Novel silk fibroin/elastin wound dressings


Contents lists available at
Acta Biomaterialia
Novel silk fibroin/elastin wound dressings
Andreia Vasconcelos Andreia C. Gomes , Artur Cavaco-Paulo ,
a Universidade do Minho, Departamento de Engenharia Têxtil, Campus de Azurém, 4800-058 Guimarães, Portugalb Centre of Molecular and Environmental Biology (CBMA), Department of Biology, Campus de Gualtar, 4710-057 Braga, Portugal
Silk fibroin (SF) and elastin (EL) scaffolds were successfully produced for the first time for the treatment
Received 28 October 2011
of burn wounds. The self-assembly properties of SF, together with the excellent chemical and mechanical
Received in revised form 12 April 2012
stability and biocompatibility, were combined with elastin protein to produce scaffolds with the ability to
Accepted 20 April 2012
mimic the extracellular matrix (ECM). Porous scaffolds were obtained by lyophilization and were further
Available online 27 April 2012
crosslinked with genipin (GE). Genipin crosslinking induces the conformational transition from randomcoil to b-sheet of SF chains, yielding scaffolds with smaller pore size and reduced swelling ratios,
degradation and release rates. All results indicated that the composition of the scaffolds had a significant
effect on their physical properties, and that can easily be tuned to obtain scaffolds suitable for biological
applications. Wound healing was assessed through the use of human full-thickness skin equivalents (Epi-
dermFT). Standardized burn wounds were induced by a cautery and the best re-epithelialization and the
fastest wound closure was obtained in wounds treated with 50SF scaffolds; these contain the highestamount of elastin after 6 days of healing in comparison with other dressings and controls. The cytocom-patibility demonstrated with human skin fibroblasts together with the healing improvement make theseSF/EL scaffolds suitable for wound dressing applications.
Ó 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
parameters in the biomaterials field. Recently, we developed silkfibroin/keratin films incorporating a synthetic inhibitor of elastase,
Skin wounds are the disruption of normal skin physiology. From
to control the high levels of this enzyme produced in a chronic
the moment the wound is created, the healing mechanism is initi-
wound environment Silk fibroin/alginate sponges demonstrate
ated to re-establish skin continuity. The healing process is complex
a higher healing effect than both components acting alone In
and involves an integrate response of different cell types and
this work, scaffolds based on silk fibroin and soluble elastin were
growth factors . Promotion of healing is often accompanied
developed and tested.
by the use of biocompatible wound dressings: these should pro-
Elastin (EL) is an insoluble extracellular matrix protein that
mote a moist environment in the wound and serve as a shield
provides elasticity and resilience to the arteries, lungs and skin
against external factors like dust and bacteria; enhance water
Due to its highly crosslinked nature, elastin is highly
and vapor permeation and promote epithelialization by releasing
insoluble and difficult to process into new biomaterials. As a con-
biological agents to the wounds. Due to its unique properties of
sequence, soluble forms of elastin including tropoelastin ,
high mechanical strength and excellent biocompatibility, silk
a-elastin and elastin-like polypeptides are fre-
fibroin has been explored for the development of wound dressings.
quently used to develop elastin-based biomaterials. Nevertheless,
The degradation rates of electrospun silk materials applied as
a crosslinking step is required to obtain an insoluble material.
wound dressings have been evaluated and the incorporation
There are several crosslinking methods for elastin including chem-
of growth factors into electrospun silk mats has been shown to
ical , enzymatic physical and c-irradia-
accelerate wound healing . Moreover, silk films have been
tion . Among them, chemical crosslinking agents are widely
shown to heal full thickness skin wounds in rats faster than tradi-
used. Aldehydes and epoxy compounds have been commonly used
tional porcine-based wound dressings
in biomaterial constructs due to their efficient formation of cross-
Blending silk fibroin with other components has been shown to
links with amino acid side chains, low antigenicity and sufficient
improve the properties of the resulting material. This allows the
mechanical strength. Despite these advantages, they exhibit high
modulation of biodegradation and release rates, important
Genipin (Ge) is a natural covalent crosslink agent isolated from
the fruits of Gardenia jasminoides Ellis that offers comparable
⇑ Corresponding author. Tel.: +351 2535 10100; fax: +351 2535 10293.
E-mail address: (A. Cavaco-Paulo).
crosslinking efficacy. It has been reported that genipin binds with
1742-7061/$ - see front matter Ó 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
biological tissues and biopolymers , leading to
2.4. Degree of crosslinking
matrices with good mechanical properties, reduced swelling extentand significantly reduced cytotoxicity when compared to synthetic
The crosslinking degree was determined by the ninhydrin assay
crosslink agents like glutaraldehyde and epoxy compounds
. Samples (6.0 ± 0.7 mg) were heated with a ninhydrin
solution (2% (w/v)) at 100 °C for 20 min. The optical absorbance
To our knowledge, elastin has been crosslinked with collagen
of the resulting solution was recorded at a wavelength of 570 nm
, fibrin and gelatin for the development of
using a Hekios c ThermoSpectronic spectrophotometer. The
biomaterials, but never with silk fibroin. In this study, we devel-
amount of free amino groups in the test sample after heating with
oped silk fibroin/elastin (SF/EL) scaffolds crosslinked with genipin.
ninhydrin is proportional to the optical absorbance of the solution.
The resulting materials were characterized by their physical–
The concentration of free NH2 groups in the sample was deter-
chemical properties and the effect of crosslinking on those proper-
mined from a standard curve of glycine concentration vs. absor-
ties was evaluated. Moreover, the wound dressing functionality of
bance. SF/EL scaffolds prepared without genipin were used as
these materials was tested with a real chronic wound exudate and
control materials. Triplicate samples were evaluated. The degree
the healing ability was assessed through the use of three-dimen-
of crosslinking was determined by the following equation:
sional (3-D) human skin equivalents.
Degree of crosslinking ð
2. Materials and methods
(NH2)nc and (NH2)c are, respectively, the mole fraction of free
NH2 in non-crosslinked and crosslinked samples.
Silk cocoons from Bombyx mori were kindly supplied from
‘‘Sezione Specializzata per la Bachicoltura'' (Padova). Elastin solu-
2.5. FTIR spectroscopy
ble from bovine neck ligament was purchased from Sigma (Spain).
Genipin is a product of Wako Chemicals (Germany). The BJ5ta cell
FTIR spectra of pure SF and SF/EL scaffolds were measured with
line (telomerase-immortalized human normal skin fibroblasts)
a Perkin-Elmer (Spectrum One FTIR) spectrometer in the spectral
was purchased from ATCC through LGC Standards.
region of 4000–650 cm�1 with a ZnSe ATR cell. Spectra were ac-
Human full-thickness skin equivalents (EpidermFT) were sup-
quired for sponges with and without methanol treatment. For EL
plied by MatTek Corporation (USA). All other reagents, including
samples, FTIR spectra were recorded in KBr pellets using a FTIR-
those used in cell culture, were analytical grade and purchased
4100 from Jasco with a resolution of 2 cm�1.
from Sigma (Spain).
2.6. Thermal analysis
2.2. Preparation of silk fibroin solution
Differential scanning calorimetry (DSC) measurements were
Silk was purified from its sericin content as previously de-
performed with a DSC-30 instrument (MettlerToledo), from room
scribed The cocoons were cut, cleaned from debris and
temperature to 120 °C, at a heating rate of 10 °C min�1, and kept
larvae and autoclaved for 30 min at 120 °C. Fibroin was then thor-
at 120 °C for 10 min, to induce dehydration of samples. The tem-
oughly washed with distilled water and dried overnight at room
perature was lowered to room temperature and increased to
temperature. Silk fibroin (SF) solution (2% (w/v)) was prepared by
500 °C at a heating rate of 10 °C min�1. Sample weight was
dissolving fibroin in 9.6 M LiBr solution at 60 °C for 3 h. The result-
2–3 mg. The open aluminum cell was swept with N2 during the
ing solution was filtered, and dialyzed against distilled water until
analysis. The analysis was performed in duplicate for scaffolds with
salts were completely removed, using cellulose tubing (Sigma,
and without methanol treatment.
Spain) (molecular-weight cut-off of 12,000–14,000 Da).
2.7. Scanning electron microscopy (SEM)
2.3. Silk fibroin/elastin blends preparation; crosslinking reaction;scaffold formation
Cross-sections were prepared by cutting the SF/EL scaffolds
with a razor blade in liquid nitrogen. Before analysis, the scaffolds
Elastin (EL) solution was prepared by dissolving the elastin
were coated with gold and examined morphologically using a
powder in distilled water. SF (2%) and EL (1%) were mixed to pre-
NOVA Nano SEM 200 FEI. The morphology was determined before
pare blends of 100/0 SF/EL, 80/20 SF/EL and 50/50 SF/EL. Genipin
and after methanol treatment.
