Neuronet.jp
0022-3565/97/2801-0471$03.00/0THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS
Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics
Printed in U.S.A.
JPET 280:471–476, 1997
Epileptic Activity Prevents Synapse Formation of HippocampalMossy Fibers via L-Type Calcium Channel Activation
In Vitro
YUJI IKEGAYA, MASATOSHI YOSHIDA, HIROSHI SAITO and NOBUYOSHI NISHIYAMA
Department of Chemical Pharmacology, Faculty of Pharmaceutical Sciences, The University of Tokyo, Tokyo 113, Japan
Accepted for publication September 13, 1996
ABSTRACT
Hippocampal slice from early postnatal rat was used to eluci-
observations using a Timm method, a histochemical technique
date the influence of epileptic activity elicited by picrotoxin on
that preferentially labels synaptic terminals of mossy fibers,
synapse formation of mossy fibers. Neurite reelongation and
revealed that picrotoxin prevented synaptogenesis in the CA3
synaptogenesis of mossy fibers transected at 8 days
in vitro
region. This inhibitory effect of picrotoxin was completely abol-
were confirmed by staining with DiI, a fluorescent membrane
ished by tetrodotoxin or nicardipine (10 mM), a L-type calcium
dye used as a neuronal tracer, and by recording field excitatory
channel blocker, but not by 2-amino-5-phosphonopentanoic
postsynaptic potentials (fEPSP) in the CA3 region evoked by
acid (50 mM), a N-methyl-D-aspartate receptor antagonist, sug-
stimulation of the dentate gyrus. Picrotoxin (50 mM), which
gesting that influx of calcium ion via L-type calcium channels
evoked spontaneous epileptiform firing in the CA3 region that
during epileptic bursts mediated the disturbance of appropriate
was occluded by tetrodotoxin (1 mM), hindered development of
synapse formation of mossy fibers.
fEPSP amplitude after a lesion of mossy fibers. Furthermore,
Because ontogenetic maturation of several regions in the
that often demonstrate aberrant sprouting of mossy fibers
central nervous system extends until early postnatal period,
into the inner molecular layer of the dentate gyrus (Babb
et
certain forms of injury or disease during this critical stage
al., 1991, Mathern
et al., 1994) or massive reduction in the
are correlated with developmental disorders. It is well known
number of dendritic spines (Mu
¨ ller
et al., 1993), it has not
that epilepsy, frequency of which is much higher in children
been reported whether epilepsy has any influences on neurite
than in adults, particularly in the first year of life, is associ-
outgrowth and synaptogenesis of mossy fibers during their
ated with a broad spectrum of cognitive deficits when it
developmental period. Fortunately, some recent reports
occurs in this postnatal period (Alpherts and Aldenkamp,
showed that developmental and physiological properties of
1990; Mizrahi, 1994; Stafstrom, 1995). However, few previ-
mossy fibers were retained in organotypic slice cultures of
ous reports identified characteristic changes in structure or
postnatal hippocampus (Dailey
et al., 1994; Robain
et al.,
function of the central nervous system of epileptic patients,
1994; Frotscher
et al., 1995). Therefore, we have asked
which may underlie such cognitive deficits.
whether epileptic activity disturbs normal neurite outgrowth
Hippocampal mossy fiber tract, axons projecting from the
and synaptogenesis of mossy fibers using hippocampal slice
granule cells in the dentate gyrus mainly to the pyramidal
culture. As a result, we found severe suppression of synapse
cells in the CA3 region, is formed very late because the
formation of mossy fibers by epileptic activity.
dentate granule cells generate postnatally (Stirling andBliss, 1978; Amaral and Dent, 1981, Gaarskjær, 1986). This
tract is believed to be involved in cognition and learningbecause its degeneration produces memory deficits (Conrad
Preparation of organotypic slice cultures. For preparation of
and Roy, 1993; Vaher
et al., 1994) and its synapses demon-
hippocampal slices, postnatal 8 day (P8) Wistar rats were decapi-tated and the brains were removed. The hippocampi were cut into
strate a high degree of functional plasticity (Bradler and
300-mm thick slices in cold glucose-enriched Gey's buffer and were
Barrionuevo, 1989; Mitsuno
et al., 1994; Malenka, 1995).
then cultivated according to the method introduced by Stoppini
et al.
Although there are numerous reports concerning dynamic
(1991). Briefly, selected sections were placed on moistened translu-
morphological plasticity of mossy fibers in epileptic seizure
cent membranes (0.4 mm Culture Plate Insert, 30 mm diameter,Millicell-CM, Millipore Corporation, Bedford, MA) that were in-serted in six-well plates (35 mm in diameter) filled with 1 ml of
Received for publication April 18, 1996.
medium (50% minimum essential medium, 25% Hanks' balanced
ABBREVIATIONS: ACSF, artificial cerebrospinal fluid; AP5, 2-amino-5-phosphonopentanoic acid; DIV, day
in vitro; fEPSP, field excitatory
postsynaptic potential; GABA, g-aminobutyric acid; NMDA, N-methyl-D-aspartate; DiI, 3,39-dilinolenyloxacarbocyanin perchlorate.
