Mintcare.sg


Clinical GuidanceInnovative Therapies Wound Treatment Systems
CAUTION: This guidance is not intended as a guarantee of results, outcome, or performance of the Innovative Therapies
Inc. (ITI) Wound Treatment Systems. These recommendations are intended to help clinicians establish patient-specific
treatment protocols. As with any application, please consult the patient's treating physician about individual conditions
and treatment, and follow all applicable instructions for use and labeling for product use and operation.
Always consult sections of this guidance along with the applicable instructions for use, labeling, and safety information
for the Innovative Therapies Inc. (ITI) Wound Treatment System before placing any Innovative Therapies Inc. (ITI) System
on a patient.
Table of Contents
1. Introduction . 2
1.1 The Innovative Therapies Inc. (ITI) Wound Treatment . 21.2 Device components . 3
2 Patient Selection . 4
2.1 Indications .42.2 Contraindications . 52.3 Precautions . 52.4 Stopping or suspending therapy .6
3. Procedure Outline . 7
3.1 Comprehensive plan of care . 73.2 Prescription / order .83.3 Preparation .83.3.1 Wound bed .83.3.2 Periwound skin.93.4 Dressings .103.4.1 Dressing procedure outline .103.4.2 Dressing removal. 113.4.3 Typical dressings and applications .123.4.3.1 TI White Foam Dressing .123.4.3.2 Other Wound contact layers .123.4.3.3 ITI Black Foam Dressing .133.4.3.4 ITI Polyurethane Drape .143.4.3.5 ITI Suction Tubing with SpeedConnect . 143.4.3.6 ITI Irrigation Tubing with SpeedConnect .153.4.4 Special dressing considerations .153.4.4.1 ITI Bridging Set .153.4.4.2 Mushroom dressings .163.4.4.3 Irregularly shaped wounds .173.4.4.4 Dressing two wounds .173.4.4.5 Securing a tight seal .183. Evaluation during treatment.193.5.1 Elements to document .193.5.2 Factors delaying healing .19
4. ITI Wound Treatment Systems Quick Reference Guide . 20
4.1 Therapy Unit Instructions . 204.2 Changing the Canister .214.3 Daily and weekly ITI device maintenance .21
5. Using an ITI Wound Treatment system in a Sterile Field . 22
5.1 Sterile dressings/aseptic canister . 225.2 Testing a seal in the operating room . 22
6.Patient Education . 23
Appendix A. Pressure/mode guidelines . 24Appendix A.1. Pressure/mode by wound type . 25Appendix A.2. Pressure/mode by wound condition . 26Appendix B. Irrigation Guidelines . 27Appendix B.1. NPWT with simultaneous irrigation . 27Appendix B.2. Suggested irrigation solutions . 28Appendix B.3. Intermittent irrigation . 29
Figure 1. A schematic diagram of an
ITI Wound Treatment Systems set-up . 3Figure 2. Typical irrigation and suction ports set-up .14Figure 3. Bridging two wounds .16Figure 4. Example of mushroom dressing .16Figure 5. Using a bridge to connect 2 wounds .17Figure 6. Connecting 2 wounds using the ITI Y-Connector .17
Table 1. Stopping or suspending therapy .6Table 2. Pressure/mode by wound type . 24Table 3. Pressure/mode by wound condition . 26Table 4. Suggested irrigation solutions . 28
1.1 The Innovative Therapies Inc. (ITI) Wound Treatment Systems
The Innovative Therapies Inc. (ITI) Wound Treatment Systems promote wound healing by removing infectious materials, exudate, and components associated with chronic wound fluid. Our proprietary negative pressure wound therapy (NPWT) system delivers negative pressure and simultaneous irrigation using patented foam dressings and accessories. The ITI Wound Treatment Systems are suitable for acute care, long-term care, nursing home or home care settings, and are eligi-ble for reimbursement under most coverage policies, Medicare, and state mandates.

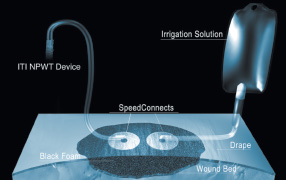
1.2 Device components
Figure 1. A schematic diagram of an ITI Wound Treatment System setup
The ITI NPWT devices: The ITI NPWT devices are the only systems on the market that deliver NPWT with simultaneous
irrigation—providing aqueous solutions to the wound without tube changes or therapy interruption. A single touch
selects pressure settings, pressure mode (continuous or intermittent pressure), and a safety lockout.
The ITI NPWT devices maintain selected pressure at canister outlet within +/- 10% of target pressure.
Intermittent pressure is on 5 minutes, off 2 minutes. The "off" interval maintains a -25 mmHg pressure.
This minimal vacuum preserves drape seal and prevents exudate backflow.
ITI Black Foam Dressing: This hydrophobic, open-cell, reticulated polyurethane foam evenly distributes negative pressure
across the wound base and allows for exudate removal. Available in four sizes, ITI Black Foam Dressing has high tensile
strength and exhibits minimal fraying when cut. Applied directly to the wound bed and to loosely fill undermined areas,
black foam can be layered as needed.
ITI White Foam Dressing: This soft, hydrophilic, open-cell polyurethane foam protects bone, tendon, ligaments, and
painful wound beds. Flexible wet or dry, ITI White Foam Dressing can be cut or rolled. Its combination of tensile strength
and pliable construction makes it appropriate for use in tunnels. White foam is applied in a single layer when used in the
wound bed. Black foam may be used on top of the white foam.
ITI Bridging Set: This bridging foam has larger pores and lower density than standard black foam—to keep cells open and
fluid moving. When a bridging technique is employed, do not allow bridging foam to come in contact with the wound bed
or intact skin.
ITI Polyurethane Drape: This clear, semi-occlusive polyurethane film with adhesive covers the wound after it has been
filled with foam. The drape seals the negative pressure environment while protecting the wound from contamination.
ITI SensiSkin™ Drape is also available for patients with friable skin.
ITI Suction Tubing with SpeedConnect:™ This 2.4 m (8 ft) tube with an adhesive connector end adheres around a hole cut
in the drape, delivering negative pressure to the wound. The distal end directly connects to the ITI NPWT device canister,
removing exudate.
ITI Irrigation Tubing with SpeedConnect: This custom 2.4 m (8 ft) tube is applied to the drape with the same technique
as the Suction SpeedConnect. It delivers aqueous fluids—removed, along with exudate, by the Suction SpeedConnect.
The distal end's Luer lock connector accommodates a wide range of commercial tubings. This tube is often called the
Irrigation SpeedConnect. Irrigation may also be applied using the ITI Irrigation Delivery Set. The Irrigation Delivery Set is
an integrated system. The irrigation SpeedConnect is melded with the tubing and irrigant container. This eliminates the
need to connect tubings.
