the
DSM-IV-TR
line and treatment follow-up: the CTRS-R:L,
School Situa-
Inattention and/or
Hyperactive-Impulsive subscales of the
tions Questionnaire-Revised (SSQ-R; DuPaul & Barkley,
Conners' Parent Rating Scales–Revised: Long Form (CPRS-
1992),
Academic Performance Rating Scale (APRS; DuPaul,
R:L) or
Conners' Teacher Rating Scales–Revised: Long
Rapport, & Perriello, 1991), and the
Side Effects Rating Scale
Form (CTRS-R:L) (Conners, 1997). Participants from this
(SERS; Barkley, 1990).
sample were excluded if they had more than one comorbid
After the initial parent and teacher meetings were
secondary diagnosis; had a history of mental retardation,
completed, each 4-week trial (baseline, placebo, low MPH
seizure disorder, brain injury, or other medical condition
dose, high MPH dose) began with a 45-minute classroom
Journal of Learning Disabilities 44(2)
observation and a 1-hour baseline assessment. The partici-
interrater reliability of the observational methods. All gradu-
pants were not medicated during baseline (B) assessment.
ate students met .90 or higher interrater reliability after receiv-
All medications and placebos were prepared by the study
ing training in the observational procedures.
pharmacist who randomly assigned children to one of six
Following protocol completion, each dependent variable
trial orders of the placebo (P), low dose (L), and high dose
was rank ordered from 1 to 4 across conditions, with a lower
(H) conditions (P-L-H, P-H-L, L-P-H, L-H-P, H-L-P, H-P-L).
rank representing better performance or behavior (i.e., ratings
For the active drug phase, the doses were calculated at
of 1 indicating good performance/behavior and ratings of
0.15 mg/kg/dose for the low dose and 0.30 mg/kg/dose for
4 indicating poor performance/behavior). As has been argued
the high dose and rounded to the nearest 2.5 mg (range 2.5 mg
in Hale et al. (1998, 2005, 2006) and Hoeppner et al. (1997),
to 30 mg per dose). The ground MPH tablet was placed in
the instruments utilized have different numbers of items (i.e.,
lactose-filled opaque capsules for the active drug conditions,
sample space) and this procedure ensures that each instrument
with lactose only for the placebo condition, and was admin-
weighs equally when determining outcome. This assessment
istered twice per day. The research assistants, teacher, parents,
approach has been found to accurately determine neuropsy-
and participants were blind as to the order of conditions. To
chological and behavioral medication response (Hale et al.,
ensure quality control and patient safety, the physician, phar-
1998, 2005, 2006; Hoeppner et al., 1997). For each partici-
macist, and principal investigator (PI) were not blind, but
pant, mean rank scores were computed separately for the cog-
they were not involved in direct data collection.
nitive and behavior ranks and displayed graphically to help
Several neuropsychological instruments were used to
evaluate individual medication response. Following rank-
assess attention, working memory, executive function, inhibi-
ordering procedures, the ordinal data was subjected to a non-
tion, and self-regulation through auditory, visual, verbal, and
parametric randomization test for ranks (NPStat; May, Masson,
motor domains. The tests were administered in the same order
Hunter, & Wells, 1990), which approximates repeated measures
on the last day of each condition by graduate students trained
multivariate analysis of variance (MANOVA) in the absence
and supervised by the first author. The instruments that were
of normal data, to determine individual MPH dose-response
administered in the following order include: the
Hale-Denckla
patterns. After the results were analyzed, the order of condi-
Cancellation Task (HDCT; Hale, Reddy, Decker, et al., 2009),
tions was revealed.
alternate forms of the
Wisconsin Selective Reminding Test (WSRT; Newby, 1999), an audiotaped version of the
Go-No
Go Test (Go-No Go; Trommer, Hoeppner, & Zecker, 1991), the
Conners Continuous Performance Test–II (CPT-II; Conners
In a previous study (Hale et al., 2005), structural equation
& MHS Staff, 2000),
Stroop Color Word Test (Stroop; Golden,
modeling (SEM) was used to develop a model of the Executive/
1978), alternate forms of the
Trail Making Test–Part B (TMT-B;
Working Memory and Self-Regulation factors, hypothesized
Reitan & Wolfson, 1985), constructed by Hale (1997), and
to reflect dorsolateral-dorsal cingulate and orbital-ventral
Test of Memory and
Learning Digits Backward subtest (DB;
cingulate frontal-subcortical circuits based on baseline, non-
Reynolds & Bigler, 1994). Baseline assessment also included
medicated, neuropsychological test performance of children
the
Wisconsin Card Sorting Test (WCST; Heaton, Chelune,
with ADHD. The nonsignificant χ2, Bentler-Bonett Non-
Talley, Kay, & Curtiss, 1993) and
Controlled Oral Word
normed Fit Index, LISREL Goodness of Fit Index, and root
Association Test (COWAT; Spreen & Benton, 1977). The utility
mean square residual values indicated the model adequately
of these reliable and valid instruments when diagnosing ADHD
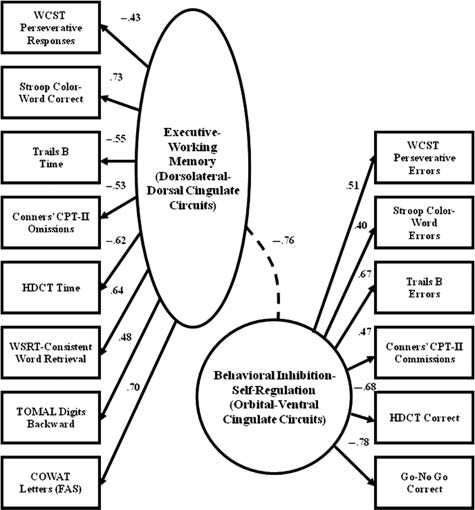
represented the obtained data (see Figure 1; Hale et al., 2005).
and determining treatments effects is well documented in the
Hale et al. (2005) hypothesized that the EWM factor would
literature (Hale & Fiorello, 2004; Pennington & Ozonoff,
be correlated with
DSM-IV-TR inattentive symptoms and the
1996; Sergeant et al., 2002; Willcutt et al., 2005), and previous
SR factor would correlate with
DSM-IV-TR hyperactive/
studies have shown no significant practice effects during medi-
impulsive symptoms, but this was not the case. Instead, both
cation trials (Hale et al., 2005, 2006; Hoeppner et al., 1997).
the EWM (
r = .502,
p = .001) and SR (
r = .327,
p = .034) factors
The assessments took place approximately 1 to 2 hours
correlated only with hyperactive/impulsive symptoms across
after medication was administered. Classroom observations
subtypes, consistent with the notion that response inhibition is
took place on the same day as neuropsychological testing,
the primary deficit associated with ADHD executive impair-
approximately 1 to 2 hours after the administration of the
ments (Barkley, 1997). As a result, regression-based saved fac-
other daily dose of medication. An adaptation of the
Restricted
tor scores derived from baseline performance were added to
Academic Task (RAT; Barkley, 1990) was used to determine
produce a combined EWM/SR impairment score, with resultant
off-task, fidgeting, vocalizing, playing with objects, and out
z scores used to calculate no apparent (N/A; +1.01 or higher),
of seat behaviors. The observational procedure included a
low (0.01 to +1.00), moderate (0.00 to −1.00), and significant
20-second momentary time sampling technique during class-
impairment (−1.01 or lower) executive impairment groups.
room instructional activities. Prior to data collection, the first
The medication trial data were subjected to a MANOVA
author used videotaped classroom recordings to measure
to determine treatment effects, with cognitive ranks and
Hale et al.
ADHD-HIT child diagnosed with comorbid ODD/CD in this group. Most of these children were boys (n = 8) and tended to be older than other groups (M = 151.11 months, SD = 39.93), with six of nine children in Grades 7 through 11. An examina-tion of the DSM-IV-TR inattentive (M = 6.60, SD = 1.94) and hyperactive-impulsive (M = 3.20, SD = 2.58) symptoms col-lected during the psychological evaluation revealed this group to have few endorsed ADHD symptoms. Their mean EWM/SR executive impairment determined by saved factor z scores was +2.33 (SD = .45).
For the low impairment group, the 8 females and 13 males
were fairly equally represented in the ADHD-CT (n = 10) and ADHD-IT (n = 9) diagnostic groups, but both children with ADHD-HIT were males. More than half of these chil-dren had comorbid diagnoses, including SLD (n = 4), ODD/CD (n = 5), and anxious/depressed (n = 3). Unlike the N/A group, these children tended to be in Grades 1 through 6 (n = 19), with a mean age of 123.19 months (SD = 24.47). The DSM-IV-TR inattentive (M = 7.22, SD = 1.69) and hyperactive-impulsive (M = 5.38, SD = 2.56) symptoms were more consistent, but many in this group failed to meet criteria for CT. EWM/SR executive impairment was +.84
Figure 1. Frontal-subcortical circuit Confirmatory Factor
(SD = .57).