Ikegaya et al.
salt solution, 25% heat inactivated horse serum). The cultures were
same ACSF. The hilus of the upper blade of the dentate granule cell
kept at 36°C in a humidified, CO -enriched atmosphere. The culture
layer was stimulated with a bipolar electrode. The evoked potential
medium was changed twice a week.
was extracellularly recorded from the CA3 pyramidal cell layer with
Lesioning of mossy fiber tract. In some slices, mossy fibers
a glass capillary microelectrode filled with 0.9% NaCl. Positive field
were transected at 8 DIV along the line linking the lips of the upper
potential (see fig. 1, B, F and H) reflected fEPSP because it was
and lower blade of the granule cell layers (see fig. 1, C, E and G, fig.
blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (10 mM), a non-
5, B, C and D). The lesion was performed under an operating micro-
NMDA receptor antagonist (data not shown). The maximal size of
scope using a manipulator with a razor blade.
fEPSP was used as an index of the number of functional synaptic
DiI labeling. Cultured slices were fixed with 0.1 M phosphate
contacts formed as a function of time after a lesion (Muller et al.,
buffer containing 4% paraformaldehyde 1 day after DiI crystal was
1993; Stoppini et al., 1993).
placed on the dentate gyrus. After a 5-wk incubation in the fixative
Timm staining. For Timm stain, cultures were washed with 0.1
at room temperature, the DiI-labeled axons were observed using a
M phosphate buffer and were then immersed for 10 min in 0.37%
fluorescent microscope (Honig and Hume, 1989).
sodium sulfide solution, immediately followed by fixation for 15 min
Extracellular recordings. Cultured slices were submerged for
with 10% (v/v) formaldehyde solution. After washed with 0.1 M
30 to 60 min in ACSF, which was composed of 124 mM NaCl, 5.0 mM
phosphate buffer, the cultures were dehydrated with 70 and 96%
KCl, 2.4 mM CaCl , 1.3 mM MgSO , 1.24 mM KH PO , 26.0 mM
ethanol, and dried. To perform the sulfide silver staining, they were
NaHCO and 10.0 mM glucose and was saturated with 95% O -5%
submerged in the physical developer according to the method of
CO , and were transferred into a recording chamber filled with the
Sloviter (1982) and were then incubated in a dark room for 50 min at
26°C. The slices were washed with distilled water at the end of thereaction.
Drugs. In slice cultures, the drugs were applied in the culture
medium on and after 8 DIV. For recording spontaneous activities,the drugs were dissolved in ACSF. All the drugs used were obtained
from commercial sources; picrotoxin (Wako Pure Chemical Industry,Ltd., Osaka, Japan), a GABA receptor channel blocker; tetrodotoxin(Sigma Chemical Co., St. Louis, MO), a voltage-sensitive sodiumchannel blocker; nicardipine (Wako), a L-type calcium channel block-er; AP5 (Sigma), a NMDA receptor antagonist; 6-cyano-7-nitroqui-noxaline-2,3-dione (Research Biochemical Incorporated, Natick,MA), a non-NMDA receptor antagonist.
Mossy fiber growth and synapse formation after a
lesion. In a series of these experiments, we investigated the
effect of epileptic activity on reformation of synapses after a
section of maturated mossy fibers because it was difficult to
know exactly when mossy fiber formation starts in vivo.
Many previous reports adopting this tissue lesion method
indicated that organotypic characteristics, developmental
processes and neuronal properties in vivo are well-preserved
in hippocampal slice cultivated after the lesion (Ga¨hwiler
and Brown, 1985; Zimmer and Ga¨hwiler, 1987, Heimrich and
Frotscher, 1993; Li et al., 1993; Stoppini, et al., 1993; Dailey
et al., 1994; Frotscher et al., 1995).