ITI Canister: Available in various volumes depending on the specific device used, the ITI canister collects exudate and
irrigation solution. Its built-in filter prevents overflows. The 300 cc and 500 cc canisters are available with an isolyzer
pack to solidify fluid. The 300 cc and 500 cc canisters are also available without an isolyzer pack. The ITI device monitors
canister volume. Audible and visual alarms occur when the canister is 90% full. While the alarms continue, therapy is
provided. The device will stop operating when the canister is 100% full. The filter size and structure provide a dual barrier
against fluids and microorganisms entering the ITI device.
ITI Y-Connector: Joins two ITI Suction Tubing with SpeedConnects to a single ITI device canister. Use when employing a single ITI NPWT device for large wounds or two wounds.
2. Patient Selection
The ITI Wound Treatment System is indicated for patients who would benefit from vacuum-assisted drainage and controlled delivery of topical wound treatment solutions and suspensions over the wound bed.
The system comprises a dressing and a powered suction pump that is designed to help promote wound healing. The system is intended for use on patients who would benefit from a suction device—including patients who would benefit from vacuum assisted drainage and removal of infectious materials or other fluids from wounds under the influence of continuous and/or intermittent suction pressure.
ITI NPWT Wound Treatment Systems are intended for patients with:
• Chronic, acute, traumatic, subacute, and dehisced wounds.
• Diabetic ulcers and pressure ulcers.
• Flaps and grafts.
2.2 Contraindications
ITI NPWT Wound Treatment Systems are contraindicated for patients with:
• Malignancy in the wound.
• Untreated osteomyelitis.
• Non-enteric or unexplored fistulas.
• Necrotic tissue with eschar present.
• Infants or other patients with low fluid volume.
DO NOT place the ITI Dressing over exposed blood vessels or organs.
DO NOT use with irrigation if irrigation fluid temperature could cause an adverse reaction, such as a change
in the patient's core body temperature. It is not recommended to deliver fluids to the thoracic cavity.
The ITI Dressings are also contraindicated for hydrogen peroxide and solutions which are alcohol- based or contain alcohol.
Use with extreme caution for:
• Patients with active bleeding, difficult wound hemostasis, or who are on anticoagulants.
• Weakened, irradiated, or sutured blood vessels or organs.
• Wounds with enteric fistula.
• Unstable thoracic wounds.
• Deep sternal wounds with unstable sternum.
DO NOT place a dressing over exposed blood vessels or organs.
When placing a dressing in close proximity to blood vessels, tendons, nerves, or organs, ensure that they are adequately protected with overlying fascia, tissue, or other protective barriers.
Bone fragments or sharp edges could puncture a dressing barrier, vessel, or organ
The ITI Wound Treatment System does not require sterile canisters. Each individually packaged ITI canister is aseptic (free of pathogenic microorganisms). Keep in mind that protocol requires ITI canisters to remain outside the sterile field.
It is not recommended to use enzymatic debridement ointments as they may clog the foam dressing.
2.4 Stopping or suspending therapy
Suspected or confirmed Discontinue device and dressing, evaluate, and treat
Therapy can resume when infection
is resolving.
Consider the following interventions, as appropriate:
Check for exposed nerves.
• Decrease pressure
If exposed nerves are found,
discontinue the device and
• Prior to dressing, irrigate with saline or topical anesthetic
• Cover wound base with white foam, topped with black
Hyperbaric oxygen
NEVER allow a negative pressure wound therapy (NPWT)
Small electrical arcs associated with
(HBO) therapy session
device—whether on or off—inside a hyperbaric oxygen (HBO) a NPWT device and dressing compo-
chamber. Device must be disconnected prior to placement in nents could be a fire hazard.
the HBO.
• If HBO therapy will exceed 2 hours—dressing must be
removed. Apply moist saline gauze dressing in its place.
• If HBO therapy is less than 2 hours—consult HBO chamber
manufacturer with respect to the presence of a dressing during therapy.
• If dressings are allowed, ITI recommends:
• Discontinue therapy. Disconnect the tubing from the
• Secure the tubing in a manner that is comfortable to
• If there is concern about electrostatic discharge due
to the synthetic materials of the dressing, cover the tubing and dressing with a towel moistened with normal saline—this will prevent electrostatic charge accumulation.
• Reconnect and restart the ITI device ONLY after the patient
has been removed from the chamber.
NEVER allow an NPWT device—whether on or off—inside an
MRI suite. The foam dressing can remain in place, clamped.
When the patient has exited the MRI suite, restart the ITI
NPWT device.
X-ray scan, CT scan
The dressing can be left in place and the device can continue ITI black foam and white foam are not
Undebrided eschar or
Delay placing the device until wound is debrided.
• Excessive slough can clog
slough covering more
foam pores, decreasing therapy
than 25% of the total
• Continuous irrigation assists
in removing slough from the wound base
• Use of enzymatic debridement
ointments may clog foam pores and impact the distribution of negative pressure across the wound base
If the dressing is in the area where equipment or pads are to
be placed, remove it. If dressing is left in place, clamp and
remove suction tubing from the canister. In either instance,
remove the ITI device from the patient area.
1. Clamp the tubing.
The ITI device is designed to not
2. Disconnect the tubing from the canister.
cause an electrical shock when in
contact in water. However, these
3. Remove the ITI NPWT device from the bathing area.
steps are a necessary precaution.
Autonomic dysreflexia
If a patient experiences autonomic dysreflexia (sudden
Discontinuing use of NPWT helps
related to spinal cord
elevation in blood pressure or heart rate in response to stim-
minimize sensory stimulation.
ulation of the sympathetic nervous system)
discontinue use of NPWT.
Table 1. Stopping or suspending therapy
3. Procedure Outline
Specific application of NPWT with ITI Wound Treatment Systems varies with each patient and wound. All wounds require close
monitoring between dressing changes. An ITI clinical representative can provide assistance with specific clinical questions.
1. Become familiar with the specific ITI device's User's Manual and these clinical guides.
2. Develop a comprehensive, individual plan of care—including NPWT using the ITI Wound Treatment System,
along with nutritional support, tissue load management, skin protection, and patient education.
3. Obtain prescription/order.
4. Prepare the wound bed.
5. Apply dressing components.
6. Begin treatment with NPWT using the ITI Wound Treatment system, selecting therapy options conforming
to the individual plan of care.
7. Educate the patient on use of the ITI Wound Treatment System.
8. Document all subsequent dressing changes and wound status, the number of foam pieces placed in,
and removed from, the wound.
Wounds should progress steadily under NPWT with the ITI Wound Treatment System. If the wound is not progressing after two weeks of therapy, reevaluate treatment options.