Analysis (CFA).
For the moderate impairment group, 14 of the 16 children
Source: Adopted from Hale, Fiorello, and Brown (2005).
were classified with ADHD-CT with one ADHD-IT and
Note: WCST = Wisconsin Card Sorting Test; CPT-I = Conners Continuous
one ADHD-HIT, and most were males (n = 12). Comorbid
Performance Test-II; HDCT = Hale-Denckla Cancel ation Task; WSRT = Wisconsin Selective Reminding Test; TOMAL =Test of Memory and Learn-
diagnoses included SLD (n = 5) and ODD/CD (n = 3), but
ing; COWAT = Controlled Oral Word Association Test.
none of the children were diagnosed with anxiety/depression. All children were in Grades 1 through 5, with a mean age of 110.63 months (SD = 16.40). The DSM-IV-TR inattentive
behavior ranks serving as dependent variables in two separate
(M = 7.28, SD = 1.48) rather than hyperactive-impulsive
equations and impairment group as the independent variables.
(M = 7.50, SD = 1.69) symptoms were comparable for this
As a result, there were effects calculated for the one within-
group. All 16 children were again in Grades 1 through 5, with
subject variable for drug conditions (B, P, L, H) and one for
a mean age of 110.63 months (SD = 16.40). The EWM/SR
the between-subject variable for impairment group and the
executive impairment factor z score was -.80, with an associ-
interaction of the two. The homogeneity of variance assump-
ated SD of .55.
tion was tested using Box's M test for the equality of homo-
For the significant impairment group (n = 10), eight of
geneity of the covariance matrices, and Mauchly Sphericity
the six males and four females were classified as having
tests were used to examine the null hypothesis that the error
ADHD-CT, with the rest ADHD-IT. Two children in this group
covariance matrices of the orthonormalized transformed
had comorbid SLD, two had comorbid ODD/CD, and surpris-
variables met sphericity assumptions. Levine's test was used
ingly one had anxious/depressed comoribidity. In Grades 1
to assess for equality of error variances. Planned contrasts
through 4, these children were the youngest of the four impair-
were used to determine treatment effects, and orthogonal
ment groups (M = 95.50, SD = 16.63). The high executive
polynomial contrasts were used to examine linear, quadratic,
impairment group had high levels of inattentive (M = 7.75,
or cubic trends as previous research has suggested that there
SD = 1.38) symptoms, but fewer hyperactive-impulsive
is sample heterogeneity in medication response with neuro-
symptoms (M = 6.87, SD = 1.46). Their EWM/SR executive
psychological response patterns related to treatment efficacy
impairment, determined by saved EWM-SR factor z scores,
(Hale et al., 1998, 2005; Hoeppner et al., 1997).
was -2.79 (SD = .72).
Cognitive and Behavioral
A cross-tabulation of impairment group by diagnosis revealed
MPH Response for Impairment Groups
that seven of nine children with N/A executive impairment
A repeated measures MANOVA was computed with drug con-
were diagnosed ADHD-IT, and comorbid diagnoses included
dition (B, P, L, H) as the within-subjects factor and impairment
SLD (n = 2) and anxious/depressed (n = 2). There was one
(N/A, low, moderate, high) serving as the between-subjects
Journal of Learning Disabilities 44(2)
Table 1. MPH Dose-Response Relationships for EWM/SR Impairment Groups
Note: Lower ranks indicates better performance and behavior; MPH = methylphenidate; EWM/SR = executive-working memory/self-regulation; N/A = no apparent EWM/SR executive impairment.
aLess than baseline with Bonferroni correction.
bLess than placebo with Bonferroni correction.
factor for the cognitive ranks. Although the Mauchly's test of
p = .04, with partial η2 = .11, and fairly adequate power = .82.
sphericity assumption for drug was met, χ2(5) = 7.52, p = .18,
Tests of within-subjects contrasts demonstrated a linear effect
as was Levene's test for the equality of error variances
for drug, F(1, 49) = 212.00, p < .001, η2= .81, and a quadratic
(p range .296 to .884), a multivariate approach to the data could
effect as well, F(1, 49) = 13.16, p < .001, η2= .21. Tests of
not be completed due to violation of the equality of covariance
between-subjects effects for impairment group was not sig-
matrices as determined by Box's M test, F(30, 2,905.05) = 2.36,
nificant, with an associated F(3, 49) of 2.38 (p = .08), suggest-
p < .001. Huynh-Feldt univariate tests of within-subjects effects
ing no uniform level of behavior impairment regardless of
showed a highly significant effect for drug, F(3, 147) = 44.83,
MPH conditions.
p < .001, η2= .47, power = 1.00. The interaction of drug and impairment was also significant, F(9, 147) = 3.11, p = .002,
MPH Dose Response
η2= .16, power = .97. Tests of within-subjects contrasts demon-strated a linear, F(1, 49) = 94.30, p < .001, η2= .65, and a cubic
Differences for Impairment Groups
effect, F(1, 49) = 16.09, p < .001, η2= .24, for drug condition,
With MANOVA results suggesting that cognitive and behav-
suggesting response curves were not uniform. The drug by
ioral dose-response curves were different based on level of
impairment interaction also demonstrated linear, F(3, 49) = 3.67,
EWM/SR impairment, repeated measures MANOVAs were
p = .01, η2= .18, and cubic, F(3, 49) = 3.14, p = .03, η2= .16,
then computed for each of the four impairment groups, with
effects, indicating different response curves for different levels
planned Bonferroni contrasts of drug conditions for cognitive
of impairment. However, there was no main effect for impair-
and behavioral ranks reported in Table 1 and graphically dis-
ment group, F(3, 49) = .41, p = .74, η2= .025, suggesting no
played in Figure 2 to facilitate interpretation. Mauchly's test
defining overall drug trial performance pattern between groups
for sphericity was nonsignificant for all analyses, so a multi-
across conditions.
variate approach to the data was utilized.
To determine if this finding was also relevant for behavioral
For the N/A group cognitive ranks, the repeated measures
MPH response, a repeated measures MANOVA was computed
MANOVA was nonsignificant for drug, F(3, 5) = 1.82, p = .26.
with drug condition (B, P, L, H) as the within-subjects factor
Power was low (.24) and partial η2 was .51. Orthogonal poly-
and impairment (N/A, low, moderate, high) serving as the
nomial tests of within-subjects contrasts revealed a linear effect
between-subjects factor for the behavioral rank data. There
for drug, F(1, 7) = 7.30, p = .03, η2= .51. For behavioral ranks,
were no violations of MANOVA assumptions, with Box's
the MANOVA was significant for drug, F(3, 5) = 8.32, p = .02,
M test, F(30, 2,905.05) = .794, p = .779, Mauchly's test of sphe-
η2= .83, power = .80. Orthogonal polynomial within-subjects
ricity, χ2(5) = 8.94, p = .112, and Levene's test for the equality
contrasts revealed a linear effect for drug, F(1, 7) = 17.06,
of error variances (p range .122 to .594) all nonsignificant.
p = .004, η2= .70, with quadratic effects approaching signifi-
Using the multivariate approach, Hotelling's trace was highly
cance, F(1, 7) = 5.45, p = .05. However, Bonferroni adjusted
significant for drug, F(3, 47) = 75.64, p < .001, partial η2 = .82,
pairwise comparisons revealed that while all blind conditions
power = 1.00, across the levels of impairment. The drug by
were lower than baseline behavior (indicating better behavior),
impairment interaction was also significant, F(9, 137) = 1.96,
none of the blind conditions (including placebo) were different
Hale et al.
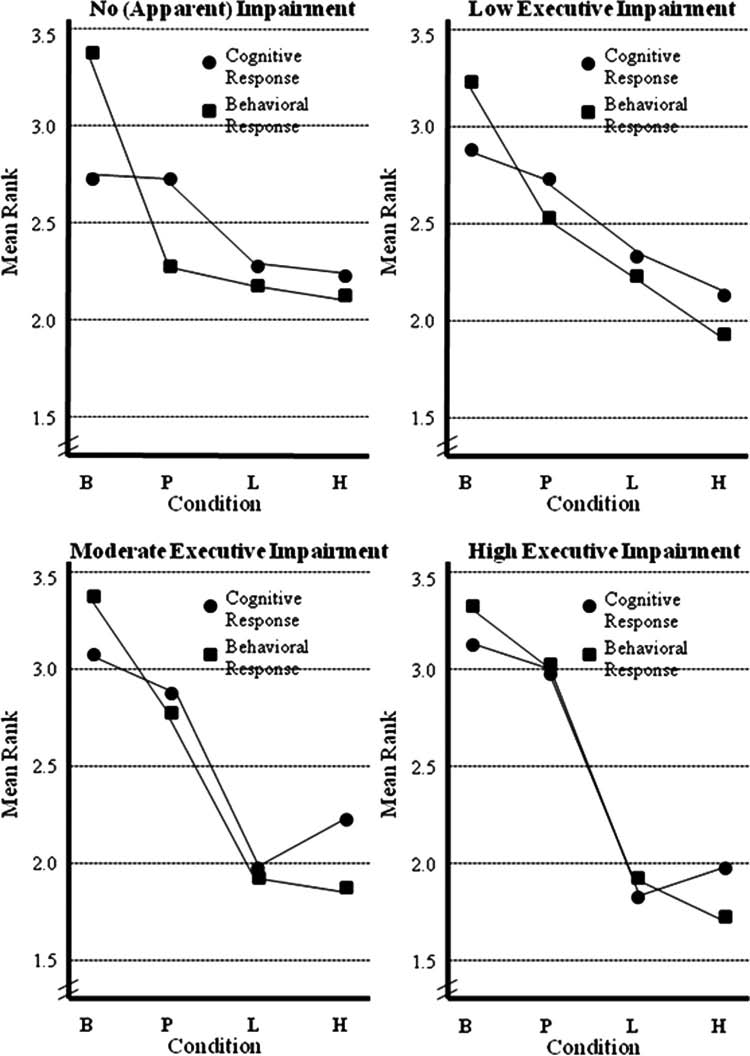
Figure 2. Medication response based on level of frontal-subcortical circuit dysfunction
Note: Lower ranks = better performance and behavior. B = baseline; P = placebo; L = low dose methylphenidate; H = high dose methylphenidate.