Mossy fibers were lesioned at 8 DIV because cultured slices
were electrophysiologically stabilized by this time (fig. 2,open circle). First, we examined reelongation and synapto-
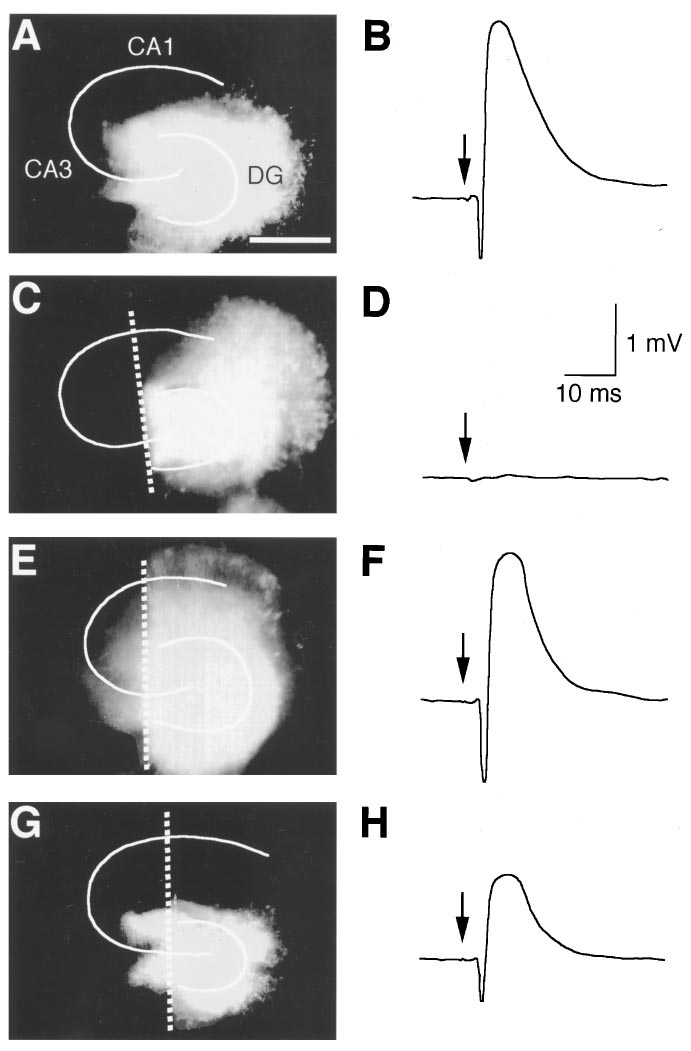
Fig. 1. Lesion-induced reorganization of mossy fibers. Fluorescent im-
ages of hippocampal slices stained with DiI were observed at 8 DIV (A),
0 day (C) or 7 days (E) after lesions at 8 DIV. White lines demonstrate the
granule cell layer of the dentate gyrus (DG) and the pyramidal cell layer
of the CA1-4 regions. Mossy fibers were transected along white broken
lines. A scale bar represents 1 mm. Right traces were typical field
potentials (average of four) in the CA3 region at 8 DIV (B), 0 day (D) or
7 days (F) after the lesion. The dentate gyrus was stimulated at the time
Fig. 2. Changes in amplitude of fEPSP recorded in the CA3 region
indicated by arrows. DiI image in G and field potential in H were
elicited by supramaximal stimulation of the dentate gyrus in intact
obtained from slices treated with picrotoxin (50 mM) for 7 days after
slices (n 5 5–9) (open circle) or slices lesioned at 8 DIV (n 5 6 –9) (closed
lesions at 8 DIV.
circle). Each point represents mean 6 S.E.M.
Epilepsy and Hippocampal Maturation
genesis of transected mossy fibers under our culture condi-
Effect of picrotoxin on mossy fiber synapse forma-
tions. Neurite outgrowth was observed by staining with DiI,
tion. For evaluating the influence of epileptic activity on
which is a fluorescent membrane dye used as a neuronal
synapse formation of mossy fibers, picrotoxin was added to
tracer (fig. 1, A, C, E and G). Although mossy fibers were
culture medium at a concentration of 50 mM immediately
completely transected by the method adopted in this study
after the lesion. Development of fEPSP amplitude after the
(fig. 1C), they elongated close to the pyramidal cell layer of
section of mossy fibers was prevented in slices cultivated in
the CA3 region beyond the transection at 7 days after the
medium containing picrotoxin (figs. 1H and 4A). This inhib-
section (fig. 1E), and formed functional excitatory synapses
itory effect of picrotoxin was completely abolished by appli-
on the pyramidal cells, which was estimated by recording
cation of tetrodotoxin (1 mM) (fig. 4B). Picrotoxin did not
synaptic responses reflecting fEPSP in the CA3 region
reduce fEPSP amplitude in intact slices (maximal response
evoked by stimulation of the dentate gyrus (fig. 1, B, D and
amplitudes in nontransected slices cultivated for 7 days in
F). Because fEPSP in the CA3 region was not observed im-
medium containing picrotoxin was 2.52 6 0.29 mV, and that
mediately after the lesion (n 5 32) (fig. 1D), it was again
in normal medium was 2.13 6 0.42 mV; means 6 S.E.M. of
confirmed that all mossy fibers were transected. At more
seven or six slices, respectively). To determine whether spon-
than 4 days after the lesion, however, fEPSP appeared in all
taneous activity in normal medium, which was rarely seen as
82 slices tested. A change in the maximal size of evoked
above described, contributed to the recovery of fEPSP after
synaptic responses was shown in figure 2. For promoting
the lesion, slices were cultivated in the medium containing
comparisons, a change in fEPSP amplitude in nontransected
tetrodotoxin for 7 days after the lesion. Tetrodotoxin (1 mM)
slices was superimposed on the same figure. An extent of
did not affect fEPSP amplitude (maximal response ampli-
maximal response in slices at 14 days after the section was
tudes in slices cultivated in normal medium for 7 days was
similar to that in DIV-matched intact slices.
