Contrast media
CONTRAST MEDIA TUTORIAL
Jessica B. Robbins, MD
Myron A. Pozniak, MD
For questions, comments, or permission to use any or all of this tutorial, please
or 608/263-8312
University of Wisconsin, Department of Radiology, Madison, WI
2010
Portions of this information are reprinted with permission of the American College of Radiology.
No other representation of this material is authorized without expressed, written permission from the ACR.
CT CONTRAST AGENTS
1. INTRODUCTION
Contrast agents are indispensable in the practice of radiology. Significant
improvements in their composition during the past few decades have made them
safer and better tolerated, as evidenced by their use in vast numbers of
examinations, often in severely ill patients. Nonetheless, risks associated with
contrast agents have not been eliminated, and adverse reactions of varying
degree continue to occur. Consequently, it is imperative for anybody
administering contrast agents to be intimately familiar with the characteristics,
indications, and potential side effects of these agents. They must be able to
recognize adverse reactions promptly and treat them effectively and rapidly. This
tutorial is designed to help residents and practicing physicians utilize contrast
agents in a manner that maximizes the safety of their patients. The information
reflects policies and practices currently in use in the Radiology Department of the
University of Wisconsin. The use of contrast agents should be determined on an
individual basis according to the clinical circumstances of each patient. The
contents of this tutorial do not guarantee that contrast agents can be used safely
in any individual situation, for the decision to use a contrast agent must be made
according to the best judgment of the physician in charge of the examination.
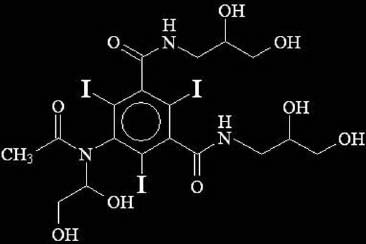
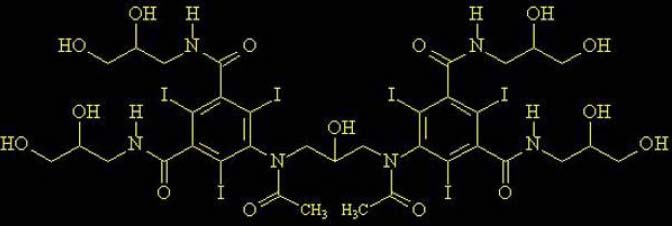
2. PHARMACOLOGY OF CONTRAST AGENTS
All intravascular iodinated contrast agents are based on a tri-iodinated benzene
ring. Three primary forms exist:
High-osmolar contrast media (HOCM) are the oldest agents. They are relatively
inexpensive, but their utility is limited. They are monomers (single benzene ring)
that ionize in solution with a valence of -1. Their cation is either sodium or
meglumine.
A major advance was the development of nonionic compounds. They are monomers that dissolve in water but do not dissociate. Hence, with fewer particles in solution, they are designated low-osmolar contrast media (LOCM).
The most recent class of agents is dimers that consist of a molecule with two benzene rings (again, each with 3 iodine atoms) that does not dissociate in water (nonionic). These compounds are designated iso-osmolar contrast media (IOCM).
The toxicity of contrast agents decreases as osmolality approaches that of serum. This has been accomplished by developing nonionizing compounds and then combining two monomers to form a dimer. Representative osmolalities are:
HOCM: Ionic monomer: diatrizoate:
1570 mosm/kg H2O
LOCM: Nonionic monomer: iohexol 240 (Omnipaque):
518 mosm/kg H2O *
LOCM: Nonionic monomer: iohexol 300 (Omnipaque):
672 mosm/kg H2O *
IOCM: Nonionic dimer: iodixanol 320 (Visipaque):
290 mosm/kg H2O *
* Agents presently used at the University of Wisconsin Hospital and Clinics.
Since the purpose of these agents is to deliver iodine in sufficient concentration for imaging, the ratio of iodine atoms to particles in solution becomes important. Ratios are:
Additional modifications that have reduced toxicity include the following: adding
calcium ions (reduces cardiac toxicity), establishing a neutral pH (low pH
predisposes to vasodilatation), and altering number and distribution of –OH ions
(decreases neural toxicity).
Currently used iodinated agents are cleared almost completely by glomerular
filtration. With reduced renal function, there is vicarious excretion primarily in bile
and through the bowel. Circulatory half life is 1–2 hours, assuming normal renal
function.
3. METHODS OF CATEGORIZING CONTRAST REACTIONS
There are two useful ways to approach contrast reactions. One is to categorize
them according to their severity. This method has immediate clinical relevance
when reactions occur and provides a framework for determining an appropriate
course of treatment. The other approach is to analyze them according to the
type of adverse reaction. This is important to understand the mechanisms of
reactions.
A. SEVERITY: The American College of Radiology has divided adverse
reactions to contrast agents into the following categories:
Mild
Signs and symptoms appear self-limited without evidence of progression
Nausea, vomiting
Shaking
Treatment: Observation and reassurance. Usually no intervention or medication
is required; however, these reactions may progress into a more severe category.
Moderate
Reactions which require treatment but are not immediately life-threatening
Tachycardia/bradycardia Hypotension
Pronounced cutaneous
reaction
Treatment: Prompt treatment with close observation
Severe
Life-threatening with more severe signs or symptoms including:
Laryngeal edema
Profound hypotension
Unresponsiveness
(severe or progressive) Convulsions
Cardiopulmonary arrest
Clinically manifest
arrhythmias Treatment: Immediate treatment. Usually requires hospitalization. Fortunately, most reactions are classified as mild. Within this category, itching, flushing, hives, nasal congestion, and swelling about the eyes and face are common. Nausea and vomiting have become less common with the use of low osmolar and iso-osmolar agents. Among the moderate reactions, bronchospasm and laryngeal edema are encountered most frequently; patients must also be monitored carefully for changes in cardiac rate and blood pressure. Severe reactions, while infrequent, can rapidly escalate to a life-threatening situation.
Delayed Contrast Reactions
Delayed contrast reactions can occur anywhere from 3 hours to 7 days following
the administration of contrast. Since patients are generally discharged from the
radiology department within 30 minutes of contrast administration, these
reactions are rarely observed by the radiologist supervising the contrast
administration. These events are often not brought to the attention of the
radiologist since the delayed event may not be ascribed to the contrast media
and these evens are often self limited. Regardless, it is important for anyone
administering intravenous contrast media to be aware of delayed reactions.