from each other. This suggests a placebo effect for behavior
For the low impairment group cognitive ranks, the repeated
ranks, but no significant MPH treatment effect. As a group,
measures MANOVA was significant for drug, F(3, 17) = 7.55,
these physician and psychologist behaviorally diagnosed
p = .002, η2= .57, power = .95, indicating a difference among
children with ADHD did not show EWM/SR impairment
cognitive/neuropsychological functioning across drug condi-
and did not appear to benefit from MPH treatment as a result.
tions. Orthogonal polynomial tests of within-subjects contrasts
Journal of Learning Disabilities 44(2)
revealed a linear effect for drug, F(1, 19) = 24.02, p < .001,
p < .001, η2= .96, effects found for dosage, and cubic,
η2= .55. Bonferroni contrasts revealed cognitive ranks were
F(1, 9) = 5.99, p = .03, η2= .40, effects found as well. As was
lower (i.e., better performance) during the active drug condi-
the case with cognitive response, Bonferroni contrasts
tions as compared to baseline, but the high dose condition
revealed that the baseline and placebo conditions were dif-
was also better than the placebo condition, indicating those
ferent from the active drug conditions, but these were not
with low impairment did respond to the higher MPH dose.
different from each other.
For behavioral ranks, the MANOVA was significant for drug, F(3, 17) = 19.34, p < .001, η2= .77, power = 1.00. Orthogonal
Analysis of Single Subject MPH Dose-
polynomial contrasts revealed a linear effect for drug, F(1, 19) = 55.04, p < .001, η2= .74, with quadratic effects approach-
ing significance, F(1, 19) = 3.95, p = .06. Bonferroni com-
With findings demonstrating differential treatment effects
parisons revealed that all blinded conditions were lower (better
based on level of EWM/SR impairment, an examination of
behavior) than baseline, and the high dose condition was lower
individual case dose-response relationships based on NPStat
than the placebo condition, suggesting that for this group,
nonparametric randomization test results was undertaken.
MPH improved cognition and behavior at the high dose only.
These single subject results were provided to parents and
However, placebo effects were also noted for this group.
referring physicians in each child's medication trial report.
For the moderate impairment group cognitive ranks, the
Although NPStat statistical response does not necessarily
repeated measures MANOVA was again significant for drug,
reflect clinical response, which was determined by referring
F(3, 12) = 16.70, p < .001, η2= .807, power = 1.00, indicating
physicians, results suggest that most of those with N/A or low
a difference among cognitive/neuropsychological functioning
executive impairment tended to be in the ADHD-IT group,
across drug conditions. Orthogonal polynomial contrasts
and often did not show a significant MPH response. However,
revealed linear, F(1, 14) = 33.67, p < .001, η2= .70, quadratic,
those with moderate or significant impairment tended to fall
F(1, 14) = 4.72, p = .048, η2= .25, and cubic, F(1, 14) = 11.57,
into the ADHD-CT group. These individuals were more likely
p =.004, η2= .45, effects for drug. This finding suggests cogni-
to show a significant cognitive and behavioral medication
tive response was not uniform across participants within this
response, with no nonresponders found in these impairment
group. Unlike the other impairment groups, Bonferroni com-
groups, regardless of behavioral ADHD diagnosis.
parisons revealed both active drug conditions to be different from both baseline and placebo, but they were not different
from one another. Similarly, there was no difference between baseline and placebo conditions. For the behavior rank
In this study, children with behaviorally diagnosed ADHD
repeated measures MANOVA, there was again a significant
underwent double-blind placebo controlled MPH trials with
within-subjects effect, F(3, 12) = 33.52, p < .001, η2= .89,
baseline cognitive/neuropsychological direct assessments of
power = 1.00, with linear, F(1, 14) = 78.79, p < .001, η2= .84,
the child, indirect behavior ratings obtained from parents and
and quadratic, F(1, 14) = 6.61, p = .022, η2= .32, effects found
teachers, and classroom observations used to calculate cogni-
for dosage. This time Bonferroni comparisons revealed all
tive and behavioral ranks. These data were subsequently sub-
blind conditions to be lower than baseline, and the high dose
mitted to NPStat nonparametric randomization tests to
to be lower than placebo, but the low dose only approached
determine statistical response. Results revealed highly signifi-
significance with placebo.
cant MPH treatment effects, but response differences emerged
For the significant impairment group, those with significant
for children based on the SEM-determined level of EWM/SR
baseline EWM/SR deficits, the effect for drug on cognitive
executive impairment. For those with N/A baseline EWM/
ranks was highly significant, F(3, 7) = 48.01, p < .001, η2= .954,
SR executive impairment, MPH response was poor. For those
power = 1.00, even with the small sample size. An examination
with significant impairment, every single child showed a sig-
of orthogonal polynomials revealed linear, F(1, 9) = 57.69,
nificant MPH response. In addition, differential cognitive and
p < .001, η2= .86, and cubic, F(1, 9) = 19.45, p = .002, η2= .68,
behavioral patterns of MPH response emerged for those with
effects, indicating nonuniform response patterns. Bonferroni
moderate and significant EWM/SR impairment, with the best
contrasts revealed that the baseline and placebo conditions
dose for cognition lower than the best dose for behavior.
were different from the active drug conditions, but these were not different from each other. However, it is interesting to
MPH Effects on "Hot" Versus "Cool"
note that the cognitive rank was qualitatively lower for the low dose than the high dose, where cognition appeared to deteriorate.
For the behavior rank repeated measures MANOVA, there
Although the exact neurophysiological explanation for these
was again a significant drug effect, F(3, 7) = 62.85, p < .001,
MPH findings is beyond the scope of this article, some specu-
η2= .964, power = 1.00, with strong linear, F(1, 9) = 226.66,
lation appears to be warranted given similar findings reported
Hale et al.
elsewhere (e.g., Arnsten, 2006a; Berridge et al., 2006; Konrad
ADHD Subtypes:
et al., 2004). Solanto et al. (2001) suggest that the tasks evalu-
Are They Distinct Disorders?
ating response inhibition or reward or punishment may reflect different types of executive functioning, which explains the
This MPH response pattern in this investigation is not surpris-
differential findings for EF tasks on and off of medication
ing given the preponderance of evidence that response inhibi-
found here. Zelazo and Muller (2002) suggest that cognitive
tion is the primary deficit in ADHD (Houghton et al., 1999;
aspects of EF may be associated with the dorsolateral pre-
Willcutt et al., 2005; Wodka et al., 2007), which secondarily
frontal cortex and are characterized as "cool" EF. In contrast,
affects attention and other more traditional executive functions
EF tasks that pull for affect (reward/punishment) are referred
(e.g., Barkley, 1997). This assumption is partially supported
to as "hot" EF and may be associated with the orbital and
by findings in this investigation, where both the EWM and SR
medial prefrontal cortices. Castellanos, Sonuga-Barke, Mil-
factors reported in Hale et al. (2005) were found to be related
ham, and Tannock (2006) suggest that cool EF is associated
to DSM-IV-TR hyperactive-impulsive symptoms, but not inat-
with more cognitively loaded types of tasks such as IQ,
tentive ones, suggesting that ADHD-IT and ADHD-CT may
response inhibition, and working memory while hot EF is
be distinct disorders (Milich, Balentine, & Lynam, 2001).