2.37 6 0.57 mV, and that in medium containing tetrodotoxin
Epileptic activity. Although epileptiform burst discharge
was 1.94 6 0.58 mV; means 6 S.E.M. of eight or nine slices,
can be elicited in acutely prepared hippocampus slices and
respectively). Inhibition of synaptogenesis by continuous ep-
cultured slices in a number of diverse ways, a simple proce-
ileptic activities was also confirmed with a Timm method, a
dure is to block inhibitory postsynaptic potentials mediatedby GABA with its receptor antagonist (Dichter and Ayala,1987; Thompson and Ga¨hwiler, 1992). At 8 DIV, 42 of 43slices (97.7%) exposed to picrotoxin (50 mM), a GABA recep-
tor channel blocker, showed spontaneous synchronized epi-leptiform bursts with a high regularity (2.05 6 0.47 bursts/min; mean 6 S.E.M. of eight slices) in the CA3 region, whichindividually consisted of 7.87 6 0.85 (mean 6 S.E.M. of eightslices) repetitive firings (fig. 3B), although epileptiform ac-tivity was not observed in normal ACSF (fig. 3A). Only 2 in 82intact slices tested (2.4%) exhibited spontaneous activity thatconsisted of a single, but not repetitive, firing. The epilepticbursts induced by picrotoxin was blocked by application oftetrodotoxin (1 mM) (fig. 3C).
Fig. 4. Inhibitory effect of picrotoxin on mossy fiber synapse forma-
tion. A, Change in size of maximal fEPSP were observed in hippocam-
pal slices cultivated in normal medium (n 5 5–11) (open circle) or in
medium containing picrotoxin (PTX, 50 mM) (n 5 5–9) (closed circle). B,
Field potentials were recorded at 7 days after the lesion in slices
cultivated in normal medium (n 5 12) (open column), in picrotoxin (50
mM, n 5 6) (hatched column) or in coexistence of picrotoxin withtetrodotoxin (TTX, 1 mM, n 5 6), nicardipine (Nic, 10 mM, n 5 7) or
Fig. 3. Typical records of epileptic activity in the CA3 region of a
2-amino-5-phosphonopentanoic acid (AP5, 50 mM, n 5 7) (closed
hippocampal slice at 8 DIV. Field potentials were recorded in normal
column). Data are means 6 S.E.M. of 6 to 12 cases. *P , .05, **P , .01
ACSF (A), in ACSF containing picrotoxin (PTX, 50 mM) (B), containing
vs. control, ##P , .01 vs. PTX: Tukey's test after analysis of variance
picrotoxin (50 mM) and tetrodotoxin (TTX, 1 mM) (C), or containing
(ANOVA). Data in A and B were obtained from different series of
picrotoxin (50 mM) and nicardipine (Nic, 10 mM) (D). A burst indicated by
experiments and were not pooled because deviation among experi-
an arrow in Ba was expanded in Bb.
ments was large.
Ikegaya et al.
recovery of fEPSP from the lesion was blocked by nicardipine(10 mM) but not by AP5 (50 mM) (fig. 4B). The ameliorativeeffect of nicardipine against picrotoxin was also confirmedmorphologically by the Timm method (fig. 5D). We thenexamined if nicardipine altered the epileptiform activity in-duced by picrotoxin. Picrotoxin (50 mM) elicited the burstingeven in nicardipine- (10 mM) containing ACSF in all eightpatients tested (fig. 3D). This epileptiform activity showedhigh regularity and its frequency was 2.35 6 0.59 min21(means 6 S.E.M. of eight slices). Each burst was consisted of6.82 6 1.02 (means 6 S.E.M. of eight slices) repetitive fir-ings. These properties were very similar to those of burstsinduced in normal ACSF. We concluded, therefore, that ni-cardipine did not change the character of picrotoxin-elicitedbursts, consistent with a previous work reporting that dihy-
dropyridine-type calcium channel blocker did not inhibit ep-ileptic discharge (van Luijtelaar et al., 1994). In addition,exposure of intact slices to nicardipine or AP5 in the absenceof picrotoxin from 8 DIV to 15 DIV did not affect fEPSPamplitude evoked in the CA3 region (data not shown, n 56–9). Taken together, it is suggested that calcium influx
through L-type calcium channels during epileptic bursts me-diated the disturbance of appropriate synapse formation ofmossy fibers.
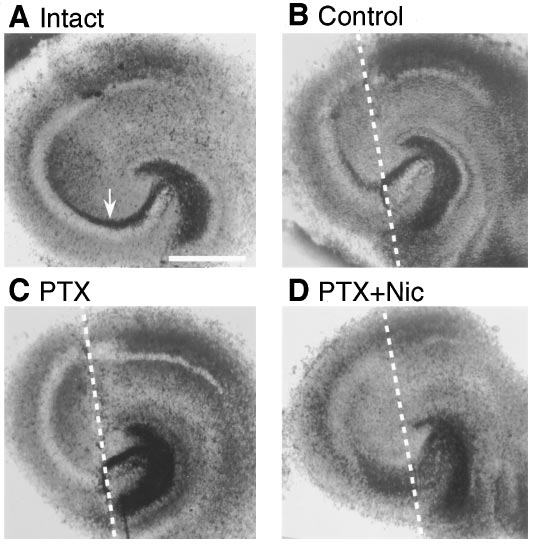
Fig. 5. Bright-field images of hippocampal slices stained with a Timm
method were obtained from an intact slice at 15 DIV (A) or slices
cultivated in normal medium (B), in picrotoxin (PIC, 50 mM) (C) or in
picrotoxin (50 mM) and nicardipine (Nic, 10 mM) (D) for 7 days after
Using hippocampal slice culture, we demonstrated that
lesions at 8 DIV. Mossy fibers were transected along white broken lines.