3.1 Comprehensive plan of care
A comprehensive evaluation of both patient and wound is essential to determine whether NPWT with irrigation is
indicated. Even if the wound meets all criteria listed in Section 2. Patient Selection, the clinician must carefully consider
whether the device is appropriate.
Patient evaluation should include:
• Health history, including any co-morbidities.
• Current medications.
• Nutritional assessment.
• Strategies for offloading weight-bearing surfaces.
• Caregiver support (for outpatient and home care patients).
Wound assessment should include:
• Wound type and location.
• Wound history.
• Length, width, depth, tunnels, undermining.
• Wound base tissue type.
• Exudate color, odor, and quantity.
• Condition of periwound skin.
• Response to previous treatments.
3.2 Prescription/order
Before therapy begins, the appropriate ITI NPWT Wound Treatment System must be specified in a signed prescription or order from a qualified clinician. The order should include:
• Wound type and location.
• Pressure setting (according to settings on device used).
• Pressure mode (continuous or intermittent pressure).
Note: The ITI device maintains selected pressure at the canister outlet within +/- 10% of target pressure.
For intermittent pressure, the "off" interval maintains a -25 mmHg pressure. A minimal vacuum preserves
drape seal and prevents exudate backflow.
• Type of irrigation solution and rate.
• Type of dressings and change frequency.
• Wound cleansing procedure.
• Any special treatment for periwound skin.
• Duration of therapy.
3.3.1. Wound bed
Thorough wound bed preparation is essential before beginning therapy. In addition, at every dressing change the wound must be thoroughly cleansed and inspected, as follows:
1. Debride non-viable and loose necrotic tissue. Remove any slough and eschar obliterating the wound base.
These tissue types prevent the foam dressing from contacting the wound base, inhibiting fluid movement. Treatment can proceed if there is scattered slough in the wound base.
Note: Continuous irrigation assists in removing scattered slough from the wound base. Use of enzymatic debridement
ointments may clog foam pores and impact the distribution of negative pressure across the wound base.
2. Thoroughly cleanse the wound and all tunnels/undermining. Flush a generous amount of irrigation solution across
the surface and any dead space (irregular contours, undermining, or tunnels) to remove surface bacteria and debris.
Note: The individual plan of care should specify a wound cleansing procedure. Syringes, catheters, and other cleansing
devices can be effective.
Continuous irrigation therapy does not substitute for wound cleansing at dressing changes.
3.3.2. Periwound skin
Periwound skin must be assessed at the onset of therapy and at each dressing change. Skin protection should be specified in the individual plan of care.
The ITI Polyurethane Drape's adhesive helps prevent maceration. The ITI SensiSkin Drape for Sensitive Skin provides additional periwound protection. Consider its use as part of the suggested interventions below.
Suggested interventions for periwound skin conditions
• Thin, fragile skin: Apply skin barrier wipe, beginning at the wound edge and
working out to coat all skin covered by drape.
• Denuded skin:
• If the skin is not weeping substantially, use a non-sting skin barrier wipe over denuded areas and
the intact periwound skin. Cover with drape as usual. If denudement does not resolve quickly, consider using the ITI SensiSkin Drape for Sensitive Skin.
• If significant weeping jeopardizes obtaining a seal with the drape, apply the non-sting skin
barrier as above and a hydrocolloid over the weeping areas.
• Skin tears:
• For small skin tears in which the surrounding skin is stable: Treat with a non-sting skin barrier and drape as usual.
• For larger or draining skin tears: Cover with appropriate wound dressings (such as hydrocolloids, adhesive foams)
and drape as usual.
• To prevent future skin tears: Carefully remove the drape at each dressing change.
• Candidiasis (skin yeast rash): Typically presenting as a macular/papular rash that may itch, candidiasis can usually
be treated with a topical antifungal product without a therapy disruption:
3. Sprinkle the skin with an appropriate antifungal powder and spread with gloved finger over and beyond the rash.
4. Seal with skin barrier wipe or spray.
5. Repeat at each dressing change until rash is resolved for at least 72 hours.
Note: If Candida is present on the periwound skin, consider whether fungus might also be in the wound base.
If fungus in the wound base is suspected, consider irrigating with an appropriate antimicrobial or antifungal product.
See Appendix B. Irrigation.
• Moist skin related to perspiration: If heavy perspiration causes the drape to dislodge:
• Sometimes a skin barrier wipe is sufficient.
• If the problem persists, sprinkle ostomy powder over the skin and spread with a gloved finger.
Seal with a skin barrier by patting with wipe or lightly applying spray.
• If additional adhesive is needed, apply a thin coating of medical adhesive onto the skin.
• Treat using the same interventions described above for moist skin.
• Consider using ITI SensiSkin Drape. With a higher moisture vapor transfer rate, MVTR,
than standard drapes, this drape may prevent maceration of the skin.
• If the maceration is severe, or does not resolve quickly, consider these steps:
1. Place therapy on hold for 48 hours to give the skin time to recover its integrity.
2. During this therapy holiday, apply appropriate moist wound dressings.
3. When therapy is resumed, consider changing the dressing application and/or pressure settings.
• Adhesive non-acceptance: If the drape does not adhere, apply a light coating of medical adhesive to the skin before
• Adhesive sensitivity (allergy):
• If a patient reports previous sensitivity to adhesives, a patch test is advised:
1. Place three small—approximately 2 sq cm (0.3 sq in)—drapes on intact skin well away from the wound and
surrounding skin.
2. Every 24 hours remove one piece.
3. If a rash occurs, it is likely that the patient is sensitive to the adhesive.
• If mild symptoms occur during the patch test or during therapy, consider using the ITI SensiSkin Drape.
A steroid spray can be prescribed to reduce itching.
• If symptoms are severe, discontinue the use of the adhesive drape and, call an ITI clinical consultant
for assistance in planning alternative cover dressings.
Because of the complex nature of wounds, clinician judgment is essential for each case. Routine dressing changes should occur every 48 to 72 hours. Dressing changes for infected wounds should be accomplished more frequently than 48 to 72 hours. Always replace with sterile disposables from unopened packages. Follow established protocols regarding clean vs. sterile technique. Consult your ITI clinical representative for assistance with specific clinical questions.
3.4.1. Dressing procedure outline
1. Carefully remove any previously applied dressing.
2. Carefully inspect—visually and manually—to ensure complete foam removal.
Consult prior documentation for number of pieces inserted.
3. Thoroughly cleanse wound.
4. Apply wound contact layer if indicated. (If a topical silver dressing is prescribed, apply the silver wound
contact layer over the wound bed—with black foam layered on top).
5. Fill wound with appropriate foam type, documenting number of pieces. Do not pack the dressing tightly into
the wound space. Foam should very lightly contact the wound edges, but not exert pressure on the wound edges (which may slow wound edge contraction).