(GE) powder, 0.1 and 0.5% (w/v) was added to blend solutionsunder constant stirring at room temperature until complete disso-lution of GE powder. The crosslinking reaction was carried out for
2.8. Swelling ratio
3, 6 and 24 h at 37 °C. The resulting solutions were cast on 96-wellplates and frozen at �20 °C for 2 days and freeze dried for 2 days to
SF/EL scaffolds, treated with methanol and completely dry
remove the solvent completely. SF/EL scaffolds without genipin
(60 °C for 24 h) were immersed in phosphate-buffered saline
were used as controls and were prepared by the same process de-
(PBS; pH 3.0, 7.4 and 11) at 37 °C for 24 h. The excess buffer was
scribed above. The control samples were identified as 100SF, 80SF
removed and the wet weight of the scaffolds was determined.
and 50SF, which correspond to 0, 20 and 50% of elastin, and cross-
The swelling ratio of the film was calculated as follows:
linked scaffolds were identified as 100SFyGE, 80SFyGE and50SFyGE, where y is the genipin concentration used. In order to in-
Swelling ratio ¼
duce the transition of SF from random coil to b-sheet structure and
consequently insolubility, scaffolds were immersed in 90% (v/v)methanol solution for 30 min and then washed in distilled water
WS is the mass of the swollen material and Wd is the initial dry
and air dried.
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
curve. Release studies were performed in triplicate samples andfor a period of 7 days. The release behavior of compounds from
The porosity of the SF/EL scaffolds with different blending ratios
polymeric systems can be determined by fitting the release data
was measured by the liquid displacement method . Hexane
to the empirical relationship given by the Ritger–Peppas equation
was used as the displacement liquid because it is a non-solvent
for SF. The scaffolds were immersed in a known volume (V1) of
hexane in a graduated cylinder for 30 min. The total volume of hex-
ane after impregnation into the scaffold was recorded as V2. The
impregnated scaffolds were then removed from the cylinder and
Mt/M1 is the fractional drug release at time t; t is the release
the residual hexane volume was recorded as V3. For all types of
time; k is the kinetic constant that measures the drug release rate,
scaffolds, experiments were carried out in triplicate. Data are
and n is the diffusion exponent that depends on the release mech-
presented as average ± SD. One-way ANOVA analysis of variance
anism and the geometry of the matrix. To determine n values, Eq.
with Bonferroni post-tests was performed, with statistically signif-
is modified in Eq. and n is determined from the slope of the
icant differences when p < 0.001. All calculations were performed
plot of log (%released) vs. log t.
using GraphPad software (version 5.03). The porosity of the scaf-
log ð%releasedÞ ¼ logðMt=M
fold (e) was calculated by the following equation:
2.12. Cytotoxicity
The scaffolds were tested for cytotoxicity according to ISO stan-
2.10. In vitro degradation
dards (10993-5, 2009). The BJ5ta cell line (normal human skinfibroblasts) was maintained according to ATCC recommendations
2.10.1. Porcine pancreatic elastase (PPE)
(four parts Dulbecco's modified Eagle's medium (DMEM) contain-
SF/El scaffolds previously treated with methanol, with and
ing 4 mM L-glutamine, 4.5 g l�1 glucose, 1.5 g l�1 sodium bicarbon-
without crosslinking, were incubated for 21 days at 37 °C in a solu-
ate, and one part of Medium 199, supplemented with 10% (v/v) of
tion containing 0.1 mg ml�1 of PPE in 100 mM Tris–HCl buffer,
fetal bovine serum (FBS), 1% (v/v) of penicillin/streptomycin solu-
pH 8.0. The control samples were incubated in PBS buffer solution
tion and 10 lg ml�1 hygromycin B). The cells were maintained at
(pH 7.4) without enzyme and submitted to the same conditions.
37 °C in a humidified atmosphere of 5% CO2. Culture medium
The solutions were replaced every 24 h.
was refreshed every 2 to 3 days.
2.10.2. Wound exudate
2.12.1. Test by indirect contact
Wound exudate was collected from pressure wounds using a
Scaffolds (£=3 mm and 6 mm thickness) were sterilized by
vacuum assisted closure system. Wound fluid was diluted ten-fold
immersion in ethanol 70% for 30 min, then hydrated and thor-
in PBS solution and centrifuged to remove cells and tissue material.
oughly rinsed with PBS. The conditioned media were obtained by
SF/EL scaffolds were incubated with exudate in the same condi-
incubating the sponges in 1 ml of DMEM in a CO2 incubator at
tions described above in a fixed ratio of exudate per mg of scaffold
37 °C for 5 days. The sponges were then removed and the condi-
of 6 mg ml�1. At designated time points, samples were washed
tioned media were obtained. Before use, the conditioned media
thoroughly with distilled water, dried in a desiccator and weighted
were filtered to remove degraded scaffolds and diluted if necessary
to estimate the extent of degradation by the following equation:
in complete cell culture medium. Complete cell culture medium
subjected to the same conditions but not exposed to the sponges
was used as a negative control, whereas a 1% (v/v) solution of Tri-
tonÒ X-100 (Sigma) prepared in fresh culture medium was used as
m0 and mf are respectively, the initial and final dry mass of the
a toxicity positive control. Cells were seeded at a density of
20 � 103 cells/100 ll/well on 96-well tissue culture polystyrene(TCPS) plates (TPP, Switzerland) the day before experiments and
2.11. In vitro release
then incubated with the conditioned media. At each defined timepoint (24, 48 and 72 h), cell viability was assessed using the Alamar
The release of a compound from SF/EL scaffolds was examined
Blue assay (alamarBlueÒ Cell Viability Reagent, Invitrogen). Resa-
by the incorporation of an antibacterial agent, gentamicin
zurin, the active ingredient of alamarBlueÒ reagent, is a non-toxic,
(2 mg ml�1). For control samples, gentamicin was dissolved in
cell-permeable compound that is blue in color and reduced to
the protein solutions and stirred for 5 min at room temperature.
resorufin, red color compound, by viable cells. The quantity of
The resulting solutions were cast in 96-well plates to prepare the
resofurin formed is directly proportional to the number of viable
SF/EL scaffolds. In the case of crosslinked samples, gentamicin
cells. 10 ll of alamarBlueÒ reagent was added to each well contain-
was dissolved in the protein solution before crosslinking reaction.
ing 100 ll of culture medium. After 4 h of incubation at 37 °C the
Before release studies, control and crosslinked scaffolds were trea-
absorbance at 570 nm was measured, using 600 nm as a reference
ted with methanol. SF/EL scaffolds were incubated at 37 °C in PBS
wavelength, in a microplate reader (Spectramax 340PC). Data are
buffer and in a solution containing 0.1 mg ml�1 of PPE. Solutions
presented as average ± SD of two independent measurements.
were changed every 24 h. At determined time points, aliquots were
Two-way ANOVA with Bonferroni post-tests was performed, with
taken and gentamicin release was determined using the o-phthal-
statistically significant differences when p < 0.001. All calculations
dialdehyde method The analysis was carried out by measur-
were performed using GraphPad software (version 5.03).
ing the maximum fluorescence of gentamicin-o-phthaldialdehydecomplex using a multiplate reader (Synergy HT W/TRF from Bio-
2.12.2. Cell proliferation
Tek) in the fluorescence mode at an emission wavelength of
Cell proliferation was determined in terms of DNA content to
456 nm. After each measurement, the samples were added back
monitor the effect of the scaffolds on fibroblast. Scaffolds, prepared
to the medium to restore the equilibrium conditions. The quantifi-
and sterilized as previously described (£=15 mm and 3 mm
cation of the release was established by a gentamicin standard
thickness), were gently placed in 24-well (TCPS) plates (TPP,
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
Switzerland), then 250 ll of cell suspension (2 � 105 cells ml�1)
was loaded onto an upper side of each scaffold and allowed to infil-
Degree of crosslinking obtained for SF/EL solutions, for the different reactionconditions, determined by Eq.
trate into the scaffold. The scaffolds were then incubated at 37 °Cunder 5% CO
Crosslinking treatment
Degree of crosslinking (%)
2 conditions for 3 h to allow for initial cell attachment.