The area indicated by an arrow is stratum lucidum, where terminals of
picrotoxin prevented reorganization of mossy fibers via L-
mossy fiber tract form synapses on the CA3 pyramidal cells.
type calcium channel activation.
¨ ller et al. (1993) found that the amplitude of evoked
histochemical technique that labels synaptic terminals of
fEPSP was depressed after chronic application of GABAA
mossy fibers because of their high zinc content (fig. 5). In
receptor blockers. In our study, however, picrotoxin had no
extrahippocampal area, subiculum and entorhinal cortex
effect on fEPSP in intact slices. This apparent contradiction
were also stained, consistent with a previous report showing
may come from the following: 1) Cultures prepared with the
that synapse boutons in these regions contained zinc (Slo-
roller-tube method they used formed a monolayer explant
mianka, 1992). In all 16 slices cultivated in normal medium,
and might be more delicate than slices cultivated with the
the stratum lucidum of the pyramidal cell layer in the CA3
static culture method we applied, which retained a few cell
region, which is indicated by an arrow in figure 5A, was
layers of thickness (Stoppini et al., 1991). The difference in
stained across the transection (fig. 5B), but this was not
slice cultivation procedures may also account for the discrep-
observed in slices cultivated in picrotoxin in all 12 cases
ancy in the extent of reinnervation in control cultures after
examined (fig. 5C). DiI labeling technique revealed that pic-
the lesion. Indeed, both our cultures and those of Stoppini et
rotoxin-treated mossy fibers grew past the lesion into the
al. (1993) showed 100% reinnervation, although in the pre-
CA3 pyramidal cell layer at 7 days after the lesion (fig. 1G) in
vious studies by Zimmer and Ga¨hwiler (1987) and Dailey et
all nine slices tested. These results suggest that picrotoxin
al. (1994) they could not produce such a high reinnervation
did not block outgrowth but inhibited synaptogenesis of
rate in slices obtained by roller-tube method. 2) Concentra-
mossy fibers. Another consistent feature in hippocampal
tion of picrotoxin Mu
¨ ller et al. (1993) applied was 500 mM
slices treated with picrotoxin was aberrant sprouting of
that was 10 times higher than ours and might exert nonspe-
mossy fibers into the molecular layer of the dentate gyrus. In
cific or toxic effects.
3 of 15 slices cultivated in control medium after the lesions,
Barbin et al. (1993) reported that blockade of GABA
this phenomenon was faintly observed (fig. 5B). This may be
ceptors reduced neurite length of cultured hippocampal neu-
due to temporary loss of target produced by lesions because
rons and suggested the involvement of GABA receptors in
some reports showed that loss of hilus interneurons, one of
neurite outgrowth. Our result that picrotoxin inhibited syn-
the main postsynaptic targets of mossy fiber tract, caused
apse formation of mossy fibers can also be interpreted as a
such aberrant sprouting (Babb et al., 1991).
consequence of prevented reelongation of transected mossy
Epileptic bursts elicit sustained depolarization shift of neu-
fibers. However, this possibility is ruled out by an observa-
ronal membrane potential that may allow influx of calcium
tion using DiI labeling technique that indicates that picro-
ion via voltage-sensitive calcium channels or NMDA receptor
toxin-treated mossy fibers extended close to the pyramidal
channels. Finally, we tested the effects of nicardipine, a L-
cell layer of the CA3 region at 7 days after a lesion. Although
type calcium channel blocker, and AP5, a NMDA receptor
we did not examine whether chronic application of picrotoxin
antagonist, on picrotoxin-induced inhibition of synaptogen-
produced epileptic activity in cultured slices, the inhibitory
esis of mossy fibers. The inhibitory effect of picrotoxin on the
effect of picrotoxin on synapse formation was probably due to
Epilepsy and Hippocampal Maturation
epileptic activity per se because it was completely canceled by
there are indications that reactive synaptogenesis may be
tetrodotoxin. In addition, aberrant sprouting of mossy fibers
involved in learning and memory (Greenough and Bailey,
into the molecular layer of the dentate gyrus, that has been
1988; Moser et al., 1994). Therefore, our results may account
typically observed in epileptic hippocampus (Babb et al.,
in part, for cognitive deficits elicited by childhood epilepsy,
1991, Mathern et al., 1994), was confirmed in picrotoxin-
and further investigation using our method will provide fur-
treated slices by a Timm method. This also suggests that
ther insights and understandings with respect to this syn-
picrotoxin actually elicited epileptiform activity in cultured
slices. Taken together, these data strongly suggest that epi-
Our results indicate that calcium ion influx through L-type
leptic activity hindered lesion-induced reorganization of
calcium channels may mediate a disorder of synapse forma-
mossy fibers.