With the exception of contrast-induced nephropathy, the more common reactions
include a cutaneous xanthem, pruritis without urticaria, nausea, vomiting,
drowsiness, and headache. While cardiopulmonary arrest has been reported, it
is probably not related to newer contrast agents. Cutaneous reactions are the
most frequent form of delayed contrast reaction with a reported incidence of 0.5-
9%.
Cutaneous reactions vary in size and presentation but are usually pruritic. For the
most part, these reactions are self-limited and symptoms can be treated with
corticosteroid creams. Rare cases may progress to become severe, some
resembling Stevens-Johnson syndrome or a cutaneous vasculitis. Consultation
with a dermatologist is appropriate for delayed cutaneous reactions.
Delayed cutaneous reactions are more common in patients who have had a
previous contrast reaction and in those who have been treated within the past 2
years, or are currently being treated with interleukin-2 (IL-2). Due to this
association, the University of Wisconsin Hospitals and Clinics screens patients
for a history of IL-2 therapy. While the exact mechanism of the delayed reaction
is unknown, they can recur if the same contrast medium is administered again.
Therefore, it is possible that these delayed reactions are T-cell mediated. As
such, prophylaxis with oral corticosteroids may not be useful.
B. MECHANISM
Anaphylactoid
Nonanaphylactoid
o Chemotoxic – organ-specific
Nephrotoxicity
Cardiovascular
Neurotoxicity
4. ANAPHYLACTOID REACTIONS
Pathophysiology
Anaphylactic reactions are events initiated when an allergen and IgE combine to
induce mast cells to release chemical mediators. Mast cells originate from bone
marrow precursors and develop in the organs in which they come to reside.
Principal locations are the skin, respiratory tract, GI tract, and blood vessels.
Allergen-specific IgE is bound on the surface of mast cells. The allergen-IgE
complex activates the mast cell and induces it to release histamine as well as
other mediators.
Histamine binds to specific receptor sites. H1 receptors are found in endothelial
and smooth muscle cells and in the central nervous system. H2 receptors are in
gastric parietal cells and in inflammatory cells.
The nature of an anaphylactic reaction depends upon the location where it
occurs. In the skin, vasodilatation produces urticaria and erythema. In mucosa,
vasodilatation produces nasal congestion and laryngeal edema. In the
respiratory tract, smooth muscle contraction produces bronchospasm. In
peripheral vessels, vasodilatation produces hypotension and shock.
Gastrointestinal reactions include nausea, vomiting, diarrhea, and cramps.
Anaphylactoid reactions are identical to anaphylactic reactions in their
manifestations, but they are not initiated by an allergen-IgE complex. Indeed, the
pathway by which the mast cells become stimulated has not yet been clarified.
Acute contrast reactions are included in this group.
The distinction between anaphylactic and anaphylactoid reactions is subtle, but it
has certain important implications for the use of iodinated contrast:
1. A reaction can occur even the first time contrast is administered. 2. The severity of a reaction is not dose-related; therefore a test dose is

3. The occurrence of a contrast reaction does not necessarily mean that it
will occur again (although the risk is greater that it may).
4. Even though the circulating contrast is systemic, the nature of the
response is variable. More than one type of reaction may occur simultaneously.
As with anaphylactic reactions, certain risk factors make patients more susceptible to iodinated contrast (anaphylactoid) reactions:
1. Allergic asthma 2. Drug allergies 3. Food allergies 4. Prior reactions to contrast
Treatment of Anaphylactoid Reactions
Anaphylactoid reactions usually occur soon after contrast is administered. They
are variable in duration and severity. They may occur abruptly and progress
rapidly. Successful management of these events depends upon early
recognition, prompt and accurate assessment, and preparedness. Because
significant reactions do not occur often, it is necessary to review them frequently
enough so that basic knowledge and management priorities remain current and
fresh.
It is of utmost importance that medications and equipment be promptly available.
An emergency box or cart should be in the immediate vicinity.
Preferably it should be sealed (not locked) so that it will be intact when needed. It must be periodically inspected (and recorded and signed on a check-off log) to insure that it is fully stocked and that none of its contents have expired. A list of medications, indications, and directions for their use and a list of emergency phone numbers should be prominently displayed. A stethoscope and blood


pressure cuff of sufficient quality for reliable clinical use should be included. There should be a bag of isotonic IV solution (normal saline or Ringer's solution) with IV tubing. An appropriate selection of needles and syringes and everything else needed to draw up medications or start additional IVs should be available. An Ambu bag™ with a proper assortment of masks, laryngoscopes with endotracheal tubes, and airways should be included. There should be a flashlight and tongue depressors. An oxygen tank and tubing should be close at hand. A clipboard with paper for flow sheets is very helpful. Additional equipment may also be necessary in your institution. All policies and procedures should be dated and periodically reviewed.
In the University of Wisconsin Hospital and Clinics, an emergency cart is maintained in the central control area of the CT complex. On the top of the cart, laminated in plastic, are lists of all of the drugs on the cart together with their adult and pediatric dose schedules. Within the cart are two sealed packages, one a respiratory kit and the other a drug kit, both with expiration dates posted on them. The drug kit is prepared by the pharmacy. The cart itself is closed (not locked) with a numbered seal. Every day, a designated nurse in CT checks the emergency cart and on an initialed and dated check-off log writes the known expiration date of the two kits inside and the number on the seal.
Verifying that the number on the seal is the same as the day before assures that the cart has remained intact. This system also permits a daily review of forthcoming expiration dates. If either the respiratory kit or the drug kit is used, it
is repacked with a new expiration date, and the cart is closed with a new
numbered seal.
When summoned to assess a patient who may be having an adverse reaction,
you must be able to act quickly, purposefully, and effectively. Ascertain from the
technologist or nurse what the problem is. Speak to the patient to obtain
additional information and determine how he/she responds. Consult information
on pertinent medical history (this should be obtained before the contrast infusion
is started). Immediately stop the contrast infusion, hook up isotonic IV fluids, and
open the IV wide. Obtain vital signs. Check the airway and breathing. Listen to
the lungs. Check skin color, temperature, and dryness. Do not hesitate to
administer oxygen by mask (6–10 liters/min), elevate the patient's legs for
hypotension, or start additional IVs as appropriate.