After the initial incubation period the wells were then filled with
250 ll of medium and placed into a cell culture incubator and
maintained at 37 °C with 5% CO2 for either 3 or 5 days. Culture
media were renewed every 2 days. After each indicated time inter-
val, cells/scaffold constructs were collected, rinsed with PBS and
cell proliferation was determined in terms of DNA content mea-
sured with Hoechst 33258 (Invitrogen). Briefly, cells were har-vested from cell-scaffold constructs by incubating with a 0.25%solution of trypsin. Cells were then collected by centrifugationand lysed in a Tris–HCl 15 mM pH 7.4 buffer with consecutivefreeze–thaw cycles. Cell lysates were incubated with equal volume
accepted mechanism is similar to that observed for amino-group
of 5 lg ml�1 Hoechest 33258 solution for 40 min at room temper-
containing compounds where the ester groups of genipin
ature in the dark. Fluorescence was determined using a FLUOROS-
interact with the amino groups of SF and elastin, leading to the for-
KAN ASCENT FL plate reader (ThermoScientific) at 350 nm
mation of secondary amide linkages. Moreover, the amino groups
excitation and 445 nm emission. The relative fluorescence unit
initiate nucleophilic attacks which result in the opening of the
value obtained from samples was interpolated against a DNA stan-
genipin dihydropyran ring. An inherent phenomenon of genipin
dard curve constructed using known number of cells, to determine
crosslinking is self-polymerization, which occurs by radical reac-
the DNA content/number of cells in each sample. Data are
tion of two amino-attached open rings Some authors
presented as average ± SD of two independent measurements.
reported that genipin preferentially reacts with the amino
Two-way ANOVA with Bonferroni post-tests was performed, with
acids lysine and arginine. SF and elastin contain respectively,
statistically significant differences when p < 0.001. All calculations
0.95% and 1.07% of these amino acids, which is a very low fraction.
were performed using GraphPad software (version 5.03).
The crosslinking sites are thus low in number, which results inlower crosslinking degrees when compared with other genipincrosslinked blend systems The highest crosslinking degree
2.13. Wound healing assay
obtained for sponges containing elastin might be related with theslightly higher fraction of lysine and arginine amino acids.
Skin equivalents (EpidermFT) were cultured at the air–liquid
The genipin crosslinking of SF/EL scaffolds might induce confor-
interface in tissue culture inserts placed in six-well plates accord-
mational changes due to the structural rearrangement of chains to
ing to manufacturer's instructions. Upon receipt the tissues were
form covalent bonds. FTIR spectra of SF and elastin, with and with-
placed into new six-well plates containing 2.5 ml of fresh culture
out crosslinking, in the range of 600–2000 cm�1 are represented on
medium, supplied with the skin equivalents, and kept at 37 °C,
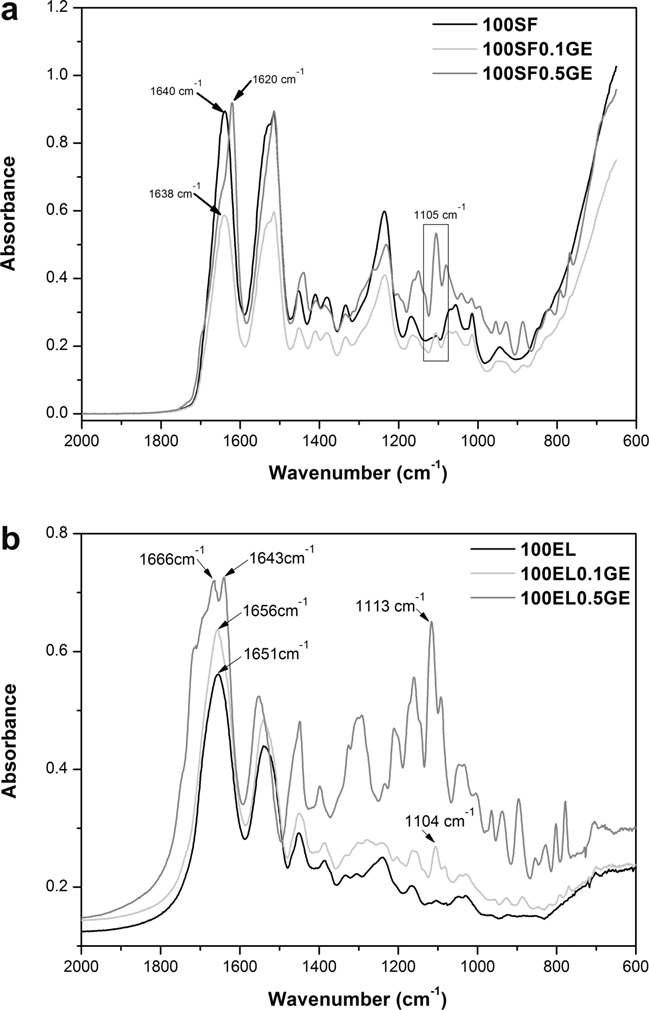
. SF protein exists in three conformations namely random coil,
5% CO2 overnight. Burn wounds were made by placing a cautery
Silk I (a-form) and Silk II (b-sheet conformation). The 100SF spec-
on top of the tissue for 10 s. The SF/EL scaffolds were then placed
trum show bands at 1640 cm�1 for amide I (C@O stretch-
over the wounded area. Two burn wounds per tissue were made
ing), 1517 cm�1 with a shoulder at 1532 cm�1 for amide II (N–H
to control wound size, and the healing was evaluated in two inde-
deformation) and 1238 cm�1 for amide III (C–N stretching, C@O
pendent assays. Skin equivalents without dressing and treated
bending vibration), indicating a random coil/Silk I conformation
with a commercial collagen dressing, Suprasorb C (Lohmann &
. SF molecules can structurally rearrange due to changes
Rauscher, Germany), were used as controls.
in the hydrogen bonding by methanol treatment acquiring a
Healing of these wounds was evaluated after 6 days by histolog-
b-sheet conformation. Genipin crosslinking is also able to induce
ical evaluation. Skin equivalents were fixed in 4% formaldehyde
b-sheet conformation of SF molecules. Comparing the SF spectra
solution at room temperature. Subsequently, paraffin-embedded
obtained after genipin crosslinking, it is clearly the transition from
tissues section of 4 lm thickness were obtained and stained with
random coil to b-sheet conformation confirmed by the shifting to
Haematoxylin and Eosin (H&E). All sections were observed under
lower wavenumbers of amide I (1620 cm�1) and amide II
a light inverted microscope (Olympus IX71).
(1514 cm�1) bands The shoulder observed at 1532 cm�1for amide II assigned to random coil, progressively disappears with
3. Results and discussion
the increase in genipin concentration. Moreover, a characteristicband of genipin at 1105 cm�1 (–COH) appeared in the spectra of
3.1. Biochemical and biophysical properties of SF/EL scaffolds
100SF0.1GE and 100SF0.5GE, confirming once again the reactionbetween genipin and SF. The FTIR results evidenced that genipin
The formation of covalent bonds on blended systems may pro-
crosslinking of SF is followed by protein conformational changes
duce stable and ordered materials with beneficial effect on their
already shown by other authors b shows the spec-
properties. To achieve such effect, genipin was used to crosslink
trum for elastin protein that was acquired in powder form using
SF/EL scaffolds. Different crosslinking conditions were tested
KBr pellets. 100EL spectrum shows characteristic protein bands
(). After 3 h of reaction a color change in the solutions is ob-
at 1651 (amide I), 1537 (amide II) and 1239 cm�1 (amide III), as-
served from light yellow to light blue, indicating the reaction be-
signed to random coil conformation . It can be seen that
tween both SF and elastin with genipin. It is described that
genipin induces in elastin structural changes into a more b-sheet
genipin reacts with amino acids or proteins to form dark blue
conformation. This was confirmed by the shifting to lower wave-
pigments associated with the oxygen-radical polymerization of
numbers of the amide I band. In addition, the appearance of a
genipin . After 6 h of reaction, the solutions became dark
new peak at 1104 cm�1 and 1113 cm�1, characteristic of genipin,
blue and the maximum crosslinking degree was reached.
confirms the crosslinking reaction. The intensity of this peak is
The exact mechanism behind the interaction of genipin with
directly proportional to the amount of genipin used for the cross-
both SF and elastin is yet to be fully described. The generally
linking. The results obtained after methanol treatment of the
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
Fig. 1. FTIR absorbance spectra (a) of pure silk fibroin (100SF) and (b) pure elastin(100EL) and crosslinked with genipin (100X0.1GE and 100X0.5GE, where X is SF orEL).