tion of mossy fibers, consistent with previous reports showing
Represa et al. (1989) found that high affinity binding sites
that calcium ion influx plays a major role in neuronal injury
for kainate increased in the CA3 region of childhood epilep-
associated with epilepsy (Wasterlain et al., 1993). However,
tics. Although their result seems to contradict our finding, it
many reports examining correlation between synaptogenesis
is known that the type of neuronal firings often determine
and calcium ion movement implied contradictive informa-
the direction of plasticity. For example, the direction of the
tion. Although most of these data demonstrate an essential
synaptic gain change depends on the membrane discharge of
role of calcium ion in synaptogenesis (Basarsky et al., 1994),
the postsynaptic cell in the hippocampus (Artola and Singer,
others suggest that synapse formation and increase in intra-
1993; Malenka, 1995). Thus, further detail examination on
cellular calcium ion is irrelevant (Verderio et al., 1994). Our
picrotoxin-induced bursts in cultured slice might elucidate
observation that blockade of calcium ion influx abolished
the difference between the preceding report and our finding.
epileptic activity-induced inhibition of synapse formation
Recovery time course of maximal fEPSP amplitude after
suggests a repressive role of calcium ion, which further com-
mossy-fiber lesions approximately matched to that of intrin-
plicated the discussion. One possible explanation is that ex-
sic formation of mossy fibers, which are completed during
cessive calcium concentration results in obstruction of syn-
postnatal 1 to 2 wk (Stirling and Bliss, 1978; Amaral and
aptogenesis although intermediate degree of calcium ion
Dent, 1981; Gaarskjær, 1986). Moreover, the maximal fEPSP
level may be required for it. Despite ambiguous role of cal-
amplitude recorded at 14 days after the section recovered to
cium ion in mossy fiber synaptogenesis, our results suggest a
an extent comparable to that in DIV-matched intact slices.
novel protective action of L-type calcium channel blockers
Additionally, synaptic terminals of regenerated mossy fibers
against disturbance of normal synaptic maturation associ-
were Timm-stain positive, that was one of the important
ated with epileptic seizure, besides its antiepileptic proper-
characteristics of mossy fibers. These observations strongly
ties as have been proposed in various models of epilepsy (van
support the idea proposed by several previous reports that
Luijtelaar et al., 1994;, Straub et al., 1994). Further investi-
developmental manner and organotypic nature in vivo are
gations on this finding may endow valuable information for
conserved in structures regenerated after the lesion (Ga¨h-
applying calcium channel blockers as prophylactics against
wiler and Brown, 1985; Heimrich and Frotscher, 1993; Li et
cognitive deficits induced by childhood epilepsy.
al., 1993; Stoppini, et al., 1993; Frotscher et al., 1995). Ac-
Finally, organotypic slice culture used in our study pre-
cordingly, process and characteristics of reorganizing mossy
serves in vivo nature to a high degree and renders a useful
fibers after a lesion in our study may correspond to those of
model for studying developmental cellular dynamics in a
developmentally programmed formation of mossy fibers
mammalian central nervous system.
(Zimmer and Ga¨hwiler, 1987; Dailey et al., 1994).
As mentioned above, mossy fibers are generated mainly in
1 to 2 wk after birth (Stirling and Bliss, 1978; Amaral and
ALPHERTS, W. C. J. AND ALDENKAMP, A. P.: Computerized neuropsychological
Dent, 1981; Gaarskjær, 1986). This postnatal period is hence
assessment of cognitive functioning in children with epilepsy. Epilepsia 31:
534–540, 1990.
a critical stage that is susceptible to injury or disease. In-
AMARAL, D. G. AND DENT, J. A.: Development of the mossy fibers of the dentate
deed, Represa et al. (1991) reported that neonatal irradiation
gyrus: I. A light and electron microscopic study of the mossy fibers and their
expansions. J. Comp. Neurol. 195: 51–86, 1981.
selectively prevented the mossy fiber formation. Whereas
ARTOLA, A. AND SINGER, W.: Long-term depression of excitatory synaptic trans-
childhood epilepsy is associated with a broad spectrum of
mission and its relationship to long-term potentiation. Trends Neurosci. 16:
cognitive deficits (Alpherts and Aldenkamp, 1990; Mizrahi,
480–487, 1993.
BABB, T. L., KUPFER, W. R., PRETORIUS, J. K., CRANDALL, P. H. AND LEVESQUE, M.
1994; Stafstrom, 1995), no reports clarified characteristic
F.: Synaptic reorganization by mossy fibers in human epileptic fascia den-
changes in structure or function of the central nervous sys-
tate. Neuroscience 42: 351–363, 1991.
tem that underlie cognitive deficits in childhood epilepsy.