associated more with risk-taking and externalizing behaviors,
Certainly, inattention, or at least intention control (Denckla,
but not inattention. Conceptualizing EF as having both cogni-
1996; Hale, Reddy, Decker, et al., 2009), is another primary
tive and affective aspects may help explain the differing find-
deficit in ADHD. Several studies have suggested that there are
ings in children with ADHD, particularly in regard to
few differences among ADHD subtypes (e.g., Geurts, Verte,
stimulant medication and, as we have demonstrated, with
Oosterlaan, Roeyers, & Sergeant, 2005; Nigg, Blaskey, Huang-
level of MPH. The phylogenetically older hot ventral circuits
Pollock, & Rappley, 2002), with inattentive symptoms associ-
are important for behavioral self-regulation or affective deci-
ated with executive dysfunction, not hyperactive impulsive
sion making, while the younger cool dorsal circuits are involved
ones (Chhabildas, Pennington, & Willcutt, 2001), and results
in deliberative executive processing and attention control
vary based on the study design and methodology. Perhaps
(Castellanos, Sonuga-Barke, Milham, & Tannock, 2006;
inconsistent findings might be in part due the behavioral defi-
Figner, Maklinlay, Wilkening, & Weber, 2009; Roiser et
nition of the ADHD populations used in these studies, where
al., 2009; Steinberg, 2008).
some children have "primary" ADHD-IT, while others have
A differential effect by MPH on the hot (e.g., SR;
secondary or "pseudo" ADHD due to other psychiatric and/or
orbital-medial-ventral cingulate) and cool (e.g., EWM;
learning problems (Hale, Reddy, Wilcox, et al., 2009).
dorsolateral-dorsal cingulate) frontal-striatal-thalamic
Results from this study suggest that there are multiple
circuits (Castellanos et al., 2006; Kelly, Sonuga-Barke,
causes of inattention symptoms in children behaviorally diag-
Scheres, & Castellanos, 2007) may explain the difference
nosed as ADHD, suggesting informant reports and behav-
in improvement in behavior versus cognition. MPH has
ioral rating scales are insufficient for differential diagnosis.
been demonstrated to increase activation in the areas asso-
Although behavior rating scales are important sources of
ciated with behavioral and risk-taking behaviors compared
information in a multimethod, multisource evaluation, they
to those involved with higher level executive functioning
remain summative behavioral judgments of children, effec-
(Zametkin et al., 1990). Support for this conclusion comes
tively intertwining subjective source opinion with objective
from an fMRI study that showed less activation in the
facts about the child (Demaray, Elting, & Schaefer, 2003;
dorsolateral region in children with ADHD, particularly
DuPaul, 2006). When multiple causes of attention problems
those with no history of medication treatment (Pliszka et al.,
are subsumed under a heterogeneous ADHD behavioral
2006). Moreover, a comparison of event-related potentials
umbrella, the diagnostic sensitivity and specificity of our
in children with a history of MPH or no history of MPH
tools is reduced, and treatment efficacy is likely attenuated
on a task requiring inhibition found less activation to
(Hale, Reddy, Decker, et al., 2009).
"failed" trials than to "successful" trials (Liotti, Pliszka,
Evidence for this assertion can be seen in the lower cor-
Perez, Glahn, & Semrud-Clikeman, 2008). In these cases
relation between EWM and SR factors and DSM-IV-TR
children with a history of MPH treatment showed improve-
inattention symptoms in this study. Also, the four EWM/SR
ment in the areas involved in inhibitory skills (behavior)
executive impairment groups differed on DSM-IV-TR
compared to those without such a history. Thus, children
hyperactive-impulsive symptoms but did not differ on DSM-
with a history of MPH at generally accepted levels were
IV-TR inattention ones, suggesting that only those with "true"
found to show improvement in the hot circuit even when
ADHD and hot circuit involvement are more likely to respond
off medication. Perhaps MPH is more likely to have a strongly
to MPH. The cool dorsolateral and dorsal anterior cingulate
linear effect on the hot circuit, but a curvilinear one for
circuits also cause attention deficits in other disorders, such
the cool circuit, where balance of catecholamines becomes
as depression (Liotti & Mayberg, 2001), so perhaps the MPH
more important.
nonresponder ADHD-IT children in this study have this type
Journal of Learning Disabilities 44(2)
of impairment or another form of "pseudo" ADHD such as
If MPH titration was based on maximizing cognitive func-
parietal lobe dysfunction (Hale, Reddy, Wilcox, et al., 2009).
tioning and adjunct behavior therapy was used to help reduce
These arguments are consistent with findings that the
problematic behaviors not adequately addressed by the lower
ADHD-IT is likely to be a heterogenous disorder (e.g., Hale,
MPH dose, perhaps then we would see long-term academic
Reddy, Decker, et al., 2009; Johansen, Aase, Meyer, &
and behavior improvement in children with ADHD.
Sagvolden, 2002) in that only some of these children display
The results presented here suggest that practitioners need
subthreshold behavioral/self-regulation/response inhibition
to reconsider use of standard indirect behavioral approaches
problems that are characteristic of children with ADHD-CT
to ADHD diagnosis and determining MPH treatment efficacy.
(Weiss, Worling, & Wasdell, 2003). A subsequent chart review
Instead, it may be useful to incorporate direct measurement
of the ADHD-IT group in this study supported this conclu-
of cognitive, neuropsychological, academic, and behavioral
sion, with children with ADHD-IT and comorbid anxiety/
functioning when conducting comprehensive evaluations to
depression often showing low executive impairment and no
ensure children referred for attention problems do indeed
MPH response, and those with higher impairment had sub-
have "true" ADHD (Hale, Reddy, Decker, et al., 2009). Even
threshold reported hyperactive-impulsive symptoms and
for those with "true" ADHD, many have comorbid behavioral
showed good MPH response. It is clear that multiple data
and academic concerns, and differential cognitive, academic,
sources, including informant reports, neuropsychological
and behavioral MPH dose-response relationships appear to
assessment of executive functions, and careful clinical evalu-
be common, so it may be useful to compare short- and long-
ation of child and family histories, is essential practice for
term MPH treatment effects on all of these critical functions.
determining the various causes of attention problems (Hale,
Multimethod, multisource evaluations using both neuropsy-
Reddy, Wilcox, et al., 2009; Reddy & Hale, 2007).
chological and behavioral assessment methods minimize the individual limitations of both approaches, and in combination
Implications for Academic
can construct a better diagnostic picture for clinicians, thereby leading to targeted interventions tailored to individual needs
Achievement in ADHD
and better treatment efficacy as a result.
When medication is considered for a child with ADHD, some teachers may be most immediately concerned about MPH
Limitations and Future Directions
effects on improving overt behavior problems. However, the results presented here and reported in the literature (e.g.,
Several study limitations are worth noting. First, the sample
Chacko et al., 2005; Evans et al., 2001; Horrigan & Barnhill,
included children aged 6 to 16, with a majority of the children
2000; Pliszka, Liotti, Bailey, Perez, Glahn, & Semrud-Clike-
in Grades K through 5. Developmental differences in ADHD
man, 2006; Teicher, Polcari, & McGreenery, 2008) suggest
neuropsychological and behavioral functioning are well
the best MPH dose for cognition may be lower than the best
known (Barkley, 1997), so future research should examine
MPH dose for behavior. These differential dose-response
if these MPH findings are consistent across age ranges. Sec-
relationships for children with ADHD could explain why
ond, the neuropsychological tests used here were not coun-
long-term treatment MPH efficacy remains limited (e.g.,
terbalanced and analyzed for order effects; however, they
Jensen et al., 2007) and that MPH has equivocal effects on
were specifically chosen because order effects have not been
academic achievement (Balthazor et al., 1991; Frankenberger
found in previous studies (e.g., Hale et al., 2005; Hoeppner et al.,
& Cannon; 1999; Van der Oord et al., 2008) because clinical
1997). Third, research assistants who conducted classroom
attention has been focused on MPH behavior control, not
observations were proficient at collecting reliable observa-
maximizing cognitive functioning. If the optimal dose for
tional data before medication trials, but future research could
behavior is chosen, children will likely struggle with learning,
also examine interrater reliability during MPH trials. Fourth,
memory, and achievement because the higher dosage limits
all participants were thought to have average intellectual func-
executive attention control/working memory functions (e.g.,
tioning by report, but only some of the children had been admin-
Berridge et al., 2006).
istered a standardized intelligence test prior to the medication
The cool dorsolateral-dorsal cingulate executive functions
trial. Although the baseline and placebo conditions in part guard
such as sustained attention, executive planning, flexible prob-
against intelligence differences, future research should at least
lem solving, fluid reasoning, processing speed, and working
include a cognitive screening of all children. Finally, it will be
memory are important predictors of academic domains such
important to consider teacher, instructional, or classroom man-
as math calculation and reasoning, reading comprehension,
agement practices in future research to determine if these vari-
written expression, higher level implicit language, and read-
ables influence or moderate MPH treatment response.
ing, math, and writing fluency (e.g., Biederman et al., 2004;
Future research could examine children with MPH titration
Bryan & Hale, 2001; Decker, Hill, & Dean, 2007; Denckla,
based on direct neuropsychological baseline function com-
1996; Goldstein & Naglieri, 2008; Hale & Fiorello, 2004).