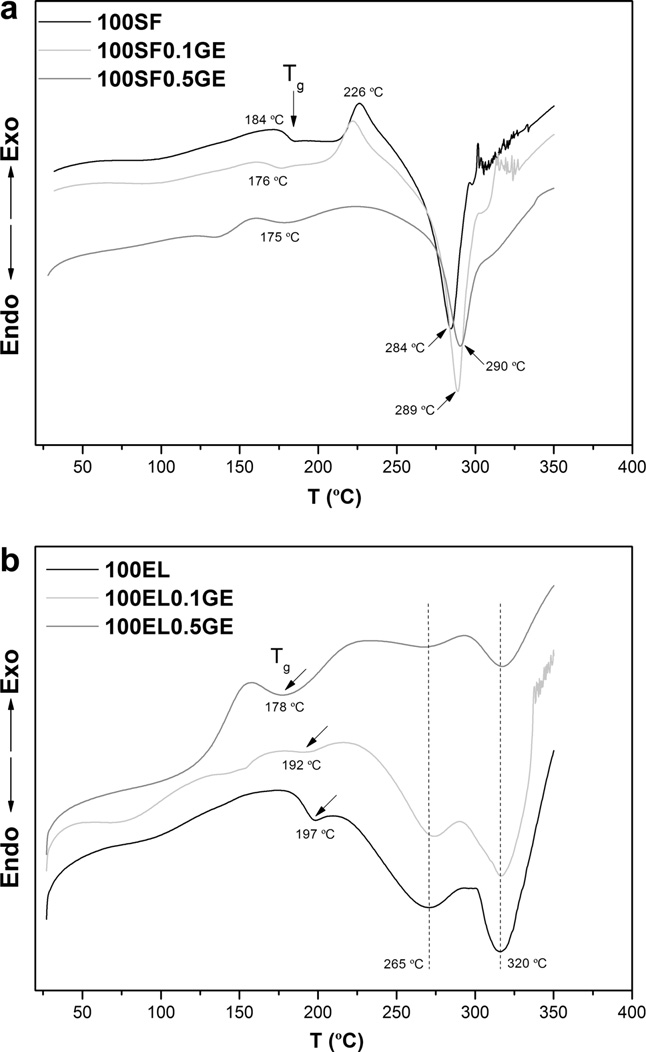
Fig. 2. DSC scans of (a) pure silk fibroin (100SF) and (b) pure elastin (100EL) andcrosslinked with genipin (100X0.1GE and 100X0.5GE, where X is SF or EL).
scaffolds (Data not shown) show no additional changes, for both
The thermal behavior of 100SF is typical of an amorphous SF
proteins, when compared with genipin crosslinked spectra.
with random coil conformation as previously shown by FTIR re-
FTIR spectra of blend systems show slightly changes of the
sults. Addition of genipin induces a small decrease in the Tg and
wavenumbers and on the areas of the bands due to mixing effects
an increase in the decomposition temperature. The increase in
of SF with elastin. The areas under the peaks for pure and blend
the thermal stability, given by the increase in Td, of 100SF scaffold
systems were calculated by integration, and the ratio AN–H (area
containing genipin is due to the increase in the extent of covalent
of N–H bending, amide II) to AC@O (area of C@O stretching, amide
crosslinks. This fact is the confirmation of the crosslinking reaction
I) (data not shown). It was shown that the addition of elastin
between genipin and SF. Furthermore, the exothermic peak at
decrease the ratio of AN–H/AC@O. Moreover, the area of C–O–C,
226 °C shifts to lower temperature (100SF0.1GE) and disappears
attributed to genipin, increases along with the ratio AN–H/AC@O
for the sample 100SF0.5GE. This result shows once again the
due to the carboxyl group from genipin. This fact is evidence of
change in the SF conformation from random coil to b-sheet after
the crosslinking reaction. The higher decrease in the ratio AN–H/
genipin crosslinking, and how this change is affected by the con-
AC@O obtained for the blend systems is the combined effect of addi-
centration of crosslinking agent.
tion of elastin and genipin crosslinking.
The DSC curve of elastin b) shows an endothermic shift at
The interaction between SF and elastin, crosslinked with geni-
197 °C assigned to the glass transition temperature of soluble
pin, was further investigated using thermal analysis (DSC). DSC
elastin peptides The thermogram is further characterized by
scans for SF and elastin are shown in and b respectively.
a weak and broad endothermic peak at 265 °C, related to the
The DSC curve for 100SF shows an endothermic shift at 184 °C that
decomposition of small aggregated structures and a more intense
corresponds to the glass transition temperature (Tg) of SF. This
endothermic peak at 320 °C related to a component decomposition
value is in the range of others previously reported for SF with a
at high temperature. Addition of genipin caused the decrease in the
random coil conformation . The exothermic peak at 226 °C
Tg and, although it was not observed, an increase in the decompo-
is related to the crystallization of amorphous SF chains caused by
sition temperature (320 °C); the weak peak at 265 °C progressively
the transition to b-sheet structure The DSC curve of
disappears with the addition of genipin. This fact indicates that the
SF is also characterized by an intense endothermic peak at 284 °C
small aggregates disappeared due to the crosslinking reaction be-
(Td) related to the decomposition of SF chains.
tween genipin and elastin. In the blend system (data not shown)
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
an increase in the decomposition temperature is observed,
the interaction between genipin and SF with conformational
suggesting once again the crosslinking effect. Nevertheless, the in-
changes that are patent of the scaffold morphology already con-
crease in Td is not dependent on blend composition because blends
firmed by FTIR and DSC results. In the blended system, it can be
with higher crosslinking degree will not have higher thermal
seen that the loose network obtained upon addition of elastin
becomes more closed and compact due to genipin crosslinking.
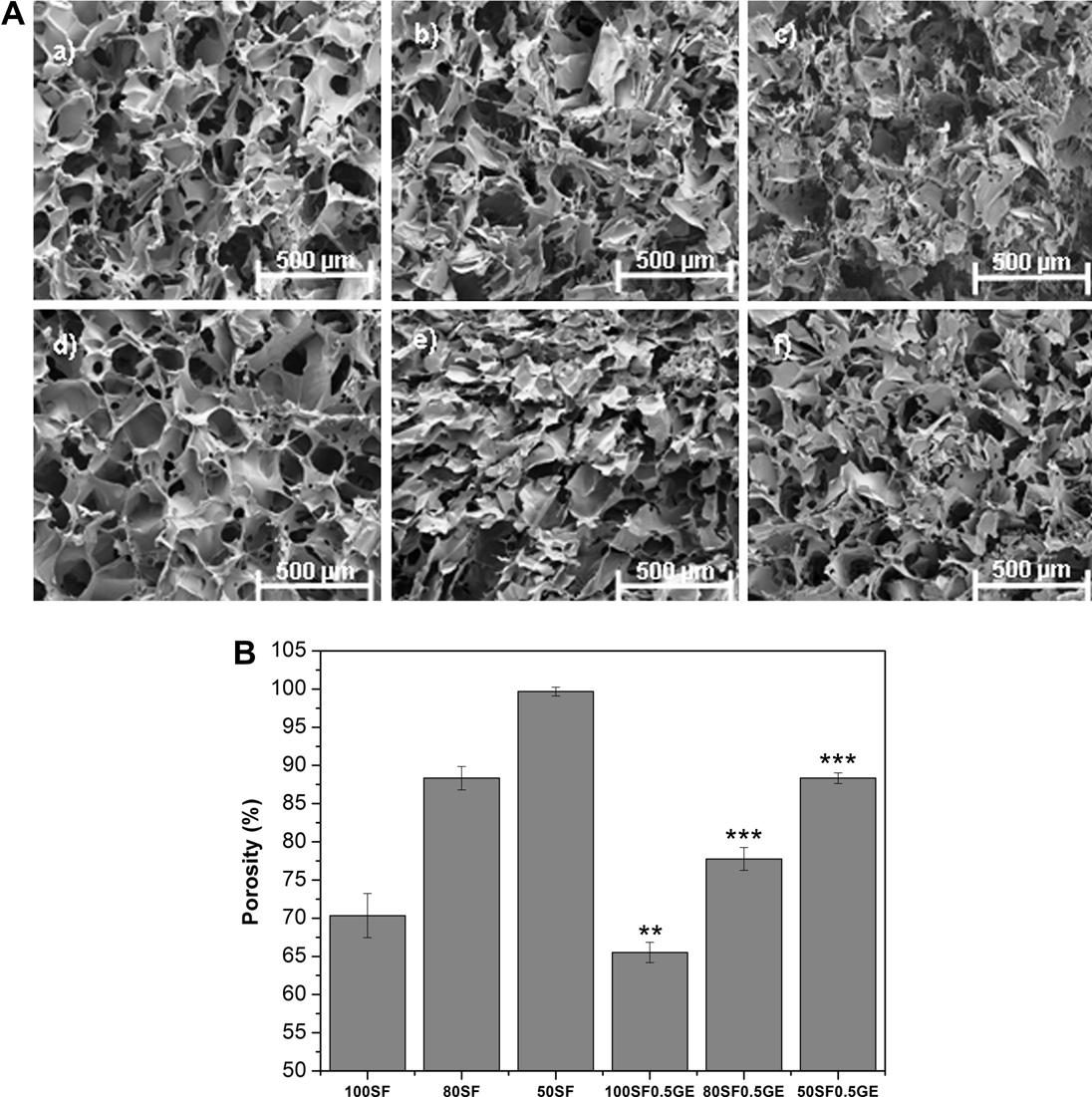
The 3-D morphology of the SF/EL scaffolds was analyzed by
The fibrils observed in 50SF scaffold disappeared after crosslinking,
SEM. The images presented are related to control and crosslinked
originating thicker walls (f).