BARBIN, G., POLLARD, H., G. A. 139 ARSA, J. L. AND BEN-ARI, Y.: Involvement of
GABA receptors in the outgrowth of cultured hippocampal neurons. Neu-
Although hippocampal plasticity in childhood epilepsy was
rosci. Lett. 152: 150–154, 1993.
reported (Represa et al., 1989; Mathern et al., 1994), it is
BASARSKY, T. A., PARPURA, V. AND HAYDON, P. G.: Hippocampal synaptogenesis
in cell culture: Developmental time course of synapse formation, calcium
unclear whether epilepsy is responsible for plasticity or plas-
influx, and synaptic protein distribution. J. Neurosci. 14: 6402–6411, 1994.
ticity contributes to epilepsy. Our result that picrotoxin pre-
BRADLER, J. E. AND BARRIONUEVO, G.: Long-term potentiation in hippocampal
vents the recovery of lesioned mossy fiber might indicate that
CA3 neurons: Tetanized input regulates heterosynaptic efficacy. Synapse 4:
132–142, 1989.
epilepsy disturbs hippocampal maturation. The hippocam-
CONRAD, C. AND ROY, D.: Selective loss of hippocampal granule cells following
pus is thought to be involved in cognition and learning (Shen
adrenalectomy: Implications for spatial memory. J. Neurosci. 13: 2582–
et al., 1994; McClelland et al., 1995), and both behavioral
DAILEY, M. E., BUCHANAN, J., BERGLES, D. E. AND SMITH, S. J.: Mossy fiber growth
(Conrad and Roy, 1993; Vaher et al., 1994) and physiological
and synaptogenesis in rat hippocampal slices in vitro. J. Neurosci. 14:
(Bradler and Barrionuevo, 1989; Mitsuno et al., 1994;
1060–1078, 1994.
Malenka, 1995) analysis suggest that mossy fibers in this
ICHTER, M. AND AYALA, G.: Cellular mechanisms of epilepsy: A status report.
Science 237: 157–164, 1987.
region participate in cognition and learning. Additionally,
FROTSCHER, M., ZAFIROV, S. AND HEIMRICH, B.: Development of identified neuro-
Ikegaya et al.
nal types and of specific synaptic connections in slice cultures of rat hip-
BEN-ARI, Y.: Development of mossy fiber synapses in hippocampal slice
pocampus. Prog. Neurobiol. 45: 143–164, 1995.
culture. Dev. Brain Res. 80: 244–250, 1994.
GAARSKJÆR R., F. B.: The organization and development of the hippocampal
SHEN, Y., SPECHT, S. M., DE SAINT GHISLAIN, I. AND LI, R.: The hippocampus: A
mossy fiber systems. Brain Res. Rev. 11: 335–357, 1986.
biological model for studying learning and memory. Prog. Neurobiol. 44:
G¨AHWILER, B. H. AND BROWN, D. A.: Functional innervation of cultured hip-
485–496, 1994.
pocampal neurons by cholinergic afferents from co-cultured septal explants.
SLOMIANKA, L.: Neurons of origin of zinc-containing pathways and the distri-
Nature 313: 577–579, 1985.
bution of zinc-containing boutons in the hippocampal region of the rat.
GREENOUGH, W. T. AND BAILEY, C. H.: The anatomy of a memory: Convergence
Neuroscience 48: 325–352, 1992.
of results across a diversity of tests. Trends Neurosci. 11: 142–147, 1988.
SLOVITER, R.: A simplified Timm stain procedure compatible with formaldehyde
HEIMRICH, B. AND FROTSCHER, M.: Slice cultures as a model to study entorhinal-
fixation and routine paraffin embedding of rat brain. Brain Res. Bull. 8:
hippocampal interaction. Hippocampus 3: 11–18, 1993.
771–774, 1982.
HONIG, M. AND HUME, R. I.: DiI and DiO: Versatile fluorescent dyes for neuronal
STAFSTROM, C. E.: Neonatal seizures. Pediatr. Rev. 16: 248–255, 1995.
labeling and pathway tracing. Trends Neurosci. 12: 333–341, 1989.
STIRLING, R. V. AND BLISS, T. V. P.: Hippocampal mossy fiber development at the
LI, D., FILED, P. M., STAREGA, U., LI, Y. AND RAISMAN, G.: Entorhinal axons
ultrastructural level. Prog. Brain Res. 48: 191–198, 1978.
project to dentate gyrus in organotypic slice co-culture. Neuroscience 52:
STOPPINI, L., BUCHS, P. A. AND MULLER, D.: A simple method for organotypic
799–813, 1993.
cultures of nervous tissue. J. Neurosci. Methods 37: 173–182, 1991.
MALENKA, R.: Synaptic plasticity in the hippocampus: LTP and LTD. Cell 78:
STOPPINI, L., BUCHS, P. A. AND MULLER, D.: Lesion-induced neurite sprouting
535–538, 1995.
and synapse formation in hippocampal organotypic cultures. Neuroscience
MATHERN, G. W., LEITE, L. P., PRETORIUS, J. K., QUINN, B., PEACOCK, W. J. AND
57: 985–994, 1993.