6. Cover with drape. As the drape's adhesive is activated by pressure and body temperature,
when applying the drape, press firmly with gloved hands to ensure a seal.
7. Cut a nickel-sized hole in the drape to apply the SpeedConnects.
8. Apply SpeedConnects: one for NPWT, and one for irrigation.
9. Select therapy settings (pressure and mode) and begin therapy.
10. Change dressings every 48 to 72 hours (more frequently for infected wounds).
3.4.2. Dressing removal
Careful dressing removal prevents damage to the wound and surrounding skin.
• To facilitate release, gently stretch and lift drape, while using a gloved index finger to hold down intact skin.
• Always make sure that all components have been removed from the wound. After removing the dressing:
• Carefully observe the visible wound base.
• Gently sweep undermined or tunneled areas with a gloved finger,
if possible, to manually check for complete foam removal.
• Consult prior documentation and then count pieces of foam to be
certain all previously inserted pieces have been removed.
Tips: If the drape does not release easily, or if the skin is very fragile, try one of the following:
• Apply a warm wet cloth to the skin as the drape is gently lifted.
• Use a medical adhesive remover. After applying this product, wash and rinse the skin thoroughly to remove
any residue that might prevent the new drape from adhering.
• If the skin is extremely fragile, carefully cut away the drape only over the open wound to enable the dressing
change, and layer a new drape over the old drape. By the next dressing change, the old drape should more readily release from the skin.
• Consider pre-medication, irrigating with a topical anesthetic before removing the dressing,
or applying a wound contact layer.
3.4.3. Typical dressings and applications
3.4.3.1. ITI White Foam Dressing
ITI White Foam Dressing is non-occlusive, hydrophilic, malleable, and provides tensile strength. The ITI white foam is soft and pliable wet or dry. Supporting fluid movement, white foam helps keep the wound interface moist.
When to consider using white foam
• To loosely fill tunnels or tracts (first, ensure a fistula is not present).
• To assuage painful wounds.
• To treat wounds with fragile granulation tissue.
• To protect exposed bone, tendons, or ligaments.
• To treat flaps and grafts, when using black foam layer on top.
Tips when using white foam
• Do not layer white foam as multiple layers may cause pooling of fluid.
Wound contact layer is usually unnecessary under white foam.
• Can be used with irrigation.
• Does not require an increased pressure setting.
• Loosely fill all dead space within wounds to prevent granulation forming a seroma or abscess.
• White foam is easily cut into strips for small spaces. Keep in mind that thin strips inherently provide
less tensile strength. As needed, wrap thin strips in a wound contact layer before placing them in a tunnel.
• Roll to fill a larger tunnel or space.
• When filling tunnels or undermined spaces, always leave a significant portion of the foam visible
in the wound base—so it is found during dressing removal. Because cutting white foam decreases tensile strength, after cutting the foam pull both ends simultaneously to check for adequate tensile strength.
• Layering of black foam over the white foam is recommended. The black foam enables NPWT to
evenly transport fluid collected by the white foam.
• Always document the number of pieces of white foam used in the wound.
3.4.3.2. Other Wound contact layers
Also known as a wound non-adherent layer, the wound contact layer is a non-adhesive, non-occlusive mesh that protects vulnerable structures, fragile granulation tissue, grafts, or suture lines. There are several brands of wound contact layers on the market, each with distinct characteristics. In some cases,
a wound contact layer can be used in place of white foam. In granular wound bases, a wound contact layer under black foam may slow the rate of granulation tissue formation.
Antimicrobial impregnated wound contact layers are available. If a topical silver dressing is prescribed, apply the silver wound contact layer over the wound bed—with black foam layered on top.
Always document the inclusion of wound contact layers.
When to consider using a wound contact layer
• If white foam is unavailable, use a wound contact layer to protect exposed bone,
tendon, ligaments, fascia, or tunnels.
• As a covering over closed incision lines (sutured or stapled) before foam is applied.
• As a wick to loosely fill small tunnels.
• In the base of painful wounds or wounds with fragile granulation tissue.
• To treat flaps and grafts, using black foam layered on top.
• To reduce wound base bioburden, apply a silver-impregnated wound contact layer.
3.4.3.3. ITI Black Foam Dressing
ITI Black Foam Dressing is hydrophobic. Designed to conform to wound surfaces, black foam evenly distributes negative pressure over the wound base, removing exudate and irrigation fluids.
When to consider using black foam
• Directly to the wound bed when no structures are visible.
• Over white foam or wound contact layer applied to the base of a wound.
• To loosely fill undermined areas.
• Over closed suture lines (apply wound contact layer first).
• Over grafts and flaps (apply a white foam layer first).
Tips for using black foam
• Never place black foam on intact skin or wound edges.
• Cut black foam slightly smaller than wound.
• Black foam can be cut to fit odd-shaped wounds. DO NOT use black foam in tunnels.
Black foam may be used in undermining. To ensure complete removal of black foam in extensive undermining,
• consider wrapping it in a wound contact layer.
• DO NOT cut black foam while holding it directly over the wound.
• Loosely fill all parts of the wound with appropriate foam, but DO NOT pack or tightly fill, which would decrease the
rate of granulation. The goal is to enable negative pressure to slightly collapse wound edges around the foam.
• Extend black foam slightly higher than skin level. This prevents negative pressure from pulling the
dressing down in a convex shape.
• There is no need to thin black foam in shallow wounds.
• Black foam may be layered in deep wounds.
• Black foam has sufficient tensile strength for undermining. When filling undermined spaces, always
leave a significant portion of the foam visible in the wound base—so it is found during dressing removal.
• Loosely fill all dead space within wounds to prevent granulation from forming a seroma or abscess.
• Always document the number of pieces of black foam used in the wound.


3.4.3.4. ITI Polyurethane Drape
ITI Polyurethane Drape should cover all foam plus approximately 4 cm (1.6 in) of surrounding skin. Before placing the SpeedConnect, cut a 2.0 cm (0.8 in) nickel-sized hole in the drape. As the drape's adhesive is activated by pressure and body temperature, press the drape firmly with gloved hands to ensure an effective seal.
Tips for using the drape
• The drape can be applied as a single piece or in strips.
• Drape strips can be overlapped.
• The drape can be used to patch air leaks.
• The ITI SensiSkin Drape is available for fragile or aging skin.
• Use of skin barrier wipe on skin prior to placing drape may assist in obtaining seal. Application of skin barrier wipe
around perimeter of drape once it is in place can enhance adherence to the skin and reduce lifting of the drape.
3.4.3.5. ITI Suction Tubing with SpeedConnect
ITI Suction Tubing with SpeedConnect removes exudate and irrigation fluids from the wound. The adhesive connector end attaches to a hole cut in the drape. The flexible blue end attaches to the suction port of the ITI canister.