Porosity measurement of scaffolds was done by the liquid dis-
(a) shows a disordered pore-like structure with a rough sur-
placement method, using hexane as a displacement liquid. Hexane
face. The pores are interconnected by a number of even smaller
was used because it permeates easily through the interconnected
pores. Addition of elastin creates a more open and loose structure
scaffold pores, causing negligible swelling or shrinkage. Porosity
with thinner walls (b and c). In the case of 50SF a fibr-
determination is important in tissue engineering as a highly porous
ilar structure can be observed. The presence of large pores in the
structure provides much surface area that promotes better cell
scaffolds facilitates cellular infiltration and growth within the
growth through the easer passage of nutrients to the growing cells.
3-D structure However, such a loose network will have
All the scaffolds, without genipin crosslinking, showed porosity
detrimental effects on mechanical, swelling and release properties.
ranging between 100 and 70%, as shown in B. Addition of
To overcome this, genipin crosslinking was performed and the
elastin increases the porosity of the scaffolds as can be seen by
results clearly evidenced that genipin changes the scaffold mor-
SEM analysis A). Crosslinking with genipin significantly
phology. 100SF0.5GE scaffold (shows a more ordered pore
(p < 0.001) decreases the porosity of the SF/EL scaffolds, and the
structure interconnected between sheets, characteristic of a
porosity increases accordingly to the elastin content in the scaffold.
b-sheet conformation In addition, the SEM images ob-
Water-binding of scaffolds is an important parameter of
tained after methanol treatment (data not shown) show the same
biomaterials properties. To study the swelling ratio in response
morphology observed with genipin crosslinking. This result shows
to external pH conditions, SF/EL scaffolds were immersed in PBS
Fig. 3. (A) SEM images of SF/EL scaffolds without genipin: (a) 100SF, (b) 80SF and (c) 50SF; after genipin crosslinking: (d) 100SF0.5GE, (e) 80SF0.5GE and (f) 50SF0.5GE. (B)Porosity percentage of SF/EL scaffolds. Each column represents the average ± SD (n = 3) (significant differences between non-crosslinked and crosslinked samples at ⁄⁄p < 0.01and ⁄⁄⁄p < 0.001).
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
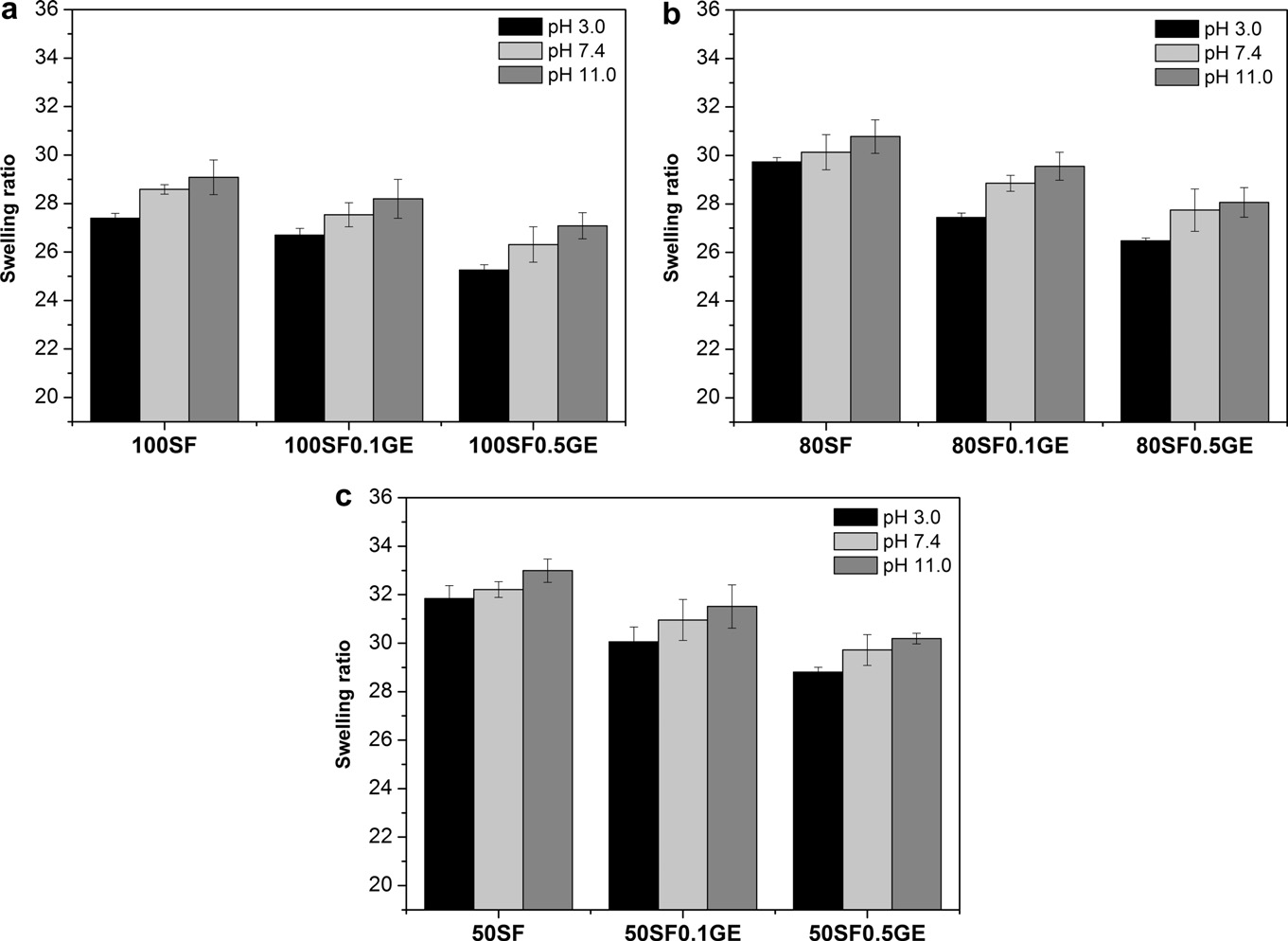
buffer solutions at pH 3, 7.4 and 11 for 24 h at 37 °C and the results
correlated with the scaffold compact structures formed after cross-
are presented in . The swelling ratio of SF/EL scaffolds was
lower in acidic conditions and became progressively higher at neu-tral and alkaline media. The lowest swelling ratio obtained at pH 3
3.2. In vitro and ex vivo biological degradation
might be attributed to the formation of hydrogen bonds betweenSF and elastin due to the presence of carboxylic acid groups
Degradation rate of matrices plays an essential role in the deter-
(–COOH) and hydroxyl groups (–OH). Increasing the pH, the car-
mination of the release of entrapped bioactive agents. The in vitro
boxylic acid groups became ionized (–COO�) and consequently,
degradation of SF/EL scaffolds was investigated by incubation in a
higher swelling ratios are observed due to a higher swelling force
isotonic, physiological pH solution (PBS, pH 7.4) and a protease rich
induced by the electrostatic repulsion between the ionized acid
medium (PPE and human exudate from chronic wounds) at 37 °C
for several days. At determined time points, samples were removed
The swelling ratio was found to be dependent on the composi-
and washed with distilled water, dried and weighed to determine
tion; 50SF scaffolds, with and without genipin, showed maximums
the extent of degradation using Eq. The results are presented in
swelling ratios ). SEM analysis indicates that 50SF
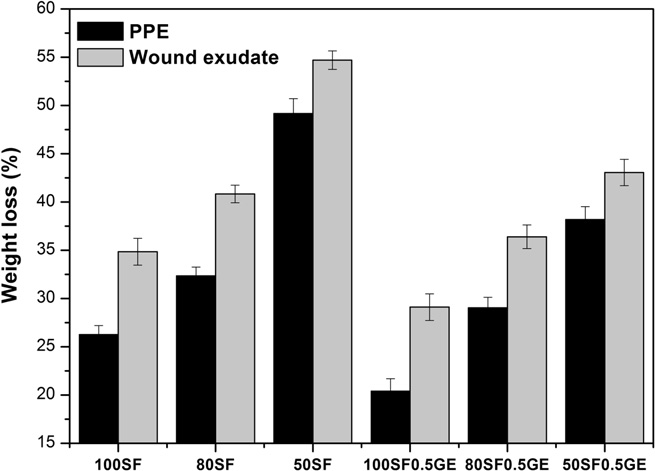
Scaffolds incubated with PBS solution showed almost no
samples presented larger pores with a loose network, still observed
degradation within 21 days. From the results it can be seen that
after crosslinking, which results in a higher hydrodynamic free
the degradation is dependent on scaffold composition. Higher
volume to accommodate more of the solvent molecules, thus
weight loss was obtained for samples containing higher amounts
increasing scaffold swelling .