pared to those who receive standard indirect behavioral titration
Hale et al.
approaches to determine if cognitive, academic, and behav-
Barkley, R. A. (1990). Attention-deficit hyperactivity disorder:
ioral outcomes are differentially affected. In addition, further
A handbook for diagnosis and treatment. New York, NY:
research examining MPH response in relation to, and in com-
bination with, other interventions is needed. While this study
Barkley, R. A. (1997). ADHD and the nature of self control.
provides additional evidence supporting evaluation of both
New York, NY: Guilford.
cognitive and academic MPH response, the exact neurophysi-
Barkley, R. A. (2006). Attention-deficit/hyperactivity disorder. In
ological nature of differential MPH response curves was not
D. A. Wolfe & E. J. Mash (Eds.), Behavioral and emotional
directly evaluated. These response curves could also differ
disorders in adolescents: Nature, assessment, and treatment
for alternative ADHD medications (e.g., Adderall, Strattera)
(pp. 91–152). New York, NY: Guilford.
or MPH dosing regimens (e.g., Concerta, Metadate). Addi-
Barkley, R. A., DuPaul, G. J., & McMurray, M. B. (1991). Attention
tional research is needed to explore MPH response for the
deficit disorder with and without hyperactivity: Clinical
different hot orbital-ventral cingulate and cool dorsolateral-
response to three dose levels of methylphenidate. Pediatrics,
dorsal cingulate circuits, how this impacts SR and EWM. This
empirical work could help determine whether MPH dose-
Bedard, A. C., Martinussen, R., Ickowicz, A., & Tannock, R.
response curves differ for the circuits and ultimately lead to
(2004). Methylphenidate improves visual-spatial memory in
better titration practices that foster academic achievement
children with attention-deficit/hyperactivity disorder. Journal
and psychosocial functioning for children with ADHD.
of the American Academy of Child and Adolescent Psychiatry, 43, 260–268.
Declaration of Conflicting Interests
Benedetto-Nash, E., & Tannock, R. (1999). Math computation,
The author(s) declared no conflicts of interest with respect to the
error patterns and stimulant effects in children with attention
authorship and/or publication of this article.
deficit hyperactivity disorder. Journal of Attention Disorders, 3, 121–134.
Berman, T., Douglas, V. I., & Barr, R. G. (1999). Effects of methyl-
The authors disclosed receipt of the following financial support for
phenidate on complex cognitive processing in attention-deficit/
the research and/or authorship of this article: This research was
hyperactivity disorder. Journal of Abnormal Psychology, 108,
made possible in part by funding from the Neuropsychiatric Research
Institute, Fargo, ND, USA.
Berridge, C. W., Devilbiss, D. M., Andrzejewski, M. E., Arnsten, A. F.,
Kelley, A. E., Schmeichel, . . Spencer, R. C. (2006). Methyl-
phenidate preferentially increases catecholamine neurotrans-
Abikoff, H., Hechtman, L., Klein, R.G., Weiss, G., Fleiss, K.,
mission with in prefrontal cortex at low doses that enhance
Etcovich, J., . . Pollack, S. (2004). Symptomatic improvement
cognitive function. Biological Psychiatry, 60, 1111–1120.
in children with ADHD treated with long-term methylpheni-
Biederman, J., Faraone, S. V., & Lapey, K. (1992). Comorbidity
date and multimodal psychosocial treatment. Journal of the
of diagnosis in attention-deficit hyperactivity disorder. In
American Academy of Child and Adolescent Psychiatry, 43,
G. Weiss (Ed.), Child and adolescent psychiatry clinics of
North America: Attention deficit hyperactivity disorder
Achenbach, T. M. (1991). Integrative guide for the 1991 CBCL/4–18,
(pp. 335–360). Philadelphia, PA: Saunders.
YSR, and TRF profiles. Burlington, VT: University of Vermont,
Biederman, J., Monuteaux, M. C., Doyle, A. E., Seidman, L. J.,
Department of Psychiatry.
Wilens, T. E., Ferraro, F., . . Faraone, S. V. (2004). Impact of
American Psychiatric Association. (2000). Diagnostic and statistical
executive function deficits and attention-deficit/hyperactivity
manual of mental disorders (4th ed., Text Rev.). Washington,
disorder (ADHD) on academic outcomes in children. Journal
of Consulting and Clinical Psychology, 72, 757–766.
Arnsten, A. F. (2006a). Fundamentals of attention-deficit/hyperactivity
Brown, R. T., & LaRosa, A. (2002). Recent developments in the
disorder: Circuits and pathways. Journal of Clinical Psychiatry,
pharmacotherapy of attention-deficit/hyperactivity disorder
(ADHD). Professional Psychology, Research and Practice, 33,
Arnsten, A. F. (2006b). Stimulants: Therapeutic actions in ADHD.
Bryan, K. L., & Hale, J. B. (2001). Differential effects of left and right
Arnsten, A. F., & Li, B. M. (2005). Neurobiology of executive func-
cerebral vascular accidents on language competency. Journal
tions: Catecholamine influences on prefrontal cortical functions.
of the International Neuropsychological Society, 7, 655–664.
Biological Psychiatry, 57, 1377–1384.
Capano, L., Minden, D., Chen, S. X., Schachar, R. J., & Ickowicz, A.
Balthazor, M. J., Wagner, R. K., & Pelham, W. E. (1991). The
(2008). Mathematical learning disorder in school-age children
specificity of the effects of stimulant medication on classroom
with attention-deficit/hyperactivity disorder. Canadian Journal
learning-related measures of cognitive processing for attention
of Psychiatry, 53, 392–399.
deficit disorder children. Journal of Abnormal Psychology, 19,
Castellanos, F. X., Sonuga-Barke, E. J. S., Milham, M. P., &
Tannock, R. (2006). Characterizing cognition in ADHD:
Journal of Learning Disabilities 44(2)
Beyond executive dysfunction. Trends in Cognitive Science,
Evans, S. W., Smith, B. H., Gnagy, E. M., Pelham, W. E., Bukstein, O.,
Greiner, A. R., . . Baron-Myak, C. (2001). Dose-response
Chacko, A., Pelham, W. E., Gnagy, E. M., Greiner, A., Vallano, G.
effects of methylphenidate on ecologically valid measures of
Bukstein, O., & Rancurello, M. (2005). Stimulant medication
academic performance and classroom behavior in adolescents
effects in a summer treatment program among young children
with ADHD. Experimental and Clinical Psychopharmacology,
with attention-deficit/hyperactivity disorder. Journal of the
American Academy of Child and Adolescent Psychiatry, 44,
Fabiano, G. A., Pelham, Jr., W. E., Coles, E. K., Gnagy, E. M.,
Chronis-Tuscano, A., & O'Connor, B. C. (2009). A meta-anal-
Chhabildas, N., Pennington, B. F., & Willcutt, E. G. (2001). A com-
ysis of behavioural treatments treatments for attention-deficit/
parison of neuropsychological profiles of the DSM-IV subtypes
hyperactivity disorder. Clinical Psychology Review, 29,
of ADHD. Journal of Abnormal Child Psychology, 29, 529–540.
Conners, K. (1997). Conners' Parent and Teacher Rating Scale–
Figner, B., Mackinlay, R. J., Wilkening, F., & Weber, E. U. (2009).
Revised. North Tonawanda, NY: Multi-Health Systems.
Affective and deliberative processes in risky choice: Age dif-
Conners, K., & MHS Staff. (2000). Conners' Continuous Perfor-
ferences in risk taking in the Columbia Card Task. Journal of
mance Test–II user's manual. Toronto, Canada: Multi-Health
Experimental Psychology: Learning, Memory, and Cognition,
Decker, S. L., Hill, S. K., & Dean, R. S. (2007). Evidence of con-
Floresco, S. B., & Magyar, O. (2006). Mesocortical dopamine
struct similarity in executive functions and fluid reasoning abili-
modulation of executive functions: Beyond working memory.
ties. International Journal of Neuroscience, 117, 735–748.
Demaray, M. K., Elting, J., & Schaefer, K. (2003). Assessment of
Frankenberger, W., & Cannon, C. (1999). Effects of Ritalin on aca-
attention-deficit/hyperactivity disorder (ADHD): A compara-
demic achievement from first to fifth grade. International Journal
tive evaluation of five commonly used, published rating scales.
of Disability, Development, and Education, 46, 199–221.
Psychology in the Schools, 40, 341–361.
Geurts, H. M., Verte, S., Oosterlaan, J., Roeyers, H., & Sergeant, J. A.
Denckla, M. B. (1996). Biological correlates of learning and atten-
(2005). ADHD subtypes: Do they differ in their executive
tion: What is relevant to learning disability and attention-deficit/
functioning profile? Archives of Clinical Neuropsychology, 20,
hyperactivity disorder? Journal of Developmental & Behavioral
Pediatrics, 17, 114–119.