It has long been recognized that anxiety plays a role in evoking and potentiating
contrast reactions. Therefore, your behavior and conduct become important
factors in your ability to successfully manage the patient. You must maintain and
display an unruffled, orderly, deliberate, capable, and effective demeanor, one
that elicits confidence and promotes a sense of well-being in the patient. These
same qualities are also important in coordinating the activities of staff members
assisting you. You must be able to take charge, assign tasks, monitor activities,
reassess the patient, and make effective decisions as circumstances change.
Those assisting should execute their duties quietly, minimizing anxiety-provoking
conversation.
Medications
When injecting medications IV through a needle inserted into a port in the IV
tubing, the IV should be running fast enough to promptly carry the medication
into the patient. It is also important to be certain that the needle is long enough
to extend into the main stream of the IV tubing. The following drugs are stocked
on the emergency cart in CT at the University of Wisconsin Hospital and Clinics
and defined by adult and pediatric dosage:
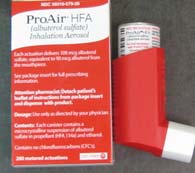


Albuterol Inhaler: A Beta-2 agonist that causes bronchodilatation
and relieves bronchospasm that may occur with asthma or as a reaction to contrast.
2 puffs to start
Atropine: A parasympatholytic agent used to treat bradycardia
that results from a vasovagal reaction (characterized by hypotension and bradycardia). The minimum adult dose is 0.6 mg, since a smaller amount can have a paradoxical reverse effect.
DOSE: 0.6–1.0
Diphenhydramine: An antihistamine which is an H-1 receptor site
blocker. In this capacity, it blocks circulating histamine from binding to target cells. It does not counteract histamine-mediated reactions that have already begun. Therefore, it should be used only to treat mild urticaria, which is likely to resolve on its own, and where it is deemed desirable to provide symptomatic relief by preventing further reactions. Diphenhydramine should not be used for severe urticaria or other more significant reactions. It does play a role in prophylaxis, as will be discussed.
25–50 mg IV or IM
Caution: Causes drowsiness. Patient should not drive or operate
machinery for 4-6 hours.
Clonidine: A drug used to treat a hypertensive crisis.
DOSE: 200 mcg (0.2 mg). Bite, chew, and swallow.
Epinephrine: A drug which is a basic sympathetic agonist with the
following effects:
Alpha–Peripheral
vasoconstriction
Beta-1–Cardiac:
contractility and heart rate (chronotropic,
Beta-2–Bronchodilatation (bronchioles)
As an alpha agonist, epinephrine is used to treat severe urticaria, facial edema, and laryngeal edema. As a beta-2 agonist, it may be needed to treat bronchospasm. It must be used with caution in patients with cardiac disease and hypertension. Epinephrine is supplied in two strengths: 1:1,000 in 1 mL vials for subcutaneous use, and 1:10,000 in 10 mL prefilled syringes for intravenous use. In each case, 1 mg of epinephrine is supplied. In most cases, the intravenous route is preferred. The subcutaneous route can be used in pediatric patients and in adults when there is inadequate IV access.
Subcutaneous: 1:1,000
DOSE: Intravenous:
1:10,000 (0.1 mg/mL)
IMPORTANT: Do NOT use the 1:1000 strength for IV injection.
Diazepam: A benzodiazepine used to treat seizures.
5–10 mg IV push
Nitroglycerin: A vasodilator used to treat acute angina.
0.4 mg sublingual
May be repeated q 5 minutes for a total of 3 doses
Pediatrics
The overall incidence of adverse reactions in children is less than that in adults.
However, children also pose unique problems due to their small size.
It is important to be familiar with pediatric dose regimens. Of note, epinephrine is
often administered to children by the subcutaneous route. Most pediatric doses
are based upon the child's weight. The Broselow-Luten system categorizes
children into one of eight color zones based on their weight and height.
Broselow-Luten charts list appropriate drug doses; these charts are part of the
emergency cart. The Broselow-Luten color zones have also been incorporated
into GE's scan protocols, which are utilized at UWHC, to ensure that the
examination is tailored to the child's size. Therefore, every child who will receive
iodinated contrast at UWHC will be assigned a color which can be used to guide
drug dosing.
The following drugs are stocked on the emergency cart in CT at the University of
Wisconsin Hospital and Clinics; the general pediatric dosages are noted below,
listed in mg/kg. Laminated cards with exact weight-based dosages are located
with the contrast reaction cart. These easy reference cards are convenient for
the team during the stress of an acute reaction and also serve to reduce the risk
of overmedication.
Albuterol: Inhalant for bronchospasm.
2 puffs --OR-- 2.5 mg in 3 mL normal saline for nebulizer if
respiratory therapist is present
Atropine: Parasympatholytic agent used to treat bradycardia that
occurs in a vasovagal reaction.
Maximum dose = 1 mg
Diphenhydramine: Used for treatment of urticaria.
Epinephrine: Used to treat laryngeal edema, severe urticaria or
facial edema, and to supplement Albuterol if needed for bronchospasm.
0.01 mg/kg/dose (0.01 mL/kg)
IMPORTANT: Do NOT use the 1:1000 strength for IV injection.
Lorazepam: A benzodiazepine for treatment of seizures.
0.01 mg/kg IV per dose
Diazepam: A benzodiazepine for treatment of seizures
0.2–0.3 mg/kg slow IV push per dose
May repeat in 5-10 minutes
5. NONANAPHYLACTOID REACTIONS
There are three categories of nonanaphylactoid reactions:
I. Chemotoxic – organ-specific
A. Nephrotoxicity
B. Cardiovascular toxicity
C. Neurotoxicity
II. Vasovagal
III. Idiopathic
I. CHEMOTOXIC
A. NEPHROTOXICITY
Physiology
The kidneys receive 20–25 percent of resting cardiac output, approximately 1.2–
1.3 liters every minute. Glomerular filtration rate is about 125 mL/min, or 180
liters per day. Urine volume is approximately 1 liter daily, indicating that the
kidneys reabsorb more than 99 percent of the glomerular filtrate.