of elastin. After 21 days of incubation, the weight loss obtained
Crosslinking with genipin also affects the swelling ratio of the
for 100SF, 80SF and 50SF in PPE solution was �26, 36 and 49%
scaffolds. Increasing genipin concentration leads to a decrease in
respectively. The low weight loss obtained for 100SF is related to
the swelling ratios. Generally, the swelling behavior of the scaf-
the crystallinity of fibroin due to the presence of b-sheet struc-
folds can be controlled by its composition and crosslinking degree.
tures. Therefore, the observed weight loss is probably due to the
In the SF/EL scaffolds, genipin crosslinking created stable struc-
degradation of the small hydrolytically peptide sequences that
tures that hinder the mobility and relaxation of the macromolecu-
remain after scaffold crystallization . Nevertheless, this effect
lar chains, lowering the swelling ratio due to water restrict
is minimized after genipin crosslinking that increases the b-sheet
mobility . This effect is more pronounced in 80SF and 50SF
content, creating a closed and compact scaffold network d).
scaffolds that attained higher crosslinking degrees when compared
This will diminish the diffusion of solution within the scaffold,
with 100SF. The decrease in the swelling ratio can also be
increasing the resistance to protease degradation. The higher
Fig. 4. The pH-dependent swelling ration of 100SF (a) 80SF, (b) and 50SF (c) scaffolds after 24 h of immersion in buffer solutions at 37 °C determined by Eq.
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
Fig. 5. In vitro degradation of SF/EL scaffolds incubated with 0.1 mg ml�1 of PPE andwound exudate (2.4 lg ml�1 of total protein content) at 37 °C for 21 days.
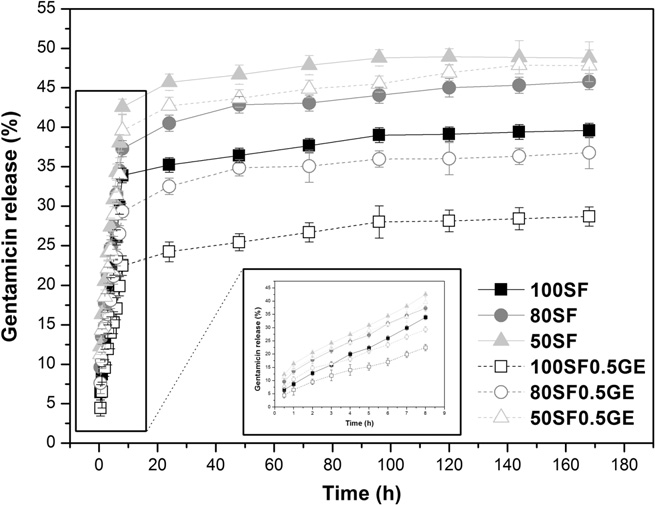
Fig. 6. Cumulative release of gentamicin from SF/EL scaffolds incubated with0.1 mg ml�1 of PPE at 37 °C for 21 days.
weight loss obtained with scaffolds containing elastin is becauseelastin is a substrate for elastase. In the human body, elastin, one
of the major components of connective tissues, is degraded by
Model compound release kinetic data obtained from fitting the experimental release
human leukocyte elastase (HLE) . In this way, SF/EL scaf-
data to Eq.
folds might be used as elastase-specific wound dressings for
Kinetic parameters
chronic wounds. Moreover, it has already been demonstrated that
elastin-based dressings promote a better wound healing either byan improvement of fibroblasts adhesion and proliferation
or by the reduction of wound contraction
The loose network observed for scaffolds containing elastin
(c) is also responsible for the higher weight loss obtained
due to the increase in the surface area. As observed before, the gen-
ipin crosslinking decreases the weight loss observed. The creationof a more compact structure between SF and elastin hinders scaf-folds degradation. These results show that genipin crosslinking
turn cause the release of higher amounts of compounds. Genipin
was effective in the control of degradation.
crosslinking induces slower release rates This is attributed
The results obtained with wound exudate show the same deg-
to the fact that genipin crosslinking enhances the decrease of pore
radation pattern but with higher values. The exudate solution used
size. In this way, the diffusion of the compounds through the
scaffold pores is more difficult and lower release is attained.
(2.4 lg ml�1 of total protein content). Nevertheless, the wound
To determine the release mechanism present in the SF/EL scaf-
exudate is a mixture of several proteases, including HLE, that act
folds, the experimental data were fitted to the semi-empirical
synergistically, increasing the hydrolysis.
power law model given by the Ritger–Peppas equation(Eq. ). This equation is further modified to determine the diffu-
3.3. In vitro release
sional exponent, n (Eq. that depends on the release mechanismand the geometry of the matrix . There are three different
The effect of scaffold composition and genipin crosslinking on
mechanisms that can be concluded from the n value. Therefore,
the release of model compounds was investigated. The release
the release, from a cylindrical geometry like the sponges devel-
behavior of gentamicin from SF/EL scaffolds in PPE solution is
oped, is purely Fickian diffusion when n = 0.45; for 0.45 < n > 0.89
shown in The release of this compound was monitored in
anomalous (non-Fickian) transport is present and, for n = 0.89 the
PBS solution (data not shown) and the release observed was low.
release is dominated by Case II transport (matrix relaxation or
Gentamicin has been used topically in the treatment of superficial
infections of the skin since it is effective against many aerobic
The results in , for control samples without crosslinking,
Gram-negative and some aerobic Gram-positive bacteria. In this
showed that the release of gentamicin is dominated by anomalous
way, the antibacterial properties of SF/EL scaffolds will also be
transport because n values are above and below 0.45. In the blends,
exploited. The release profile shown can be divided into three
increasing elastin content, the values for release rate, k, became
parts: an initial burst release in the initial 24 h, due to the release
progressively higher. This indicates that the addition of elastin im-
of the compound bound to the surface of the scaffold; a continuous
proves the release of drugs from the scaffolds probably due to the
phase release from 24 to 72 h; and a stagnant phase release for the
increase in swelling ratio and degradation rate, for higher elastin
remaining period of time. Furthermore, it was observed that higher
content as previously discussed. On the other hand, the decrease
release was obtained for scaffolds containing higher amounts of
in the values for diffusional exponent, n, closest to 0.45, suggests
elastin. The release of a compound from a matrix is governed by
that the addition of elastin also improves the diffusion of drugs
several factors such as nature and size of the compound, degree
from the scaffolds.
and density of crosslinking and pore size among others. From the
Addition of genipin gradually changes the mechanism from
SEM results discussed previously, it was concluded that higher
anomalous transport to Fickian diffusion, especially for the sample
elastin content leads to scaffolds with higher pore size which in
80SF0.5GE (n = 0.451). Furthermore, the crosslinking effect on the
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
Fig. 7. Viability of human normal skin fibroblasts after 24 h, 48 h and 72 h ofcontact with conditioned medium (culture medium where scaffolds were incu-bated). Only the positive control (treatment with Triton detergent) revealed
Fig. 8. Number of human normal skin fibroblasts cells, determined in terms of DNA
diminished cell viability. (⁄⁄⁄ = significantly different from all the other tested
content, after 3 and 5 days of direct contact (significantly different from cells
conditions, p < 0.001).
control after 3 days of incubation at ⁄p < 0.05, ⁄⁄p < 0.01 and ⁄⁄⁄p < 0.001; signifi-cantly different from cells control after 5 days of incubation at #p < 0.05,##p < 0.01and ###p < 0.001).
scaffold morphology (compact and closed structure with smallerpores) also influences the release rate. It is observed that therelease rate becomes slower (lower k values) for higher amounts
production of low levels of the inflammatory mediator TNFa by
of elastin, due to the higher crosslinking degree obtained for these
these cells after 48 h of incubation when compared to PMA
samples as explained before. The release results clearly support the
(phorbol 12-myristate-13 acetate), which is known to differentiate
notion that the release from SF/EL scaffolds is affected by its com-
THP-1 cells into macrophage-like cells and mimic the intrinsic acti-
position and that genipin crosslinking can be used to modulate the
vation and differentiation signals that macrophages encounter dur-
release mechanism and rate of the compounds.
ing the foreign body reaction This additional observationfurther supports the notion that SF/EL scaffolds are non-immuno-genic and represent a safe alternative biomaterial for the treatment
3.4. Cytocompatibility SF/EL scaffolds
Biocompatibility of SF/EL scaffolds, with and without genipin
crosslinking, was assessed in human skin fibroblasts in in vitro cul-
3.5. Wound healing
tures. The results of the indirect contact study after fibroblast incu-bation with material extracts showed no cytotoxicity caused by
To determine the effect of SF/EL scaffolds on the wound healing,
medium conditioned by the scaffolds regardless of the incubation
materials were applied on the top of the wound immediately after
time. represents the viability results for cells in contact with
causing the burn. Histological evaluation of the healing pro-
undiluted conditioned media. In all cases, the metabolic activity of
cess after a period of 6 days revealed that SF/EL scaffolds induced
cells in contact with the conditioned media was statistically similar
fibroblasts and keratinocytes proliferation and migration to the
or higher than the one obtained with negative control (complete
wound site, especially for wounds treated with scaffolds contain-
culture medium). This result constitutes a preliminary study of
ing elastin (and b). The healing improvement obtained with
the biocompatibility of SF/EL sponges, indicating that these mate-
SF/EL scaffolds is similar to the commercial collagen dressing,
rials are not cytotoxic.