After cutting a 2.0 cm (0.8 in) hole in the drape, remove the backing from the Suction SpeedConnect and press it firmly onto the drape—centered over the hole.
Exudate Irrigation Solution
Figure 2. Typical irrigation and suction ports set-up.
Tips for using Suction SpeedConnect
• In large wounds, two suction SpeedConnects may provide optimal treatment.
Use a Y-connector with two SpeedConnects and attach to a single therapy unit.
• Consider the best location for placing the Suction SpeedConnect. For example, for an irregularly shaped wound,
the Suction SpeedConnect could be placed over the area of greatest depth. For abdominal wounds in which the patient is frequently sitting or is ambulatory, place the Suction SpeedConnect in the lower third of the wound.
• Consider the best direction for placement of the Suction SpeedConnect tubing. To prevent pressure ulcers from
forming under tubing, consider changing direction of the tubing at each dressing change.
• If additional surface is needed to place the Suction SpeedConnect, use a bridge to increase dressing surface.
• Some fluid in the tube is normal during operation.
Tips for optimal drainage
• Place the ITI device at or below wound level.
• During dressing changes look for fluid pooling in the wound bed. Pooling is caused by thick exudate,
fibrin or serosanguinous fluid clogging the white foam, black foam, or ITI Suction Tubing with SpeedConnect. This can be resolved by the use of simultaneous irrigation.
• Consider, if medically advised, setting the ITI NPWT device to intermittent mode. Fluctuating pressure changes
help move fluids out of the tubing.
3.4.3.6. ITI Irrigation Tubing with SpeedConnect and ITI Irrigation Delivery Set
ITI Irrigation Tubing with SpeedConnect delivers irrigation solution to the wound. The tubing's adhesive connector end attaches to the drape in the same manner as the Suction SpeedConnect. The distal end's Luer lock connector accommodates a wide range of commercial tubing sets. When applying NPWT with irrigation to small wounds, the two SpeedConnects can be placed close to each other.
The ITI Irrigation Delivery Set is an integrated system. The irrigation SpeedConnect is melded with the tubing and irrigant container. This eliminates the need to connect tubings. Instructions for set up and irrigation delivery are on the irrigant container.
See Appendix B. Irrigation.
3.4.4. Special dressing considerations
3.4.4.1. ITI Bridging Set
Bridging foam has larger pores and lower density than standard black foam. This keeps cells open and fluid moving. The bridging foam's size is optimized for maximum flow.
When to consider bridging
• To increase dressing surface area in a very small wound.
• To dress multiple small wounds: Bridge one wound to another.
• To locate the SpeedConnect away from a bony prominence that the patient is unable to offload—
such as the sacrum or heel—or contours, or uneven skin surfaces.
• If needed, employ two bridges: One for suction and the other for irrigation.
Tips for bridging
• The ITI Bridging Set is designed for optimal fluid movement.
• Bridge foam should never touch intact skin. Always drape the skin before placing a foam bridge over it.
• Bridge foam should never directly contact the wound base.
• Bridge foam must directly contact the foam that is covering or filling the wound. This maintains negative pressure.
• When bridging wounds from the sacral area to a lateral aspect, avoid placing the bridge over the iliac crest or
other bones. If the patient lies on lateral bones, the bridge can be obstructed, blocking suction to the wound, which can dislodge the dressing.


• DO NOT increase pressure in an effort to move more fluid across the bridge. Increased pressure does not fix chronic
blockage. Consider, if medically advised, setting a lower pressure or setting to intermittent mode.
• Avoid using a bridge narrower than 3.5 cm (1.4 in). For extremities, DO NOT construct a bridge to create a circumfer-
ential dressing. This could create a tourniquet effect that can constrict blood flow.
Figure 3. Bridging two wounds.
3.4.4.2. Mushroom dressings
A mushroom dressing may be appropriate for deep, small wounds. If these wounds are dressed conventionally, negative pressure may overcollapse the foam, impeding the removal of fluid. The mushroom dressing's larger foam surface reduc-es the risk of foam collapse.
1. Fill the wound as usual.
2. Drape the periwound skin circumferentially, extending approximately 4 cm (1.6 in) from the wound opening.
3. Cut a piece of ITI Black Foam Dressing, extending approximately 2.5 cm (1 in) from the wound opening
(this looks like a mushroom cap).
4. Lay the black foam over the filled wound and complete dressing as usual.
5. Thin the width of the ITI Black Foam as necessary.
Figure 4. Example of mushroom dressing.
3.4.4.3. Irregularly shaped wounds
To more easily conform black foam dressing to a wound with a very irregular shape, cut to shape.
3.4.4.4. Dressing two wounds
To apply therapy to two wounds using a single ITI Wound Treatment System device, either:
Use a bridge to connect both wounds to a single Suction SpeedConnect, or
Dress each wound separately with its own Suction SpeedConnect and Y-connect both SpeedConnects to the canister. Replace the ITI Y-Connector at each dressing change.
Figure 5. Using a bridge to connect 2 wounds.
Figure 6. Connecting 2 wounds using the ITI Y-Connector.
3.4.4.5. Securing a tight seal
Sometimes a wound's location makes it hard to achieve an air-tight drape seal. Consider using one of the products listed
below. Also see 3.4.2. Periwound skin.
• Protective Skin Barrier wipes or sprays: Use on skin before placing drape. Use of skin barrier wipes or sprays on skin
prior to placing drape may assist in obtaining a seal. Application of skin barrier wipes or sprays around the perimeter of the drape once it is in place, can enhance adherence to the skin and reduce snagging or lifting of the drape.
• A border or "picture frame" placed around the drape edge with 1 in tape is useful to prevent drape from snagging on
bedding/clothing or rolling up in friction prone areas. In large wounds, it is useful to hold drape edges down, thereby preventing several micro-leaks that together can trigger the leak alarm.
• Ostomy Paste: Use as "caulk" to seal leaks:
• Before draping the leak-prone area: Place a bead or line of paste on the skin.
• If a leak occurs after the drape is placed: Remove the plunger from a syringe, fill the syringe with paste,
and replace the plunger. Then, placing the hub of the syringe into the leak, discharge paste from the syringe t o fill the leaky area.
• Ostomy paste strips or cohesive seals: Shape these materials to fill creases or difficult-to-seal areas.
For best results, lay the material on intact skin before applying the drape. Mold by hand after applying the drape.
• Hydrocolloid strips: Cut hydrocolloid dressings into strips and layer to fill creases or uneven skin.
For best results, lay hydrocolloid directly on dry skin before applying the drape.
3.5 Evaluation during treatment
The patient, wound, and NPWT device must be carefully monitored during therapy. Frequent observation is essential even when patients have been receiving therapy for a long time.