Iodinated contrast agents have a molecular weight in the range of 600–1650
g/mol. From the vascular compartment, they pass through capillaries into the
extracellular space. Until eliminated, they remain in the vascular and interstitial
compartments, normally entering only cells of the proximal convoluted tubule.
Clearance is almost entirely by glomerular filtration. Contrast agents can easily
pass through the glomeruli, which can filter molecules up to 40,000 mw.
Pathogenesis
It is well established that iodinated contrast can exert a nephrotoxic effect. Three
general types of mechanisms have been described.
1. Vascular changes. Primarily a hyperosmotic effect where hypertonic
solution in the tubules inhibits water reabsorption, causing the tubules to swell and intrarenal pressure to rise. As a result, both renal blood flow and glomerular filtration decrease.
2. Tubular injury. Evidence for a toxic effect is based on observations of
reduced clearance of paraaminohippurate under certain conditions and also a rise in the urinary excretion of enzymes found in proximal tubular cells.
3. Tubular obstruction. This theory cites older studies that reported
precipitation of Bence-Jones proteins with earlier contrast agents, causing tubular obstruction.
Diagnosis of Renal Insufficiency
In most clinical settings, renal function is monitored by serum creatinine, which is
not a sensitive marker. Creatinine is a product of muscle metabolism and is
therefore proportional to muscle mass. The glomerular filtration rate (GFR) must
decline by about 50 percent, to 60 mL/min, before the serum creatinine rises
above 1.5 mg/dL. Thus, by the time the serum creatinine becomes abnormal,
significant renal dysfunction may already be present.
There is no universal agreement regarding what value of serum creatinine
indicates significant renal insufficiency. However, a level of 1.5 mg/dL is a widely
accepted figure. A cut-off below 1.5 mg/dL may classify individuals with normal
renal function, but large muscle mass, as having renal insufficiency. A cut-off
greater than 1.5 mg/dL may exclude individuals who actually have renal
insufficiency. A serum creatinine of greater than 1.5 mg/dL indicates renal
insufficiency.
GFR is a more sensitive indicator of renal function than serum creatinine alone.
Because creatinine clearance, as derived from 24-hour urine collection, is a
cumbersome test and has been shown to overestimate the true GFR by as much
as 20% and alternative predictor of GFR is ideal. The estimated GFR (eGFR) is
significantly easier to obtain as it is calculated from serum creatinine, age,
gender, and ethnicity using the MDRD (Modification of Diet in Renal Disease)
calculation. Additionally, eGFR has been shown to be a more accurate predictor
of GFR than serum creatinine alone.
With respect to GFR, moderately decreased renal function is defined as GFR 30-
59, severely decreased renal function is defined as GFR 15-29 and renal failure
is defined as GFR<15.
Blood urea nitrogen (BUN) is not an adequate measure of renal function. When
used in conjunction with serum creatinine, it can provide information about the
hydration status of a patient.
Screening for Renal Disease
While a thorough history is invaluable in evaluating patients for intravenous
contrast, screening for underlying renal disease with serum creatinine is also
important. Every inpatient requires a serum creatinine measurement within 1
week of the proposed dose of contrast. Outpatients with at least one of the
following risk factors require a serum creatinine measurement within 1 month of the proposed dose of contrast:
1. Age > 65 years 2. Diabetes treated with insulin or other prescribed medication 3. Receiving chemotherapy or aminoglycoside within the past 1 month 4. Diagnosis of a collagen vascular disease 5. Diagnosis of a paraproteinemia syndrome/disease (e.g. multiple
6. History of a kidney transplant, renal tumor, renal surgery, or single
7. History of endstage liver disease 8. History of severe congestive heart failure
Of course, these requirements can be waived under appropriate circumstances,
such as a life-threatening trauma that requires emergent evaluation. As serum
creatinine is not a precise marker of renal function, in cases of doubt a creatinine
clearance might be needed.
Renal Toxicity Due to Contrast Agents
Acute renal failure is a clinical entity characterized by an abrupt decline in renal
function. Among hospitalized patients, contrast agents have been listed as the
third most common cause of acute renal failure for inpatients, behind
hypotension and surgery.
Although institutional criteria vary, in general acute renal failure is defined when
the serum creatinine raises 25–50 percent or 0.5–1 mg/dL. Serum creatinine
peaks in 3–5 days but may be elevated as early as the first day. Clinical
manifestations are highly variable and may be absent or proceed to oliguria
(urine output < 400 mL/24h). Most effects are temporary and completely
reversible. In mild cases, serum creatinine returns to normal in 2 weeks. When
severe, dialysis may be necessary.
Predisposing Factors
NORMAL RENAL FUNCTION: For individuals with normal renal function and no
additional risk factors, contrast-induced nephrotoxicity is an uncommon event.
For these individuals, no significant advantage with regard to acute renal failure
has been demonstrated by using LOCM instead of HOCM.
RENAL IMPAIRMENT: For individuals with renal impairment, whether due to
intrinsic renal disease or to renal vascular insufficiency, several studies have
shown that LOCM agents are less nephrotoxic than HOCM. Adverse effects of
contrast agents are intensified in DEHYDRATED patients, so it is important to
ensure that these patients are adequately hydrated before a contrast study is
performed.