Suprasorb C, used in several types of wounds including burn
Direct contact study was performed by seeding the cells on the
scaffolds to evaluate the effect of SF/EL scaffolds on fibroblasts pro-
Microscopic observations of the wounds indicated that the con-
liferation. The results presented in showed a time-dependent
trol sample is characterized by the absence of epithelium
increase in the number of cells that may suggest an increase in cell
and the dermis is covered with crust from burning. After 6 days of
proliferation. Human skin fibroblasts continued to increase in
healing, the crust had disappeared from the control sample
number over the period examined, indicating that the scaffolds
(and from samples treated with different materials. In
are able to support fibroblasts proliferation without producing
addition, wounds treated with dressings (collagen and SF/EL scaf-
toxic effects. It can also be observed that the presence of elastin
folds) induced keratinocyte and fibroblast migration from the
on the scaffolds favors cell proliferation. Especially after 5 days of
margins to the wound ground, which should result in a faster re-
incubation. This fact is explained by the increase of hydrophilicity
epithelialization and wound closure. Partial-thickness burn
introduced by the presence of elastin that enhances cell adhesion
wounds heal almost entirely by epithelialization from the skin
and subsequent activity. For scaffolds crosslinked with genipin a
periphery to the wound core which was also observed in
decrease in the number of cells is observed when compared to
this study. For this reason, histological examination was done with
non-crosslinked scaffolds, which might be related with the de-
sections obtained in the center of the wound, so the results pre-
crease in the porosity caused by the genipin crosslinking that
sented and the differences obtained are related with the healing
inhibits cell infiltration.
improvement by SF/EL scaffolds and not by natural artifacts. From
Preliminary studies on the immunogenicity of SF/EL scaffolds
the histological results obtained it is also visible that wounds trea-
were performed in vitro by measuring TNFa production by
ted with scaffolds containing higher amounts of elastin (50SF;
THP-1 human macrophages exposed to these materials (data not
are almost completely closed and covered with new epithe-
shown). The results suggested that SF/EL scaffolds induce
lium, which was not the case in controls. The results indicated that
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
Fig. 9. Histological analysis of burn wound tissues stained with H&E: (a) control wound (no dressing) immediately after burning; (b) control wound (no dressing) after 6 daysof healing; (c) wound treated with commercial collagen dressing, Suprasorb C, after 6 days of healing; (d) wound treated with 100SF scaffold after 6 days of healing; (e)wound treated with 80SF scaffold after 6 days of healing; (f) wound treated with 50SF scaffold after 6 days of healing. Bars = 100 lm.
wound size reduction was significantly greater in the order of
containing higher amount of elastin accelerates re-epithelializa-
50SF > 80SF > 100SF = Suprasorb C > No dressing.
tion and wound closure. The results presented are important in
The characterization results presented earlier indicated that
the design and application of tailor-made biomaterials for wound
scaffolds containing higher amounts of elastin become more swel-
lable, flexible and elastic. These characteristics suggest that theattachment of the cells within the wound to the dressing is im-
proved, resulting in a faster re-epithelialization.
Elastin is the major constituent of skin elastic fibers and is ben-
We would like to acknowledge FCT – Portuguese Foundation for
eficial for dermal regeneration Several studies have explored
Science and Technology for the scholarship conceded to Andreia
the application of elastin containing materials for wound healing,
Vasconcelos; European FP6 project Lidwine, contract no. NMP2-
such as scaffolds of collagen and solubilized elastin or dermal
CT-2006-026741 and PEst-C/BIA/UI4050/2011.
substitutes coated with elastin Silk fibroin based-biomate-rials have also been used in this field with promising results; nevertheless, the present study exploits for the first time
Appendix A. Figures with essential colour discrimination
the combination of silk fibroin and elastin for the production ofwound dressing scaffolds.
Certain figures in this article, particularly Fig. 9, are difficult to
interpret in black and white. The full colour images can be foundin the on-line version, at
Novel SF/EL scaffolds crosslinked with genipin were success-
fully obtained. The genipin crosslinking results in the conforma-tional transition of SF chains from random coil to b-sheet
[1] Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Percoraro RE, Rodeheaver G,
conformation. The SF/EL scaffolds presented different pore sizes
et al. Definitions and guidelines for assessment of wounds and evaluation of
and distinct morphologies which are related with the elastin ratio
healing. Wound Repair Regen 1994;2:165–70.
[2] Robson MC. Wound infection: a failure of wound healing caused by an
and genipin crosslinking. The biochemical and biophysical proper-
imbalance of bacteria. Clin N Am 1997;77:637–50.
ties of the scaffolds such as higher thermal stability, pH-swelling
[3] Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of
dependence and reduced biological degradation and drug release
chronic cutaneous wounds. AM J SUR 1998;176:26S–38S.
rates were obtained after genipin crosslinking with a concentration
of 0.5%. A very important technical approach of this study was the
[5] Park JE, Barbul A. Understanding the role of imune regulation in wound
validation of SF/EL scaffolds using human wound exudates. Degra-
healing. AM J SUR 2004;187:S11–6.
dation was evaluated using wound exudates, and it was observed
[6] Wharram SE, Zhang X, Kaplan DL, McCarthy SP. Electrospun silk material
systems for wound healing. Macromol Biosci 2010;10:246–57.
that genipin crosslinking reduces susceptibility to degradation in
[7] Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. Biofunctionalized
this particular context. Moreover, SF/EL scaffolds showed no cyto-
electrospun silk mats as a topical bioactive dressing for accelerated wound
toxicity and are able to support cell proliferation in vitro in human
healing. Acta Biomater 2009;5:2570–8.
[8] Sugihara A, Sugiura K, Morita H, Ninagawa T, Tubouchi K, Tobe R, et al.
skin fibroblasts. Dermal burn healing experiments using human
Promotive effects of a silk film on epidermal recovery from full-thickness skin
skin equivalents have shown that the application of SF/EL scaffolds
wounds. Proc Soc Exp Biol Med 2000;225:58–64.
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
[9] Vasconcelos A, Pêgo AP, Henriques L, Lamghari M, Cavaco-Paulo A. Protein
[39] Vasconcelos A, Freddi G, Cavaco-Paulo A. Biodegradable materials based on
matrices for improved wound healing: elastase inhibition by a synthetic
silk fibroin and keratin. Biomacromolecules 2009;10:1019.
peptide model. Biomacromolecules 2010;11:2213–20.
[40] Friedman M. Applications of the ninhydrin reaction for analysis of amino acids,
[10] Roh D-H, Kang S-Y, Kim J-Y, Kwon Y-B, Young Kweon H, Lee K-G, et al. Wound
peptides, and proteins to agricultural and biomedical sciences. J Agric Food
healing effect of silk fibroin/alginate-blended sponge in full thickness skin
defect of rat. J Mater Sci Mater Med 2006;17:547–52.
[41] Yuan Y, Chesnutt BM, Utturkar G, Haggard WO, Yang Y, Ong JL, et al. The effect
[11] Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and
of cross-linking of chitosan microspheres with genipin on protein release.
aging. Microsc Res Tech 1997;38:428–35.
Carbohydr Polym 2007;68:561–7.
[12] Faury G. Function–structure relationship of elastic arteries in evolution: from
[42] Silva SS, Motta A, Rodrigues M, Pinheiro AFM, Gomes ME, Mano JF, et al. Novel
microfibrils to elastin and elastic fibres. Pathol Biol 2001;49:310–25.
genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering
[13] Martyn C, Greenwald S. A hypothesis about a mechanism for the programming
of blood pressure and vascular disease in early life. Clin Exp Pharmacol Physiol
[43] Nazarov R, Jin H-J, Kaplan DL. Porous 3-D scaffolds from regenerated silk
fibroin. Biomacromolecules 2004;5:718–26.