Gimpel, G. A., Collett, B. R., Veeder, M. A., Gifford, J. A.,
Dickstein, S. G., Bannon, K., Castellanos, F. X., & Milham, M. P.
Sneddon, P., Bushman, B., . . Odell, J. D. (2005). Effects of
(2006). The neural correlates of attention deficit hyperactivity
stimulant medication on cognitive performance of children
disorder: An ALE meta-analysis. Journal of Child Psychology
with ADHD. Clinical Pediatrics, 44, 405–411.
and Psychiatry, 47, 1051–1062.
Golden, J. C. (1978). Stroop Color and Word Test. Chicago, IL:
Dommett, E. J., Henderson, E. L., Westwell, M. S., & Greenfield, S. A.
(2008). Methylphenidate amplifies long-term plasticity in the hip-
Gorman, E. B., Klorman, R., Thatcher, J. E., & Borgstedt, A. D.
pocampus via noradrenergic mechanisms. Learning & Memory,
(2006). Effects of methylphenidate on subtypes of attention-
deficit/hyperactivity disorder. Journal of the American Academy
Douglas, V. I., Barr, R. G., Desilets, J., & Sherman, E. (1995). Do
of Child and Adolescent Psychiatry, 45, 808–816.
high doses of stimulants impair flexible thinking in attention-
Greenhill, L. L., Swanson, J. M., Vitiello, B., Davies, M.,
deficit/hyperactivity disorder? Journal of the American Acad-
Clevenger, W., Wu, M., . . Wigal, T. (2001). Impairment and
emy of Child and Adolescent Psychiatry, 34, 877–885.
deportment responses to different methylphenidate doses in chil-
DuPaul, G. J. (2006). Academic achievement in children with
dren with ADHD: The MTA titration trial. Journal of the Ameri-
ADHD. Journal of the American Academy of Child and Ado-
can Academy of Child and Adolescent Psychiatry, 40, 180–187.
lescent Psychiatry, 45, 766–767.
Goldstein, S., & Naglieri, J. (2008). The school neuropsychology of
DuPaul, G. J., & Barkley, R. A. (1992). Situational variability of
ADHD: Theory, assessment, and intervention. Psychology in
attention problems: Psychometric properties of the Revised
the Schools, 45, 859–874.
Home and School Situations Questionnaires. Journal of Clinical
Hale, J. B. (1997). Development of alternate forms of the Hale
Child Psychology, 21, 178–188.
Cancellation Task and Trail-Making Test for use in ADHD
DuPaul, G. J., Rapport, M. D., & Perriello, L. M. (1991). Teacher rat-
medication trials. Unpublished manuscript, Rochester Institute
ings of academic skills: The development of the Academic Perfor-
of Technology.
mance Rating Scale. School Psychology Review, 20, 284–300.
Hale, J. B., Blaine-Halperin, D., & Beakley, K. (2007, February).
DuPaul, G. J., & Stoner, G. (2004). ADHD in the schools: Assessment
Executive impairment determines ADHD response to methylphe-
and intervention strategies (2nd ed.). New York, NY: Guilford.
nidate treatment. Paper presented at the 35th Annual Meeting of
Engert, V., & Pruessner, J. C. (2008). Dopaminergic and noradren-
the International Neuropsychological Society, Portland, OR.
ergic contributions to functionality in ADHD: The role of methyl-
Hale, J. B., & Fiorello, C. A. (2004). School neuropsychology:
phenidate. Current Neuropharmacology, 6, 322–328.
A practitioner's handbook. New York, NY: Guilford.
Hale et al.
Hale, J. B., Fiorello, C. A., & Brown, L. (2005). Determining medi-
Jensen, P. S., Arnold, L. E., Swanson, J. M., Vitiello, B., Abikoff, H. B.,
cation treatment effects using teacher ratings and classroom
Greenhill, L. L. . Hur, K. (2007). 3-year follow up of the
observations of children with ADHD: Does neuropsychological
NIMH MTA study. Journal of the American Academy of Child
impairment matter? Educational and Child Psychology, 22,
and Adolescent Psychiatry, 46, 989–1002.
Johansen, E. B., Aase, H., Meyer, A., & Sagvolden, T. (2002).
Hale, J. B., Hoeppner, J. B., DeWitt, M. B., Coury, D. L.,
Attention-deficit/hyperactivity disorder (ADHD) behaviour
Ritacco, D. G., & Trommer, B. (1998). Evaluating medication
explained by dysfunctioning reinforcement and extinction pro-
response in ADHD. Journal of Learning Disabilities, 31,
cesses. Behavioural Brain Research, 130(1-2), 37–45.
Julien, R. M. (2005). A primer of drug action. New York, NY: Worth
Hale, J. B., Mulligan, C. A., & Simmerman, K. L. (2006, February).
Quantifying medication response in ADHD: Does neuropsycho-
Kelly, C., Sonuga-Barke, E. J. S., Scheres, A., & Castellanos, F. X.
logical impairment matter? Poster presentation at the 34th
(2007). Functional neuroimaging of the reward and motiva-
Annual Meeting of the International Neuropsychological Society,
tional pathway in ADHD. In M. Fitzgerald, M. Bellgrove, &
M. Gill (Eds.), Handbook of Attention Deficit Hyperactivity
Hale, J. B., Reddy, L. A., Decker, S. L., Thompson, R., Henzel, J.,
Disorder. (pp. 209–235). Hoboken, NJ: John Wiley & Sons.
Teodori, A., . . Denckla, M. B. (2009). Development and vali-
Kemner, C., Jonkman, L. M., Kenemans, J. L., Bocker, K. B.,
dation of an executive function and behavior rating screening
Verbaten, M. N., & Engeland, H. (2004). Sources of auditory
battery sensitive to ADHD. Journal of Clinical and Experimental
selective attention and the effects of methylphenidate in chil-
Neuropsychology, 1, 1–16.
dren with attention-deficit/hyperactivity disorder. Biological
Hale, J. B., Reddy, L. A., Wilcox, G., McLaughlin, A., Hain, L.,
Psychiatry, 55, 776–778.
Stern, A., . . Eusebio, E. (2009). Assessment and intervention
Keulers, E. H. H., Hendriksen, J. G. M., Feron, F. J. M.,
for children with ADHD and other frontal-striatal circuit dis-
Wassenberg, R., Wuisman-Frerker, M. G. F., Jolles, J., &
orders. In D. C. Miller (Ed.), Best practices in school neuro-
Vles, J. S. (2006). Methylphenidate improves reading perfor-
psychology: Guidelines for effective practice, assessment and
mance in children with attention deficit hyperactivity disorder
evidence-based interventions (pp. 225–280). New York, NY:
and comorbid dyslexia: An unblinded clinical trial. European
Journal of Paediatric Neurology, 11(1), 21–28.
Heaton, R. K., Chelune, G. J., Talley, J. L., Kay, G. G., & Curtiss,
Kobel, M., Bechtel, N., Weber, P., Specht, K., Klarhofer, M.,
G. (1993). Wisconsin Card Sorting Test (WCST) manual
Scheffler, K., . . Penner, I. K. (2008). Effects of methylpheni-
revised and expanded. Odessa, FL: Psychological Assessment
date on working memory functioning in children with attention
deficit/hyperactivity disorder. European Journal of Paediatric
Hoeppner, J. B., Hale, J. B., Bradley, A., Byrns, M., Coury, D. L.,
Neurology, 13, 516–523.
& Trommer, B. L. (1997). A clinical protocol for determining
Konrad, K., Gunther, T., Hanisch, C., & Herpertz-Dahlmann, B.
methylphenidate dosage levels in ADHD. Journal of Attention
(2004). Differential effects of methylphenidate on attentional
Disorders, 2, 19–30.
functions in children with attention-deficit/hyperactivity disor-
Hood, J., Baird, G., Rankin, P. M., & Isaacs, E. (2005). Immediate
der. Journal of the American Academy of Child and Adolescent
effects of methylphenidate on cognitive attention skills of chil-
Psychiatry, 43, 191–198.
dren with attention-deficit/hyperactivity disorder. Developmental
Kuhle, H., Kinkebur, J., Andes, K., Heidorn, F., Zeyer, S.,
Medicine & Child Neurology, 47, 408–414.
Rautzenberg, P., . . Jansen, F. (2007). Self-regulation of visual
Horrigan, J. P., & Barnhill, L. J. (2000). Low-dose amphetamine
attention and facial expression of emotions in ADHD children.
salts and adult attention deficit/hyperactivity disorder. Journal
Journal of Attention Disorders, 10, 350–358.
of Clinical Psychiatry, 61, 414–417.
Lajoie, G., Anderson, V., Anderson, P., Tucker, A. R., Robertson, I. H.,
Houghton, S., Douglas, G., West, J., Whiting, K., Wall, M.,
& Manly, T. (2005). Effects of methylphenidate on attention
Langsford, S., . . Carroll, A. (1999). Differential patterns of
skills in children with attention deficit/hyperactivity disorder.
executive function in children with attention-deficit hyperactiv-
Brain Impairment, 6(1), 21–32.
ity disorder according to gender and subtype. Journal of Child
Langleben, D. D., Monterosso, L., Elman, I., Ash, B., Krikorian, G.,
Neurology, 14, 801–805.