DIABETES increases the risk of contrast-induced renal toxicity, even when serum creatinine is normal. The effect is magnified, however, in those patients with both diabetes and renal disease. Additionally, insulin-dependent diabetics are likely at higher risk than non-insulin-dependent diabetics. Therefore, we set a lower cutoff for the use of IV contrast in diabetics. The following table contains the current UW guidelines for IV contrast, accounting for renal disease and diabetes (creatinine in mg/dL):
Diabetic
Among all predisposing factors, diabetics with pre-existing renal disease constitute the group at highest risk for nephrotoxicity (acute renal failure) from iodinated contrast agents. PARAPROTEINEMIAS or DYSPROTEINEMIAS are a group of diseases involving the monoclonal production of abnormal immunoglobulin; hence they are also termed MONOCLONAL GAMMOPATHIES. The most important is MULTIPLE MYELOMA; others include Waldenstrom's macroglobulinemia, heavy chain disease, primary amyloidosis, and monoclonal gammopathy of uncertain significance (MGUS). Most of these patients (except those with heavy chain disease) manifest BENCE-JONES proteins in the urine. These are light chain proteins, either kappa or lambda, that are small enough to be filtered. Old reports of renal failure with HOCM in multiple myeloma documented tubular obstruction due to precipitated Bence-Jones proteins. More recently, it has been postulated that such precipitation is not likely with the newer classes of agents, if patients are not dehydrated. If an iodinated contrast study is needed in such a patient, adequate hydration and a LOCM are mandatory. TAMM-HORSEFALL proteins are produced by cells in the ascending Loop of Henle. With low flow, they form hyaline casts of the tubules that appear in the urine. In patients with normal renal function, they signify only dehydration. When renal disease is present, red blood cells pass through the damaged glomerulus, become enmeshed in the Tamm-Horsefall protein precipitate, and appear as red
cell casts. They are laboratory indicators of renal injury, but are not employed directly to determine the use of contrast agents. GOUT (a disease arising from hyperuricemia) affects the kidneys by precipitation of uric acid crystals in acidic urine (acute urate nephropathy) or by formation of sodium urate tophi in the renal parenchyma (chronic urate nephropathy). Hyperuricemia was implicated in early studies as a cause of acute renal failure in iodinated contrast nephrotoxicity, but it has never been proven to be a stand-alone factor. Nonetheless, it continues to be seen as a relative risk factor, and if the contrast study cannot be postponed, patients with acute gout must at least be adequately hydrated. With the added presence of renal insufficiency (serum creatinine > 1.5 mg /dL), an alternative study should be considered. METFORMIN (GLUCOPHAGE™) is an oral antihyperglycemic medication used to treat diabetes. It is eliminated unchanged through the kidneys, likely by glomerular filtration and tubular excretion. As a biguanide, it stimulates intestinal production of lactic acid. There are now multi-ingredient oral antihyperglycemics, many of which contain metformin. Therefore, it is important to obtain a list of ALL of the oral medications a patient uses to treat his or her diabetes and to search for metformin or metformin-containing formulations. Appendix 2 lists many of the metformin-containing medications. Lactic acidosis can be fatal. Conditions that reduce metformin excretion or increase serum lactate include:
1. Renal disease–decreases metformin excretion 2. Liver disease–decreases lactic acid metabolism 3. Heart disease–increases anaerobic metabolism
Accordingly, in order to avoid lactic acidosis due to acute renal failure, the following precautions should be taken:
1. Metformin should be withheld at the time iodinated contrast is used. 2. It may be resumed in 48 hours only if renal function is shown to be
B. CARDIOVASCULAR TOXICITY
Patients with underlying cardiac disease have an increased incidence and/or
severity of cardiovascular side effects. Pulmonary angiography and intracardiac
and coronary artery injections carry the highest degree of risk. Possible
reactions include hypotension, tachycardia, and arrhythmias. More severe, but
uncommon reactions include congestive heart failure, pulmonary edema, and
cardiac arrest.
C. NEUROTOXICITY
Iodinated contrast agents cause a change in the blood-brain barrier due to their
hypertonicity. These risks are reduced when low or iso-osmolar agents are used.
Potential reactions include headache, confusion, seizures, altered
consciousness, visual disturbances, and dizziness.
II. VASOVAGAL REACTIONS
Vasovagal reactions are characterized by bradycardia and hypotension.
Initial resuscitation should include elevating the legs and/or placing the patient in
a Trendelenburg position and administering oxygen at the rate of 6–10
liters/minute.
Atropine may be used in the initial treatment of bradycardia. Epinephrine may be
necessary. See section on treatment of anaphylactoid reactions for appropriate
doses.
IV fluids are used to treat hypotension and should be administered rapidly. Large
volumes may be required. Normal saline and Lactated Ringer's are appropriate
choices.
It is important to monitor vital signs frequently to titrate the amount of medications
and fluids that are used.
6. RISK FACTORS
There are certain diseases or conditions that place a patient at added risk for an
adverse reaction to contrast. It is important that they be recognized in order that
appropriate precautionary measures and safeguards may be taken. To this end,
a careful and accurate history and review of pertinent laboratory data are vital.
Age–This is not an important factor in and of itself. However, it should be
remembered that significant illness is common among the elderly. Additionally,
they may have decreased reserve that makes them less able to tolerate an
adverse reaction.
Allergies–A history of allergies doubles the risk of an adverse reaction.
Anxiety–It has long been recognized that there is an increase in both the rate
and the severity of contrast reactions in anxious or fearful patients. Therefore it
is important that all medical personnel involved in a contrast procedure, and
especially the radiologist, conduct themselves in a manner that bespeaks
calmness, self-assurance, and competence, one that calms patients and allays
fears. It may be necessary to premedicate some patients. At the University of
Wisconsin Hospital and Clinics, midazolam 2 mg IV titrated up to a 5 mg
maximum dose is recommended (note this is contraindicated in patients with
glaucoma).
Asthma–A history of allergic asthma increases the risk of reaction up to five
times.
Cardiac Disease–Severe cardiac disease carries an increased risk of reaction.
Pertinent conditions include congestive heart failure, angina, cardiomyopathy,
aortic valvular disease, and pulmonary hypertension. Whenever possible, the
total dose of contrast should be reduced.
Debility–Debilitated patients are at added risk of adverse reaction due to
decreased ability to cope with the chemotoxicity of contrast agents.
Dehydration–Dehydration has the potential to increase nephrotoxicity,
particularly in patients with impaired renal function or multiple myeloma. If it is
absolutely necessary for a patient with multiple myeloma, sickle cell disease,
homocystinuria, or gout to receive iodinated contrast, it is imperative that the
individual be adequately hydrated prior to the scan.
Diabetes–See section on nephrotoxicity.
Dialysis–Patients who are on dialysis for potentially reversible acute renal failure
may receive iodinated contrast if deemed necessary by the referring physician. It
seems that the insult to the renal parenchyma occurs during the first pass of
contrast. Therefore, the current opinion is that the timing of hemodialysis, with
respect to contrast administration, is irrelevant. Rather, it is more important to
ensure that the patient is adequately hydrated prior to contrast administration.