BABB, T. L.: Children with severe epilepsy: Evidence of hippocampal neuron
STRAUB, H., K ¨OHLING, R. AND SPECKMANN, E. J.: Picrotoxin-induced epileptic
losses and aberrant mossy fiber sprouting during postnatal granule cell
activity in hippocampal and neocortical slices (guinea pig): Suppression by
migration and differentiation. Dev. Brain Res. 78: 70–80, 1994.
organic calcium channel blockers. Brain Res. 658: 119–126, 1994.
MCCLELLAND, J. L., MCNAUGHTON, B., LAND AND O'REILLY, R. C.: Why there are
complementary learning systems in the hippocampus and neocortex: In-
HOMPSON, S. M. AND G ¨
AHWILER, B. H.: Comparison of the actions of baclofen at
pre- and postsynaptic receptors in the rat hippocampus in vitro. J. Physiol.
sights from the successes and failures of connectionist models of learning
and memory. Psychol. Rev. 102: 419–457, 1995.
Lond. 451: 329–345, 1992.
VAHER, P., LUINE, V., GOULD, E. AND MCEWEN, B.: Effects of adrenalectomy on
ITSUNO, K., SASA, M. ISHIHARA, K., ISHIKAWA, M. AND KIKUCHI, H.: LTP of mossy
fiber-stimulated potentials in CA3 during learning in rats. Physiol. Behav.
spatial memory performance and dentate gyrus morphology. Brain Res. 656:
55: 633–638, 1994.
71–78, 1994.
IZRAHI, E. M.: Seizure disorders in children. Curr. Opin. Pediatr. 6: 642–646,
ERDERIO, C., COCO, S., FUMAGALLI, G. AND MATTEOLI, M.: Spatial changes in
calcium signaling during the establishment of neuronal polarity and synap-
togenesis. J. Cell. Biol. 126: 1527–1536, 1994.
OSER, M. B., TROMMALD, M. AND ANDERSEN, P.: An increase in dendritic spine
density on hippocampal CA1 pyramidal cells following spatial learning in
VAN LUIJTELAAR, E. L., ATES, N. AND VAN DER STAAY, F. J.: The effects of chronic
adult rats suggests the formation of new synapses. Proc. Natl. Acad. Sci.
treatment with a calcium channel antagonist on two types of generalized
U.S.A. 91: 12673–12675, 1994.
epilepsies in rats. Pharmacol. Biochem. Behav. 48: 575–579, 1994.
MULLER, D., BUCHS, P. A. AND STOPPINI, L.: Time course of synaptic development
WASTERLAIN, C. G., FUJIKAWA, D. G., PENIX, L. AND SANKAR, R.: Phathophysi-
in hippocampal organotypic cultures. Dev. Brain Res. 71: 93–100, 1993.
ological mechanisms of brain damage from status epilepticus. Epilepsia 34:
M ¨ULLER, M., G¨AHWILER, B. H., RIETSCHIN, L. AND THOMPSON, S. M.: Reversible
S37–S53, 1993.
loss of dendritic spines and altered excitability after chronic epilepsy in
IMMER, J. AND G ¨
AHWILER, B. H.: Growth of hippocampal mossy fibers: A lesion
hippocampal slice cultures. Proc. Natl. Acad. Sci. U.S.A. 90: 257–261, 1993.
and coculture study of organotypic slice cultures. J. Comp. Neurol. 264:
REPRESA, A., DESSI, F., BEAUDOIN, M. AND BEN-ARI, Y.: Effects of neonatal g-ray
1–13, 1987.
irradiation on rat hippocampus. I. Postnatal maturation of hippocampal
cells. Neuroscience 42: 137–150, 1991.
Send reprint requests to: Dr. Nobuyoshi Nishiyama, Department of Chem-
REPRESA, A., ROBAIN, O., TREMBLAY, E. AND BEN-ARI, Y.: Hippocampal plasticity
ical Pharmacology, Faculty of Pharmaceutical Sciences, The University of
in childhood epilepsy. Neurosci. Lett. 99: 351–355, 1989.
Tokyo, 7–3-1 Bunkyo-ku, Tokyo 113, Japan.
ROBAIN, O., BARBIN, G., BILLETTE D. E. VILLEMEUR, T., JARDIN, L., JAHCHAN, T. AND
Source: http://neuronet.jp/pdf/O_011.pdf
Available online at Biofouling of crypts of historical and architectural interest at La Plata Cemetery Patricia S. Guiamet , Vilma Rosato , Sandra Gómez de Saravia , Ana M. García , Diego A. Moreno a Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas (INIFTA), Departamento de Química, Facultad de Ciencias Exactas, UNLP, CCT La Plata- CONICET. C.C. 16, Suc. 4,
Isabell Hensel, Gunther Teubner Matrix Reloaded. Critica dell'effetto orizzontale dei diritti fondamentali centrato sullo Stato sull'esempio del publication bias (errore sistematico di pubblicazione) Versione in tedesco: http://www.jura.uni-frankfurt.de/49069887/KJ_Teubner_Hensel.pdf Matrix Reloaded Critica dell'effetto orizzontale dei diritti fondamentali centrato sullo Stato sull'esempio del