& Austin, G. (2006). Effects of methylphenidate on Stroop
Huang, Y., Chao, C., Wu, Y., Chen, Y., & Chen, Y. (2007). Acute
Color-Word task performance in children with attention-deficit/
effects of methylphenidate on performance during the Test
hyperactivity disorder. Psychiatry Research, 141, 315–320.
of Variables of Attention in children with attention deficit/
Lichter, D. G., & Cummings, J. L. (Eds.). (2001). Frontal-subcortical
hyperactivity disorder. Psychiatry and Clinical Neurosciences,
circuits in psychiatric and neurological disorders. New York,
NY: Guilford.
Isles, A. R., & Humby, T. (2006). Modes of imprinted gene action in
Liotti, M., & Mayberg, H. S. (2001). The role of functional neuro-
learning disability. Journal of Intellectual Disability Research,
imaging in the neuropsychology of depression. Journal of
Clinical and Experimental Neuropsychology, 23, 121–136.
Journal of Learning Disabilities 44(2)
Liotti, M., Pliszka, S. R., Perez, R., Glahn, D. C., & Semrud-
Pennington, B. F., & Ozonoff, S. (1996). Executive functions and
Clikeman, M. (2008). Electrophysiological correlates of response
developmental psychopathology. Journal of Child Psychology
inhibition in children and adolescents with ADHD: Influence of
and Psychiatry, 37, 51–87.
gender, age, and previous treatment history. Psychophysiology,
Pietrzak, R. H., Mollica, C. M., Maruff, P., & Snyder, P. J. (2006).
Cognitive effects of immediate-release methylphenidate in chil-
Lufi, D., Parish-Plass, J., & Gai, E. (1997). The effect of methyl-
dren with attention-deficit/hyperactivity disorder. Neuroscience
phenidate on the cognitive and personality functioning of
and Behavioral Reviews, 30, 1225–1245.
ADHD children. Journal of Psychiatry and Related Services,
Pliszka, S. R., Glahn, D. C., Semrud-Clikeman, M., Franklin, C.,
Perez, R., Xiong, J., & Liotti, M. (2006). Neuroimaging of
May, R. B., Masson, M. E. J., Hunter, M. A., & Wells, J. (1990).
inhibitory control areas in children with attention deficit hyper-
NPStat 3.01 [Computer software]. Victoria, Canada: University
activity disorder who were treatment naive or in long-term
of Victoria.
treatment. American Journal of Psychiatry, 163, 1052–1060.
Mayes, S. D., & Calhoun, S. L. (2006). Frequency of reading, math,
Pliszka, S. R., Liotti, M., Bailey, B. Y., Perez, R., Glahn, D., &
and writing disabilities in children with clinical disorders.
Semrud-Clikeman, M. (2007). Electrophysiological effects of
Learning and Individual Differences, 16, 145–157.
stimulant treatment on inhibitory control in children with atten-
McInnes, A., Bedard, A. C., Hogg-Johnson, S., & Tannock, R.,
tion-deficit/hyperactivity disorder. Journal of Child and Ado-
(2007). Preliminary evidence of beneficial effects of methylphe-
lescent Psychopharmacology, 17, 356–366.
nidate on listening comprehension in children with attention-
Polanczyk, G., & Rohde, L. A. (2007). Epidemiology of attention-
deficit/hyperactivity disorder. Journal of Child and Adolescent
deficit/hyperactivity disorder across the lifespan. Current
Opinion in Psychiatry, 20, 386–392.
McKenzie, I., & Wurr, C. (2004). Diagnosing and treating atten-
Powers, R. L., Marks, D. J., Miller, C. J., Newcorn, J. H., &
tional difficulties: A nationwide survey. Archives of Diseases in
Halperin, J. M. (2008). Stimulant treatment in children with
Childhood, 89, 913–916.
attention-deficit/hyperactivity disorder moderates adolescent
Milich, R., Balentine, A. C., & Lynam, D. R. (2001). ADHD
academic outcome. Journal of Child and Adolescent Psycho-
combined type and ADHD predominantly inattentive type are
pharmacology, 18, 449–459.
distinct and unrelated disorders. Clinical Psychology, Science
Reddy, L. A., & De Thomas, C. (2006). Assessment of ADHD
and Practice, 8, 463–488.
children and adolescents. In S. R. Smith & L. Handler (Eds.),
Newby, R. F. (1999). Wisconsin Selective Reminding Test. Milwaukee,
The clinical assessment of children and adolescents: A practitio-
WI: Medical College of Wisconsin.
ner's guide. (pp. 367–387). Mahwah, NJ: Lawrence Erlbaum.
Nigg, J. T., Blaskey, L. G., Huang-Pollock, C. L., & Rappley, M. D.
Reddy, L. A., & Hale, J. (2007). Inattentiveness. In A. R. Eisen
(2002). Neuropsychological executive functions and DSM-IV
(Ed.), Treating childhood behavioral and emotional disorders:
ADHD subtypes. Journal of the American Academy of Child
A step-by-step evidence-based approach (pp. 156–211).
and Adolescent Psychiatry, 41, 59–66.
New York, NY: Guilford.
Northup, J., Fusilier, I., Swanson, V., Roane, H., & Borrero, J.
Reitan, R. M., & Wolfson, D. (1985). Neuroanatomy and neuro-
(1997). An evaluation of methylphenidate as a potential estab-
pathology: A guide for neuropsychologists. Tucson, AZ: Neuro-
lishing operation for some common classroom reinforcers.
psychology Press.
Journal of Applied Behavior Analysis, 30, 615–625.
Reynolds, C. R., & Bigler, E. D. (1994). Test of memory and learning.
Ozonoff, S., & Jensen, J. (1999). Specific executive function pro-
Austin, TX: Pro-Ed.
files in three neurodevelopmental disorders. Journal of Autism
Roiser, J. P., Cannon, D. M., Ghandi, S. K., Taylor-Tavares, J.,
& Developmental Disorders, 29, 171–177.
Erickson, K., Wood, S., . . Drevets, W. C. (2009). Hot and
Pearson, D. A., Santos, C. W., Casat, C. D., Lane, D. M., Jerger, S. W.,
cold cognition in unmedicated depressed subjects with bipolar
Roache, J. D., . . Cleveland, L. A. (2004). Treatment effects
disorder. Bipolar Disorders, 11, 178–189.
of methylphenidate on cognitive functioning in children with
Rubia, K., Noorloos, J., Smith, A., Gunning, B., & Sergeant, J.
mental retardation and ADHD. Journal of the American Acad-
(2003). Motor timing deficits in community and clinical boys
emy of Child and Adolescent Psychiatry, 43, 677–685.
with hyperactive behavior: The effect of methylphenidate on
Pearson, D. A., Santos, C. W., Roache, J. D., Casat, C. D.,
motor timing. Journal of Abnormal Child Psychology, 31,
Loveland, K. A., Lachar, D., . . Cleveland, L. A. (2003). Treat-
ment effects of methylphenidate on behavioral adjustment in
Scheffler, R. M., Brown, T. T., Fulton, B. D., Hinshaw, S. P., Levine,
children with mental retardation and ADHD. Journal of the
P., & Stone, S. (2009). Positive association between attention-def-
American Academy of Child and Adolescent Psychiatry, 42,
icit/ hyperactivity disorder medication use and academic achieve-
ment during elementary school. Pediatrics, 123, 1273–1279.
Pelham, W. E., & Gnagy, E. M. (1999). Psychosocial and combined
Semrud-Clikeman, M. (2005). Neuropsychological aspects for
treatments for ADHD. Mental Retardation and Developmental
evaluating learning disabilities. Journal of Learning Disabilities,
Disabilities Research Reviews, 5, 225–236.
Hale et al.
Semrud-Clikeman, M., Pliszka, S., & Liotti, M. (2008). Executive
Teicher, M. H., Polcari, A., & McGreenery, C. E. (2008). Utility of
functioning in children with attention-deficit/hyperactivity dis-
objective measures of activity and attention in the assessment
order: Combined type with and without a stimulant medication
of therapeutic response to stimulants in children with attention-
history. Neuropsychology, 22, 329–340.
deficit/hyperactivity disorder. Journal of Child and Adolescent
Sergeant, J. A., Geurts, H., & Oosterlaan, J. (2002). How specific is a
deficit in executive functioning for attention-deficit/hyperactivity
Trommer, B. L., Hoeppner, J. B., & Zecker, S. G. (1991). The go-
disorder? Behavioural Brain Research, 130, 3–28.
no-go test in attention deficit disorder is sensitive to methyl-
Smith, A. B., Taylor, E., Brammer, M., Toone, B., & Rubia, K.
phenidate. Journal of Child Neurology, 6(Suppl.), S128–S131.