Patients who undergo routine hemodialysis for end-stage renal disease can
receive non-ionic contrast. It is not imperative that dialysis be performed
immediately following the contrast procedure. However, it is preferable that the
study be scheduled so that the next routine dialysis is within 24 hours following
the examination.
Gout– See section on nephrotoxicity. If a contrast-enhanced examination is
absolutely necessary in a patient with acute gout, the individual must be well
hydrated.
Interleukin-2 Immunotherapy–Individuals who are on interleukin-2 or who have
taken it in the past, as long as 2 years previously, are at risk for delayed
reactions occurring several hours after the injection, similar to those that occur
with interleukin-2 itself. University of Wisconsin Hospital and Clinics guidelines
require that patients be monitored for 2 hours after the examination has been
completed.
Metformin (Glucophage)–See section on nephrotoxicity. Patients who receive
iodinated contrast may resume metformin 48 hours after the iodinated contrast
injection IF their renal function remains normal.
Multiple Myeloma–See section on nephrotoxicity. If these patients absolutely
must have iodinated contrast, they must be well hydrated.
Pheochromocytoma–Patients with pheochromocytoma are at risk for a
hypertensive crisis during the administration of contrast. Blood pressure must be
carefully monitored. University of Wisconsin Hospital and Clinics protocol
requires baseline blood pressure and pulse recordings and subsequent checks
just after injection and then every 5 minutes for 30 minutes or until it is deemed
safe to stop.
Previous Contrast Reaction–Without premedication (see section on
Premedication), the risk of reaction is increased 3 to 8 times.
Sickle Cell Disease–In-vitro studies have shown that red blood cell (RBC)
morphology may be affected by intravenous contrast material. In patients with
sickle cell disease, altered RBC morphology can lead to sickling. It seems that
the higher the osmolality, the more profound the effect and less sickling is seen
with LOCM as compared to HOCM. However, there is insufficient evidence of a
clinically significant increase risk to the sickle cell patient if LOCM is utilized. If
iodinated contrast is absolutely necessary, these patients must be adequately
hydrated.
7. EXTRAVASATION OF CONTRAST
Extravasation of small amounts of contrast usually results in only minimal
symptoms, including swelling, erythema, and pain. These symptoms usually
abate with no lasting effect.
Depending on the agent used, severe reactions can occur. They take the form of
skin ulceration and necrosis. The primary underlying mechanism is believed to
be the hyperosmolality of the contrast agent. Mechanical compression due to a
compartment syndrome may also occur. The following factors increase the
severity of reactions:
1. Vascular insufficiency–arterial, venous, lymphatic. 2. Type of contrast agent–ionic more harmful than nonionic. 3. Volume of extravasation; depending on location, significant reactions
can occur from even small volumes (< 10 mL).
4. Dorsum of hand, foot, and ankle where there is less subcutaneous
tissue or where nerves and vessels are near.
The inflammatory reaction usually reaches a maximum in 24–48 hours. Recommendations for observation and treatment vary, although each institution should develop its own protocol. A summary of the protocol currently in place in the University of Wisconsin Hospital and Clinics policy is as follows:
1. Immediate cessation of injection when a problem is detected. 2. Notification of responsible physicians. 3. Initial treatment:
a. Elevate the affected extremity above the level of the heart b. Intermittent, brief compression to milk the extravasate centrally,
can be applied with an ace wrap. (DO NOT leave the wrap on for more than 1 minute to avoid compartment syndrome.)
c. Observation d. Notification of the referring physician if the patient becomes
4. Indications for plastic surgery consultation (any one of the following):
a. Progressive swelling or pain b. Decreased capillary refill c. Altered sensation d. Skin ulceration or blistering.
5. Instructions are given to the patient. 6. Follow-up calls. 7. Documentation.
a. The physician evaluating the patient should fill out the contrast
extravasation form provided by the technologist
b. The extravasation event and treatment, if any provided, should
be included in the dictated report.
8. PREMEDICATION
Overall, patients who are at increased risk for an anaphylactoid reaction benefit
from premedication. Such patients include those with asthma, allergies, or a
history of a prior moderate or severe reaction to contrast. It should be noted,
however, that premedication is not a fail-safe guarantee that an iodinated
contrast-enhanced examination will be performed without complication.
The premedication regimen at the University of Wisconsin Hospitals and Clinics
includes:
1.
Methylprednisolone:
DOSE: 32 mg by mouth 12 and 2 hours before contrast.
2. Diphenhydramine
a. 50 mg IM or PO 1 hour before contrast, OR b. 50 mg (or 25 mg per height/weight indication) IV 15–20 minutes
before contrast.
Note: Patients may not drive or operate heavy machinery for 4–6 hours after receiving diphenhydramine.
In addition, these patients should receive nonionic contrast agents.
9. CLINICAL USE OF HIGH OSMOLAR IODINATED CONTRAST AGENTS
While most contrast-enhanced examinations are now performed with either low-
or isoosmolar iodinated contrast agents a high osmolar contrast agent is rarely
indicated. Iodipamide Meglumine (Cholografin®) is an intravenous contrast
agent which can be utilized for CT cholangiography. We will be seeing more CT
cholangiography in the preoperative work-up of potential living liver donors.
Iodipamide nearly completely ionizes in bodily fluids resulting in its high
osmolality. While older literature reports reaction rates as high as 15%, newer
series are reporting minor contrast reaction rates of only 1-3%. The decreased
reaction rate may be attributable to slow infusion rates and pretreatment with
intravenous diphenhydramine. As a result, we adhere to the following protocol
when injecting iodipamide:
1. Diphenhydramine 25 mg IV 2. 20 cc Iodipamide diluted into 100cc 0.9 Normal Saline (total
volume 120 cc) infused over 30 minutes.
3. Continual monitoring for potential contrast reaction 4. CT 30 minutes after completion of Iodipamide infusion
Iodipamide is primarily excreted into the bile with less than 5% of dose excreted into the urine. The biliary excretion explains why Iodipamide is used to image the biliary system. However, when the patient's hepatic function is compromised, a larger portion of the contrast is excreted by the kidneys, placing the patient at increased risk for renal toxicity.