At each dressing change, carefully evaluate and document the wound and surrounding skin. Perform a complete wound assessment at least every 7 days. The rate of healing varies with wound type and patient co-morbidities.
3.5.1. Elements to document
• Number of pieces removed of each type of foam.
• Wound contact layer removed, if applicable.
• Wound base appearance.
• Periwound skin condition.
• Amount and color of exudate.
• Odor, if present after cleansing.
• How wound was cleansed.
• Number of pieces applied of each type of foam.
• Pressure setting and mode.
• Irrigation solution used and application rate.
• Dressing pulled down and stable.
• Reported pain with related interventions.
• Patient education for the wound and The ITI Wound Treatment System.
3.5.2. Factors delaying healing
If a wound fails to progress, or if negative progress is noted, consider these possible factors:
• Internal factors include: Infection, biofilm formation, inadequate nutrition, anemia, dehydration,
and vascular insufficiency.
Wound colonization can run the spectrum from scattered free-floating colonies, to colonies that form biofilms, to
frank infection. NPWT with continuous irrigation helps keep bacterial load reduced. See Appendix B. Irrigation.
• If a topical silver dressing is prescribed, apply the silver wound contact layer over the wound bed—with black foam
layered on top. Keep in mind that although topical silver products decrease bacterial load, they do not replace the need to thoroughly debride and cleanse the wound at each dressing change.
• External factors include: Pressure over the wound, trauma, and therapy turned off for prolonged periods.
If, after correcting these factors, wound healing still does not progress, consider discontinuing NPWT therapy and imple-menting another dressing/therapy regimen.
4. Device Quick Reference Guide
Please refer to the appropriate ITI Wound Treatment System device User's Manuals for detailed operating instructions
and safety tips. IMPORTANT: As with any prescription medical device, failure to follow product instructions or changing
settings and performing therapy applications without the express direction and/or supervision of a trained clinical
caregiver, may lead to improper product performance and the potential for serious or fatal injury.
4.1 Therapy Unit Instructions
1. Ensure that an ITI Fluid Collection Canister is properly placed in the receptacle of the Therapy Unit.
Note: Always use a new canister with a new patient.
2. Inspect the adhesive tubing connectors to ensure that they are properly connected to the ITI Foam Dressing
and that the connections are well sealed.
3. Connect the distal end of the SpeedConnect tubing with the blue tapered connector to the suction port of the
Canister. Gently twist and push the connector on just enough to secure and seal it. Also, ensure that the clamp on the SpeedConnect tube is open.
4. Attach or slide the canister into place making sure the 2 device ports are properly lined up and/or connected.
(Refer to the User's Manual for the specific device you are using for complete instructions).
5. Plug the Therapy Unit's A.C. Adapter into a suitable 120 VAC, 60 Hz, outlet. Insert the power plug into the Power
Jack on the side of the Therapy Unit. The green "power" light on the unit indicates that the unit is receiving power. The amber light below the green "power" light indicates that the battery is charging.
6. Press the ON button to begin therapy. Set the device to desired pressure. Select the desired mode, either continuous
or intermittent. Refer to the appropriate ITI NPWT device's User Manual for instructions on how to set intermittent mode. The unit will remember the mode setting when the power is turned OFF and ON. During intermittent mode operation, the unit will provide therapeutic (set) pressure for 5 minutes (on phase) and approximately -25 mmHg for 2 minutes (off phase). By maintaining this lower pressure during the "off" phase of the cycle, the dressing seal is never compromised. This method of applying intermittent pressure also increases patient comfort.
7. Set pressure lockout if desired. Consult user's manual for device specific lockout instructions.
8. Dressing should collapse, indicating the presence of a vacuum. If you are using irrigation, once target pressure is
achieved, turn the irrigation fluid supply on and adjust flow rate for desired therapy.
9. Carefully check dressing for an adequate seal. If air leak(s) is present, repair with additional ITI Semipermeable
drape material as needed.
10. 1The therapy unit should be operated at least 22 out of every 24 hours to prevent possible infection.
4.2 Replace the ITI Canister
1. Close the clamp on the suction tubing.
2. Turn the device off.
3. If using irrigation, clamp the roller clamp on the fluid delivery tubing.
4. Remove tubing connector from top of ITI Canister. Place canister cap onto canister.
5. Read volume in canister and document, press canister release button and withdraw canister from the unit.
6. Dispose of the canister according to local, state, and federal regulations as well as institutional protocols.
7. Place a new ITI canister into the ITI device making sure the 2 ports on the canister are properly lined up and
connected to the appropriate openings on the device. Attach the distal end of the SpeedConnect tubing to the suction tubing port.
8. Turn the device on and open the tubing clamp. Ensure that pressure and mode are correct.
9. If using irrigation, unclamp the roller clamp on the fluid delivery tubing and resume irrigation
at the ordered flow rate.
4.3 Daily and weekly ITI NPWT device maintenance
• Daily: Visually inspect the device. Check to ensure the device is functioning properly. Check for any sign of
• If the unit is not functioning properly, refer to the appropriate User's Manual Operating Instructions/Alarm
Troubleshooting guide, or contact ITI to have the unit serviced.
• If the device appears contaminated or dirty, follow the Weekly instructions below.
• Weekly: Wipe the ITI device with either a diluted solution of 5 ml bleach in 1 liter of warm water (approximately
1 teaspoon bleach in 1 quart water) or a mild disinfectant. Use a coarse cloth and wring out any excess solution until the cloth is damp and not dripping. Other cleaning fluids are not recommended, as they can damage the finish of the enclosure.
Follow standard safety precautions when cleaning the unit.
Cleaning solutions that are recommended include bleach and water, Clorox Wipes, Dispatch, Sani Cloths, CaviWipes, Viraguard®, OxyFect™ H, CLINI-TECH®, 3M™ Quat Disinfectant and any mild hospital-grade disinfectant.
Inspect the AC adapter for damage and/or unusual wear. Replace damaged or worn power supply immediately. AC adapters are available from ITI. Do not substitute with other vendors' adapters—this could cause serious electrical damage.
5. Using an ITI Wound Treatment system in a Sterile Field
5.1 Sterile dressings / aseptic canister
All ITI dressings are packaged sterile and are latex-free. ITI employs ethylene oxide (EtO) sterilization for our foam and drape products. This avoids radioactivity issues associated with sterilization by gamma irradiation and its associated waste materials.
The ITI Wound Treatment System does not require sterile canisters. Each individually packaged canister is aseptic.
Aseptic canisters are acceptable because the sterile ITI Suction Tubing with SpeedConnect, linking the ITI device to the canister, is 2.4 m (8 ft) long and does not connect in-line or mid-line. The separation between canister and wound eliminates the need for sterilized canisters.