(2006). Task-specific hypoactivation in prefrontal and tempo-
Tucha, O., & Lange, K. W. (2001). Effects of methylphenidate on
roparietal brain regions during motor inhibition and task
kinematic aspects of handwriting in hyperactive boys. Journal
switching in medication-naïve children and adolescents with
of Abnormal Child Psychology, 29, 351–356.
attention-deficit/hyperactivity disorder. American Journal of
Tucha, O., & Lange, K. W. (2005). The effect of conscious control
Psychiatry, 163, 1044–1051.
on handwriting in children with attention-deficit/hyperactivity
Solanto, M. V., Abikoff, H., Sonuga-Barke, E. J. S., Schachar, R. J.,
disorder. Journal of Attention Disorders, 9, 323–332.
Logan, G. D., Wigal, T., . . Turkel, E. (2001). The ecological
Vance, A., Maruff, P., & Barnett, R. (2003). Attention deficit hyper-
validity of delay aversion and response inhibition as measures
activity disorder, combined type: Better executive function perfor-
of impulsivity in AD/HD: A supplement to the NIMH multi-
mance with longer-term psychostimulant medication. Australian
modal treatment study of AD/HD. Journal of Abnormal Child
and New Zealand Journal of Psychiatry, 37, 570–576.
Psychology, 29, 215–228.
Van der Oord, S., Prins, P. J., Oosterlaan, J., & Emmelkamp, P. M.
Solanto, M. V., & Wender, E. H. (1989). Does methylphenidate con-
(2008). Efficacy of methylphenidate, psychosocial treatments
strict cognitive functioning? Journal of the American Academy
and their combination in school-aged children with ADHD: A
of Child and Adolescent Psychiatry, 28, 897–902.
meta-analysis. Clinical Psychology Review, 28, 783–800.
Spencer, T. J. (2006). ADHD and comorbidity in childhood. Journal
Volkow, N. D., Fowler, J. S., Wang, G. J., & Swanson, J. M. (2004).
of Clinical Psychiatry, 67, 27–31.
Dopamine in drug abuse and addiction: Results from imaging
Sprague, R. L., & Sleator, E. K. (1976). Methylphenidate in hyper-
studies and treatment implications. Molecular Psychiatry, 9,
kinetic children: Differences in dose effects on learning and
social behavior. Science, 198, 1274–1276.
Waxmonsky, J., Pelham, W. E., Gnagy, E., Cummings, M. R.,
Spreen, O., & Benton, A. L. (1977). Neurosensory center compre-
O'Connor, B., Majumdar, A., . . Robb, J. A. (2008). The effi-
hensive examination for aphasia (NCCEA). Victoria, Canada:
cacy and tolerability of methylphenidate and behavior modifi-
University of Victoria Neuropsychology Laboratory.
cation in children with attention-deficit/hyperactivity disorder
Steinberg, L. (2008). A social neuroscience perspective on adoles-
and severe mood dysregulation. Journal of Child and Adolescent
cent risk-taking. Developmental Review, 28, 78–106.
Swanson, J. M., Cantwell, D., Lerner, M., McBurnett, K., &
Weiss, M. D., Worling, D. E., & Wasdell, M. B. (2003). A chart
Hanna, G. (1991). Effects of stimulant medication on learning
review study of the inattentive and combined types of ADHD.
in children with ADHD. Journal of Learning Disabilities, 24,
Journal of Attention Disorders, 7, 1–9.
Willcutt, E. G., Doyle, A. E., Nigg, J. T., Faraone, S. V., &
Swanson, J. M., Flockhart, D., Udrea, D., Cantwell, D., Connor, D.,
Pennington, B. F. (2005). Validity of the executive function
& Williams, L. (1995). Clonodine in the treatment of ADHD:
theory of attention-deficit/hyperactivity disorder: A meta-
Questions about safety and efficacy. Journal of Child and
analytic review. Biological Psychiatry, 57, 1336–1346.
Adolescent Psychopharmacology, 5, 301–304.
Wilson, H. K., Cox, D. J., Merkel, R. L., Moore, M., & Coghill, D.
Tamm, L., & Carlson, C.L. (2007). Task demands interact with sin-
(2006). Effect of extended release stimulant-based medications
gle and combined effects of medication and contingencies on
on neuropsychological functioning among adolescents with
children with ADHD. Journal of Attention Disorders, 10,
attention-deficit/hyperactivity disorder. Archives of Clinical
Tannock, R., Ickowicz, A., & Schachar, R. (1995). Differential
Wodka, E. L., Mahone, E. M., Blanker, J. G., Gidley-Larson, J. C.,
effects of methylphenidate on working memory in ADHD chil-
Fotedar, S., Denckla, M.B., & Mostofsky, S. H. (2007). Evidence
dren with and without comorbid anxiety. Journal of American
that response inhibition is a primary deficit in ADHD. Journal
Academy of Child and Adolescent Psychiatry, 34, 886–896.
of Clinical and Experimental Neuropsychology, 29, 345–356.
Tannock, R., Schachar, R., & Logan, G. (1995). Methylphenidate
Zametkin, A. J., Nordahl, T. E., Gross, M., King, A. C., Semple, W. E.,
and cognitive flexibility: Dissociated dose effects in hyperac-
Rumsey, J., . . Cohen, R. M. (1990). Cerebral glucose metabo-
tive children. Journal of Abnormal Child Psychology, 23,
lism in adults with hyperactivity of childhood onset. New England
Journal of Medicine, 323, 1361–1366.
Journal of Learning Disabilities 44(2)
Zelazo, P. D., & Muller, U. (2002). Executive function in typical
professor at Michigan State University with a joint appointment
and atypical development. In U. Goswami (Ed.), Blackwell
in Psychology and Psychiatry.
handbook of childhood cognitive development (pp. 445–469). Malden, MA: Blackwell Publishing.
Lisa A. Hain, PsyD, NCSP, ABSNP, is an Assistant Professor and
Practicum Coordinator in the Department of Psychology at the
About the Authors
Philadelphia College of Osteopathic Medicine. Dr. Hain's research
James B. Hale, PhD is an Associate Professor of Clinical
interests center on the application and integration of neuropsycho-
Neuropsychology in the Department of Psychology at the
logical principles in the field of school psychology.
University of Victoria. He specializes in applying brain-behavior relationships to high incidence disorders, and linking neuropsy-
James Whitaker, PsyD, is a consultant with the Pearson Clinical
chological assessment data to academic and behavioral
Assessment group and an adjunct instructor in the school psychol-
ogy programs at Philadelphia College of Osteopathic Medicine and Bucknell University.
Linda A. Reddy, PhD is an Associate Professor in the Graduate
School of Applied and Professional Psychology at Rutgers
Jessica Morley, EdS, NCSP, is a School Psychologist at the Chester
University. She specializes in the assessment and intervention of
County Intermediate Unit in Pennsylvania. She provides psycho-
ADHD and ADHD-related disorders, test development and valida-
educational services to students with high incidence disorders in
tion, and school interventions.
secondary alternative education settings.
Margaret Semrud-Clikeman, PhD received her doctorate from
Kyle Lawrence is a graduate student in the School Psychology
the University of Georgia in 1990 and completed an internship
Program at the Philadelphia College of Osteopathic Medicine.
and postdoctoral fellowship at the Massachusetts General Hospital/Harvard Medical school (MGH). She continues her research inter-
Alex Smith is a graduate student in the School Psychology Program
ests in the areas of ADHD and educational neuroscience. Dr.
at the Philadelphia College of Osteopathic Medicine.
Semrud-Clikeman was awarded the 1999 Early Career Contributions award from the National Academy of
Nicole Jones is a graduate student in the School Psychology Program
Neuropsychology. She has published more than 60 articles, 45
at the Philadelphia College of Osteopathic Medicine. She works as
chapters and five books. Dr. Semrud-Clikeman is currently a
a therapist for young children with Autism Spectrum Disorders.
Source: http://medpsych.biz/pdfs/Hale%20et%20al.%202011.pdf
Infektionskrankheiten Seite 1 – LZK Thüringen – Stand: September 2013 1. Einleitung und Rechtsgrundlagen Eine Infektionskrankheit ist eine durch Erreger hervorgerufene Erkrankung. Sie ist aber nicht einer Infektion gleichzusetzen, da nicht jede Infektion notwendigerweise zu einer Erkrankung führt. Infektionskrankheiten zeigen ein breites Spektrum von zeitlichen Verläufen und Symp-tomen.
Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer Jennifer S. Temel, M.D., Joseph A. Greer, Ph.D., Alona Muzikansky, M.A., Emily R. Gallagher, R.N., Sonal Admane, M.B., B.S., M.P.H., Vicki A. Jackson, M.D., M.P.H., Constance M. Dahlin, A.P.N., Craig D. Blinderman, M.D., Juliet Jacobsen, M.D., William F. Pirl, M.D., M.P.H., J. Andrew Billings, M.D., and Thomas J. Lynch, M.D.