MRI CONTRAST AGENTS
1. INTRODUCTION
Contrast agents used in MR imaging are gadolinium-based, non-nephrotoxic and
are safe and effective for diagnostic applications. The three contrast agents used
at the UWHC are MultiHance® (gadobenate dimeglumine), Eovist® (gadoxetate)
and Omniscan® (gadodiamide). The rate of allergic reaction to gadolinium based
contrast agents is much less than to iodinated contrast agents. The types of
adverse reactions are similar between CT and MR contrast agents, and the
treatments of these reactions are identical.
Although gadolinium based contrast agents are very safe in general, certain
patient populations are at risk for a rare disease called nephrogenic systemic
fibrosis (NSF). NSF was first described in 1997. NSF is an irreversible fibrotic
system disorder, with variable progression, causing disabling limitation of
movement, swallowing problems, and breathing difficulties. There is no effective
treatment for NSF. At our institution, NSF has exclusively occurred in
INPATIENTS with renal disease and exposure to Omniscan® who were also very
ill with co-existing pro-inflammatory conditions (see HIGH RISK CRITERIA
below). To date there are no documented cases of NSF at our institution in
OUTPATIENTS or LOW RISK INPATIENTS receiving Omniscan® or
MultiHance®.
Currently MultiHance® is used exclusively in both inpatients and outpatients with
renal insufficiency. There have been no reported cases of NSF at the UWHC
since switching to MultiHance® in November 2006.
2. ASSESSING PATIENTS FOR NSF RISK FACTORS
All INPATIENTS who are scheduled for contrast enhanced MR scans must
complete the UWHC Clinical NSF High Risk Screening Questionnaire before
their procedure. The Questionnaire will be filled out by a nurse, physician or
other healthcare provider. This is not a requirement for outpatients undergoing
contrast enhanced MRI.
OUTPATIENTS:
OUTPATIENTS at very low or no risk for NSF. In
OUTPATIENTS, we use MultiHance® if the patient has a history of renal
disease, per the screening sheet. The case does not need to be discussed
with the ordering physician. We do not require eGFRs on OUTPATIENTS
and they are not screened with the INPATIENT questionnaire.
SCREENING INPATIENTS:
Case-based clinical screening guidelines minimize the risk of NSF while
continuing to provide the option of contrast enhanced magnetic resonance (CE-MR) imaging to patients. Below are the UWHC Department of Radiology current guidelines, as reviewed at the yearly MR Safety Committee meeting.
Currently we only consider INPATIENTS at high risk, and only if they fail the
below screening questionnaire.
INPATIENTS are considered at high risk for NSF if they meet criteria from BOTH
section 1 and section 2 of the following questionnaire:
1. Does the patient meet the following criteria:
a. Inpatient with kidney or liver transplant (tx) with eGFR <60?
b.
Inpatient with native kidneys and eGFR <30?
Inpatient with acute renal failure?
2. Does the patient have a recent (1 month) history of:
a. Major infection (pneumonia/sepsis/osteomyelitis)?
b. Vascular ischemia of the extremities (arterial thrombosis/gangrene/amputation)?
c. Venous or arterial thrombosis (PE/hepatic artery thrombosis)?
d. Major surgery or vascular procedure (Vascular/CABG/Amputation/Transplantation)?
e. Multi-organ system failure?
If the INPATIENT fails the NSF screening and is deemed high risk, MultiHance®
may be used if the benefits of a CE-MRI/A with MultiHance® outweigh the
theoretical risk of NSF with MultiHance®. In these cases, the ordering attending
physician should discuss the theoretical risk of NSF with the patient and inform
the patient that there are no reported cases of NSF with MultiHance®, here or
elsewhere.
UWHC data in patients with renal insufficiency, acquired from November 2006 to April 2009, revealed no
cases of NSF in 466 patient exams where MultiHance® was administered (Figure 1).
Figure 1:
Nov 2006-April 2009: 466 patient exams
<30 ml/min/1.73m2
NO NSF CASES
Cases of NSF have been associated with other gadolinium based contrasts, including Omniscan®, which is not currently used at UWHC in patients with renal disease. IF the exam is deemed medically necessary by the physician and radiologist, and the patient is on hemodialysis, the patient should be dialyzed promptly post-MRI/A. If the patient is not on hemodialysis, the patient should not have dialysis. The radiologist approving the MR exam should ask the referring service to notify the dialysis unit that the patient will need dialysis after the CE-MR exam and that MR staff will help coordinate the timing of the CE-MRI/A and dialysis.
3. CONCLUSION Gadolinium based contrast agents can be used safely for imaging patients in most settings. The risk of allergic reaction is less than with CT agents, and MR contrast agents are not nephrotoxic. However, extremely ill inpatients with renal insufficiency may be a risk for a rare systemic disease, NSF. Carefully considering the risks and benefits of a contrast enhanced MR examination can minimize the possibility of developing NSF in high risk inpatients.
CONCLUSIONS
Although contrast agents are widely used with safe outcomes and little or no side effects, adverse reactions nonetheless may occur. They may be severe, and they may progress rapidly. Successful patient management during contrast-enhanced examinations requires all of the following:
1. Knowledge of the patient's medical history. 2. Patient preparation, including premedication, if necessary. 3. Proper selection of the agent to be used. 4. Knowledge of the pathophysiology of contrast reactions. 5. Prompt recognition and accurate assessment of reactions. 6. Immediate availability of necessary equipment and drugs. 7. Adequate prior planning and training. 5. Current knowledge of medications and other treatment options.
In summary, vigilance and attention to detail are key. Expect the unexpected and be prepared.
APPENDIX 1: GENERIC AND TRADE NAMES OF DRUGS AND CONTRAST
AGENTS
Note: The trade names listed here are examples of ones commonly in use; other trade name products may also be available.