Protocol requires canisters to remain outside the sterile field.
5.2 Testing a seal in the operating room
To test a seal in the operating room, follow these steps:
1. In the OR, apply negative pressure wound dressing.
2. Attach the ITI Suction Tubing with SpeedConnect to low wall suction.
3. After the seal is confirmed, clamp and cover the end of the tubing with a drape or place it inside a sterile glove.
4. After the patient is transported to a post-operative area, attach the ITI Suction Tubing with SpeedConnect
to the ITI device. Open the clamp, turn the device on and proceed with setting pressure and mode.
6. Patient Education
For the full set of ITI clinical resources, go to the Innovative Therapies Inc. website www.itimedical.com or contact your ITI representative.
For at-home use, the FDA mandates that patients and their caregivers be instructed on how to safely operate a device—and how to get help if needed. Patients must demonstrate—and the clinician must document—satisfactory competence.
• Use The Innovative Therapies patient education guides for home use of ITI NPWT. ITI recommends that one copy be
kept in the medical record and one copy be given to the patient and/or caregiver. The teaching plan includes:
• How to plug the therapy unit in and know that it is charging.
• How to turn the therapy unit off and on.
• How to read and troubleshoot the alarms.
• How to change the canister.
• How to reseal a leak in the drape.
• Actions to take if a dressing must be removed.
• How to monitor for excessive bleeding.
• Emergency action plan.
• How to reorder supplies.
• Who to call if help is needed or to answer questions.
• Also provide the patient and caregiver with the appropriate instruction guide for using the ITI Wound Treatment
System at home. This guide supplements (but does not replace) one-on-one patient education.
Appendix A. Pressure / Mode Guidelines
The ITI Wound Treatment Systems are indicated for patients with chronic, acute, traumatic, subacute, and dehisced wounds, diabetic ulcers, pressure ulcers, flaps and grafts.
Note: This guidance was developed based on manufacturer's knowledge of performance of dressing components in cer-
tain wound types. As with any application, please consult the patient's treating physician about individual conditions and
treatments, and follow all applicable instructions for use and labeling for product use and operations.
Appendix A.1. Pressure/mode by wound type
Wound Type
Suggested Pressure/Mode Notes
Acute WoundsTraumatic injuries
Pressure: -120/125mmHg
Irrigation provides continuous
Mode: Continuous Consider inter-
cleansing and decreases pain.
mittent mode once exudate volume
Closed incisions for
Pressure: -120/125mmHg
Place a 4 cm (1.6 in) drape border
patients at risk for wound
around the incision—directly adja-
cent to sutures or staples.
Skin and muscle flap
Apply wound contact layer over
incisions are included in
suture line, extending approximate-
ly 1 cm (0.4 in) beyond that line.
Cover with black foam, drape, and
apply SpeedConnect as usual.
Therapy is generally discontinued
after 1 week.
Typically: Black foam
Pressure: -70/75 to -120/125 mmHg Apply wound contact layer or white
For some cases, the
foam over graft. Then apply black
clinician can choose
Therapy is generally discontinued
after 1 week.
For bioengineered skin products,
check with the manufacturer for
specific guidance prior to using
Surgically debrided
Typically: Black foam
Pressure: -120/125 mmHg
If hemostasis is achieved in the
If structures exposed:
Mode: Continuous until exudate
OR, the device can be placed at
Single layer white foam
decreases, then intermittent
with black foam on top.
After placing the device, frequently
monitor the dressing and exudate
to detect any bleeding.
Wound Type
Suggested Pressure/Mode Notes
Chronic WoundsPressure ulcers
Typically: Black foam
Pressure: -120/125 mmHg
Irrigation helps stalled wounds
If structures exposed:
begin normal healing progression.
Single layer white foam
with black foam on top.
Lower extremity ulcers,
Pressure: -120/125 mmHg
In some cases, edema manage-
such as venous leg ulcers
Mode: Continuous.
ment is required. If compression
wraps are used, they should overlie
Consider switching to intermittent
the dressing, but must not overlie
when exudate decreases.
the SpeedConnect. Select appropriate topical irrigation
Lower extremity ulcers
Typically: Black foam
Pressure: -120/125 mmHg
In cases when wounds are dry and
due to arterial disease
If structures exposed or
If wound pain occurs, decrease
prone to infection, irrigation helps
pain occurs: Single layer pressure to lower setting.
promote granulation and remove
of white foam with black
foam on top.
Typically: Black foam
Pressure: -120/125 mmHg
Patients with bone exposed or
If structures exposed:
Mode: Continuous.
close to wound surface should be
Single layer of white
evaluated for osteomyelitis.
Consider switching to intermittent
foam with black foam on when exudate decreases.
If periwound skin is particularly vul-
nerable, take preemptive measures
to protect skin from moisture.
Dehisced surgical
Pressure: Consider lower pressures If bowel is close to wound surface,
-70/-75 mmHg until wound bed
delay therapy due to the risk of fis-
is fully granular, then increase if
tula development.
The following dressing technique
Mode: Continuous.
may assist in wound closure:
Consider switching to intermittent
1. Completely fill the depth
mode when exudate decreases.
(ensure foam is in contact with
wound base) and underfill the
2. Pull wound edges towards
each other, and apply drape
in strips to hold traction while
negative pressure is applied.
When therapy begins, select appro-
priate topical irrigation solution.
Caution: It is not recommended to
deliver fluids to the thoracic cavity.
Explored enterocutane-
White foam over fistula
Pressure: -120/125 mmHg
Irrigation thins the effluent, pre-
ous fistulas within chron- opening with black foam or -150 mmHg.
venting foam from becoming
ic abdominal wounds
clogged. Consider placing the
SpeedConnect in the area of the
fistula opening. If neither fascia nor
solid granulation covers the wound
base, delay therapy to avoid creat-
ing other fistulas.
Table 2. Pressure/mode by wound type.
Appendix A.2. Pressure/mode by wound condition
Wound Type
Suggested Pressure / Mode Notes
Large volume of exudate
Increase pressure and/or
Although increased pressure may help fluid movement,
intermittent mode.
pressure that is too high may overcollapse the foam,
causing fluid to pool in the wound. By setting intermittent mode, the foam intermittently
contracts and relaxes in the wound, enhancing flow.
Intermittent mode.
Irrigation thins the exudate and cleanses the foam.
Increase pressure.
Do not use one ITI NPWT device on more than two wounds.
Decrease pressure.
A single layer of white foam or wound contact layer with
black foam on top may decrease pain.
Wound granulation is
Irrigate with an appropriate topical solution.
Consider silver wound contact layer.
Consider patient co-morbidities.
Black foam overcollapse
Decrease pressure and/or switch
Consider a thicker piece of foam, mushroom technique
to intermittent mode.
for small wounds or bridging.
Table 3. Pressure/mode by wound condition.
Appendix B. Irrigation Guidelines
Appendix B.1. NPWT with simultaneous irrigation
The ITI Wound Treatment System provides NPWT with simultaneous irrigation—delivering aqueous solutions over the wound bed. Irrigation cleanses all areas in contact with foam: the wound base and any tunnels or undermined areas. By keeping the wound base moist, irrigation promotes wound healing. In addition, by cleansing the foam itself, irrigation helps maintain uniform pressure.
Irrigation is applied through the ITI Irrigation Tubing with SpeedConnect or the ITI Irrigation Delivery Set, placed in the same manner as the ITI Suction Tubing with SpeedConnect. The two SpeedConnects can be in close proximity for small wounds, or at some distance from each other for large wounds.
Typically, continuous irrigation is applied at a rate of 25 to 30 cc/hr. For intermittent irrigation procedures,
see Appendix B.3. Intermittent irrigation.
Clinicians should carefully investigate indications, contraindications, and usage guidelines before prescribing any solution. This document is intended to provide general information about topical irrigation solutions used with wounds. It is not intended to be used as a guideline for prescribing the solutions.
Tips for using irrigation with ambulatory patients
• Tape together the suction and irrigation tubes.
• As needed, shorten irrigation tubing as follows:
1. Cut tubing with sanitized scissors.
2. Remove the Luer lock connector and cap from the severed tubing end.
3. Insert the Luer lock connector and cap into the working shortened tube.
Appendix B.2. Suggested irrigation solutions
Irrigation
Clinical
Mafenide acetate
Commonly used to
Systemically absorbed.
prevent and treat burn
Patients sensitive to
gramnegative and gram- wound infections.
other sulfonamides,
Administer within 24
positive organisms,
Activity not altered by
furosemide, thiazide
hours of mixing.
including Pseudomonas the presence of blood,
diuretics, sulfonylureas, Deliver using 0.22
and certain strains of
serum, leukocytes, or
or carbonic anhydrase
micron filter.
inhibitors may be sensi-
tive to this product.
Does not reduce fungal
Can penetrate non-via-
Monitor for metabolic
acidosis. Metabolites excreted
via urine—use caution
in renal-compromised
Antiseptic/ antimicro-
Stable derivative of
Non-toxic, non-allergen-
0.4% solution is gen-
sodium (Clorpactin)
hypochlorous acid.
ic when used at +recom- erally used for wound
Effective against bacte-
Only effective when
mended strength.
ria, yeast, fungi, viruses, in contact with viable
Stable for 5 days at
spores, and antibioticre- wound base.
room temperature.
sistant organisms.
In vivo toxicity is debat-
0.05% solution is gen-
non-viable tissue.
erally used for wound
Effective against bacte-
Is a weak hypochlorite
No data indicate that
irrigation or dressings.
ria, yeast, fungi, viruses, solution buffered with
solution is toxic or caus- Integrity and potency
spores, and antibioticre- sodium bicarbonate.
es allergic reactions.
may be affected by
sistant organisms.
Produced both commer-
exposure to light, heat,
cially and by patients at and loosely sealed con-
clinician direction.
May cause irritation to
May cause irritation to
intact skin.
intact skin, especially at
stronger dilutions.
Infrequently used;
Application is painless.
0.5% solution is
generally selected in
Hypoallergenic, but can generally used.
Effective against broad
full thickness burns in
pull electrolytes from
Powder must be mixed
spectrum of bacteria
patients who are aller-
with sterile water.
gic to other topical anti
Stains intact skin.
Mixed solution is light
Does not penetrate
Anesthetic agent.
May be helpful in
Patients may experience Irrigation dilution
reducing pain with
allergic reaction, toxici-
should be individual-
dressing change.
ty, and other drug asso-
ized—generally ranges
ciated side effects.
from 25 cc 1% lidocaine
Monitor cardiovascular
to 250 cc normal saline.
status carefully during
Generally not used for
and after administra-
continuous irrigation—
rather as local anes-
thetic prior to dressing
Wound irrigation solu-
Does not impact micro-
Discard unused fluid
0.9% solution is
organism viability.
within 72 hours of
generally used.
Useful for wound bed
opening container.
Hypoallergenic, non-
Table 4. Suggested irrigation solutions.
Appendix B.3. Intermittent irrigation
This section addresses intermittent irrigation.
For some care settings—such as outpatient or home care—payors may not compensate for the extra canisters required for collecting irrigation fluids, or may not pay for fluids and setups not used for IV administration. In those settings, intermittent irrigation may provide a solution.
The prescribing clinician specifies the volume and frequency of intermittent irrigation.
1. Pour the prescribed amount of irrigation solution into a clean, sterile (or sanitized), disposable container.
2. Remove the cap from the end of the Irrigation SpeedConnect.
3. Place the Irrigation SpeedConnect into the container. The irrigation solution will be pulled through the tubing
and into the dressing. Alternatively, a syringe may be used to draw the fluid and deliver it via the SpeedConnect. The syringe may be refilled to deliver the amount of irrigation solution prescribed.
4. Replace the cap on the end of the irrigation SpeedConnect.
5. Repeat 2 to 3 times daily or as prescribed.
Svedman and SVED are registered trademarks of Innovative Therapies Inc.
Innovative Therapies Inc. owns, uses, or has applied for the following trademark(s) or service mark(s): Quantum, Svedman, SVED, SensiSkin, Speed Connect, "Outcomes Focused Wound Therapy" and "Wound Care. Anywhere". All other trademarks are trademarks of their respective owners or holders. 2013 Innovative Therapies Inc.
Please refer to the Innovative Therapies product manual for indications, contraindications, precautions and safety information.
Always consult a physician and product instructions for use prior to application.
Caution: Federal law restricts these devices to sale by or on the order of a physician.
24-9000 09/13 Rev C
3770 Park Central Boulevard North Pompano Beach, Florida 33064
Call our customer support
www.itimedical.com
professionals at 1-866-484-6798
Source: http://mintcare.sg/?wpdmact=process&did=MzYuaG90bGluaw==
2.54cm or 1inch margin on all Font: Time New Roman, Size: 14, Style: Bold, Small Title case (Should es for the whole manuscript not be Caps), Align: Center Simultaneous estimation of fluticasone propionate, azelastine hydrochloride, phenylethyl alcohol and benzalkonium chloride by
ha identificado que el metabolismo de esta droga se produce presentarse en las primeras semanas y, generalmente, disminuyen con el principalmente en el hígado e incluye la O-metilación, hidroxilación y uso continuo de la medicación. El profesional indicará a los pacientes si ha identificado que el metabolismo de esta droga se produce principalmente en el hígado e incluye la O-metilación, hidroxilación y