Generic Name
Trade Name
Gadobenate dimeglumine.Multihance Gadoxetate.Eovist Metformin……………….…….Glucophage
APPENDIX 2: METFORMIN-CONTAINING MEDICATIONS
Metformin formulations:
Glucophage
Glumetza
Fortamet
Glucophage XR
Multi-ingredient medications containing metformin (trade name):
Metformin/glipizide (Metaglip)
Metformin/glyburide (Glucovance)
Metformin/pioglitazone (ActoPlus Met, ActoPlus Met XR)
Metformin/repaglinide (PrandiMet)
Metformin/rosiglitazone (Avandamet)
Metformin/sitagliptin (Janumet)
BIBLIOGRAPHY
1. American College of Radiology. Manual on Contrast Media, Verision 7. 2010. 2. Aspelin PR, Aubry P, Fransson SG, et al. Nephrotoxic Effects in High-Risk
Patients Undergoing Angiography. N Eng J Med 2003; 348:491-499.
3. Atwell TD, Lteif AN, Brown DL, McCann M, Townsend JE, Leroy AJ. Neonatal
thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am J Roentgenol 2008;191:268-271
4. Barrett BJ, Carlisle EJ. Metaanalysis of the Relative Nephrotoxicity of High- and
Low-Osmolality Iodinated Contrast Media. Radiology 1993; 188:171-178.
5. Barrett BJ, Parfrey PS, et al. Nonionic Low-Osmolality versus Ionic High-
Osmolality Contrast Material for Intravenous Use in Patients Perceived to be at High Risk: Randomized Trial. Radiology 1992; 183:105-110.
6. Becker JA. Evaluation of Renal Function. Radiology 1991; 179:337-338. 7. Berk RN, Ferrucci JT, Leopold GR. Radiology of the gallbladder and bile ducts :
diagnosis and intervention. Philadelphia: W.B. Saunders, 1983:ix, 585 p
8. Bettman MA, Ellis JH. Radiologists Can Help Prevent Contrast-Related
Nephropathy. RSNA News, January, 2004.
9. Bush WH, Swanson, DP. Acute Reactions to Intravascular Contrast Media:
Types, Risk Factors, Recognition, and Specific Treatment. AJR 1991; 157:1153-1161.
10. Cohan RH, Ellis JH, Garner WL. Extravasation of Radiographic Contrast
Material: Recognition, Prevention, and Treatment. Radiology 1996; 200:593-604.
11. Cohan RH, Dunnick NR, Bashore TM. Treatment of Reactions to Radiographic
Contrast Material. AJR 1988; 151:263-270.
12. Dunnick NR. Questions and Answers. AJR 1996; 166:209-213. 13. Ellis JH, Cohan RH, Sonnad SS, Cohan NS. Selective Use of Radiographic Low-
Osmolality Contrast Media in the 1990s. Radiology 1996; 200:297-311.
14. Freed KS, Leder RA, Alexander C, DeLong DM, Kliewer, MA. Breakthrough
Adverse Reactions to Low-Osmolar Contrast Media After Steroid Premedication. AJR 2001; 176:1389-1392.
15. Hesley G and Hartman R. Review of common and uncommon contrast media
reactions. Applied Radiology 2008;: 20-24.
16. Jakobsen JA, Berg KL, Waaler B, Andrew E. Renal Effects of the Non-Ionic
Contrast Medium Iopentol After Intravenous Injection in Healthy Volunteers. Acta Radiol 1990; 31:87-91.
17. Jakobsen JA, Lundby B, Kristoffersen DT, et al. Evaluation of Renal Function
with Delayed CT after Injection of Nonionic Monomeric and Dimeric Contrast Media in Healthy Volunteers. Radiology 1992; 182:419-424.
18. Katzbert RW. Urography into the 21st Century: New Contrast Media, Renal
Handling, Imaging Characteristics, and Nephrotoxicity. Radiology 1997; 204:297-312.
19. Lalli AF. Urographic Contrast Media Reactions and Anxiety. Radiology 1974;
20. Lautin EM, Freeman NJ, Schoenfeld AH, et al. Radiocontrast-Associated Renal
Dysfunction: Incidence and Risk Factors. AJR 1991; 157:49-58.
21. Lautin EM, Freeman NJ, Schoenfeld AH, et al. Radiocontrast-Associated Renal
Dysfunction: A Comparison of Lower-Osmolality and Conventional High-Osmolality Contrast Media. AJR 1991; 157:59-65.
22. Moss AA. Double-blind comparison of iodipamide and lodoxamate using direct
and drip infusion intravenous cholangiography. AJR 1977;128:931-933
23. National Kidney Foundation Kidney Disease Outcome Quality Initiative
Guidelines, 2002.
24. Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast Material-Induced Renal
Failure in Patients with Diabetes Mellitus, Renal Insufficiency, or Both. N Eng J Med 1989; 320:143-149.
25. Rodesch F, Camus M, Ermans AM, Dodion J, Delange F. Adverse effect of
amniofetography on fetal thyroid function. Am J Obstet Gynecol 1976;126:723-726
26. Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of Ionic and Nonionic
Contrast Media in 1196 Patients: A Randomized Trial. Kidney International 1995; 47:254-261.
27. University of Wisconsin Computed Tomography Policy and Procedures Manual,
28. Webb JA, Stacul F, Thomsen HS, et al. Late adverse reactions to intravascular
iodinated contrast material. Eur Radiol 2003; 13: 1650-1655.
29. Yeh BM, et al. Biliary tract depiction in living potential liver donors: comparison of
conventional MR, mangafodipir trisodium-enhanced excretory MR, and multi-detector row CT cholangiography--initial experience. Radiology 2004;230:645-651.
Source: https://www.radiology.wisc.edu/fileShelf/contrastCorner/ContrastAgentsTutorial.pdf?buster=1454986961
Examen : BEP Date de l'épreuve : Spécialité/option : Carrières sanitaires et sociales Repère de l'épreuve : EP2 Épreuve : Sciences et technologie (En majuscules, suivi s'il y a lieu du nom d'épouse) Prénoms : N° du candidat (le numéro est celui qui figure sur la convocation ou la
Documento descargado de http://www.elsevier.es el 07/03/2013. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. Histeroscopia en el tratamiento de los po ´lipos placentarios Luis Alonso Pacheco Miguel Rodrigo Olmedo, Juan Larracoechea Barrionuevo y Rafael Gonzalez de gor Crooke Unidad de Endoscopia, Centro Gutenberg, Ma