[14] Mithieux SM, Rasko JEJ, Weiss ASAS. Synthetic elastin hydrogels derived from
massive elastic assemblies of self-organized human protein monomers.
spectrophotometric methods for the analysis of tobramycin and other
aminoglycosides. J Pharm Sci 1990;79:428–31.
[15] Leach JB, Wolinsky JB, Stone PJ, Wong JY. Crosslinked [alpha]-elastin
[45] Ritger PL, Peppas NA. A simple equation for description of solute release II.
biomaterials: towards a processable elastin mimetic scaffold. Acta Biomater
Fickian and anomalous release from swellable devices. J Controlled Release
[16] Annabi N, Mithieux SM, Weiss AS, Dehghani F. The fabrication of elastin-based
[46] Mi FL, Sung HW, Shyu SS. Synthesis and characterization of a novel chitosan-
hydrogels using high pressure CO2. Biomaterials 2009;30:1–7.
based network prepared using naturally occurring crosslinker. J Polym Sci, Part
[17] Urry DW, Chi-Hao L, Parker TM, Gowda DC, Prasad KU, Reid MC, et al.
A: Polym Chem 2000;38:2804–14.
Temperature of polypeptide inverse temperature transition depends on mean
[47] Sung HW, Chang Y, Liang IL, Chang WH, Chen YC. Fixation of biological tissues
residue hydrophobicity. J Am Chem Soc 1991;113:4346–8.
with a naturally occurring crosslinking agent: fixation rate and effects of pH,
[18] Urry DW. Free energy transduction in polypeptides and proteins based on
temperature, and initial fixative concentration. J Biomed Mater Res
inverse temperature transitions. Prog Biophys Mol Biol 1992;57:23–57.
[19] Urry DW. Physical chemistry of biological free energy transduction as
[48] Liang HC, Chang WH, Liang HF, Lee MH, Sung HW. Crosslinking structures of
gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J
Appl Polym Sci 2004;91:4017–26.
[20] Vieth S, Bellingham CM, Keeley FW, Hodge SM, Rousseau D. Microstructural
[49] Silva SS, Maniglio D, Motta A, Mano JF, Reis RL, Migliaresi C. Genipin-modified
and tensile properties of elastin-based polypeptides crosslinked with genipin
silk-fibroin nanometric nets. Macromol Biosci 2008;8:766–74.
and pyrroloquinoline quinone. Biopolymers 2007;85:199–206.
[50] Chen X, Knight DP, Shao Z, Vollrath F. Regenerated Bombyx silk solutions
[21] McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of
studied with rheometry and FTIR. Polymer 2001;42:9969–74.
enzymatically cross-linked elastin-like polypeptide gels for cartilaginous
[51] Chen X, Shao Z, Marinkovic NS, Miller LM, Zhou P, Chance MR. Conformation
tissue repair. Tissue Eng 2005;11:1768–79.
transition kinetics of regenerated Bombyx mori silk fibroin membrane
[22] Nagapudi K, Brinkman WT, Leisen J, Thomas BS, Wright ER, Haller C, et al.
monitored by time-resolved FTIR spectroscopy. Biophys Chem 2001;89:25–34.
Protein-based thermoplastic elastomers. Macromolecules 2004;38:345–54.
[52] Hu X, Kaplan D, Cebe P. Determining beta-sheet crystallinity in fibrous
[23] Nagapudi K, Brinkman WT, Thomas BS, Park JO, Srinivasarao M, Wright E, et al.
proteins by thermal analysis and infrared spectroscopy. Macromolecules
Viscoelastic and mechanical behavior of recombinant protein elastomers.
[53] Chen X, Shao Z, Knight DP, Vollrath F. Conformation transition kinetics of
[24] Mithieux SM, Tu Y, Korkmaz E, Braet F, Weiss AS. In situ polymerization of
Bombyx mori silk protein. Protein: Struct, Funct, Bioinformatics 2007;68:223–
[54] Wise SG, Mithieux SM, Weiss AS, Alexander M. Engineered tropoelastin and
[25] Lee J, Macosko CW, Urry DW. Mechanical properties of cross-linked synthetic
elastin-based biomaterials. Advances in Protein Chemistry and Structural
elastomeric polypentapeptides. Macromolecules 2001;34:5968–74.
Biology: Academic Press; 2009. p. 1–24.
[26] Sung HW, Huang DM, Chang WH, Huang RN, Hsu JC. Evaluation of gelatin
[55] Wise SG, Weiss AS. Tropoelastin. Int J Biochem Cell Biol 2009;41:494–7.
hydrogel crosslinked with various crosslinking agents as bioadhesives: in vitro
[56] Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials derived from silk-
study. J Biomed Mater Res 1999;46:520–30.
tropoelastin protein systems. Biomaterials 2010;31:8121–31.
[27] Chang Y, Tsai C-C, Liang H-C, Sung H-W. In vivo evaluation of cellular and
[57] Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone
acellular bovine pericardia fixed with a naturally occurring crosslinking agent
morphogenetic protein-2 decorated silk fibroin films induce osteogenic
(genipin). Biomaterials 2002;23:2447–57.
differentiation of human bone marrow stromal cells. J Biomed Mater Res A
[28] Fujikawa S, Nakamura S, Koga K. Genipin, a new type of protein crosslinking
reagent from gardenia fruits. Agric Biol Chem 1988;52:869–70.
[58] Lu Q, Zhang X, Hu X, Kaplan DL. Green process to prepare silk fibroin/gelatin
[29] Sung HW, Huang RN, Huang LLH, Tsai CC, Chiu CT. Feasibility study of a natural
biomaterial scaffolds. Macromol Biosci 2010;10:289–98.
crosslinking reagent for biological tissue fixation. J Biomed Mater Res
[59] Samouillan V, André C, Dandurand J, Lacabanne C. Effect of water on the
molecular mobility of elastin. Biomacromolecules 2004;5:958–64.
[30] Sung HW, Liang IL, Chen CN, Huang RN, Liang HF. Stability of a biological tissue
[60] Annabi N, Mithieux SM, Boughton EA, Ruys AJ, Weiss AS, Dehghani F. Synthesis
fixed with a naturally occurring crosslinking agent (genipin). J Biomed Mater
of highly porous crosslinked elastin hydrogels and their interaction with
fibroblasts in vitro. Biomaterials 2009;30:4550–7.
[31] Butler MF, Ng YF, Pudney PDA. Mechanism and kinetics of the crosslinking
[61] She Z, Zhang B, Jin C, Feng Q, Xu Y. Preparation andin vitro degradation of
reaction between biopolymers containing primary amine groups and genipin. J
porous three-dimensional silk fibroin/chitosan scaffold. Polym Degrad Stab
Polym Sci, Part A: Polym Chem 2003;41:3941–53.
[32] Chen H, Ouyang W, Lawuyi B, Martoni C, Prakash S. Reaction of chitosan with
[62] Rokhade AP, Patil SA, Aminabhavi TM. Synthesis and characterization of semi-
genipin and its fluorogenic attributes for potential microcapsule membrane
interpenetrating polymer network microspheres of acrylamide grafted
characterization. J Biomed Mater Res A 2005;75A:917–27.
dextran and chitosan for controlled release of acyclovir. Carbohydr Polym
[33] Chang Y, Tsai C-C, Liang H-C, Sung H-W. Reconstruction of the right ventricular
outflow tract with a bovine jugular vein graft fixed with a naturally occurring
[63] Mandal BB, Kapoor S,
crosslinking agent (genipin) in a canine model. J Thorac Cardiovasc Surg
interpenetrating network hydrogels for controlled drug release. Biomaterials
[34] Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun
[64] Bajpai AK, Giri A. Water sorption behaviour of highly swelling (carboxy
methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate
as agrochemical. Carbohydr Polym 2003;53:271–9.
[35] Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes
[65] Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, et al. Stabilization of enzymes
I, et al. Electrospinning of collagen and elastin for tissue engineering
in silk films. Biomacromolecules 2009;10:1032–42.
applications. Biomaterials 2006;27:724–34.
[66] Havemann K, Gramse M. Physiology and pathology of neutral proteinases of
[36] Bonzon N, Carrat X, Deminière C, Daculsi G, Lefebvre F, Rabaud M. New
human granurocytes. Adv Exp Med Biol 1984;164:1–20.
artificial connective matrix made of fibrin monomers, elastin peptides and
[67] Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-
type I + III collagens: structural study, biocompatibility and use as tympanic
bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative
membranes in rabbit. Biomaterials 1995;16:881–5.
mechanism by which neutrophils focus and preserve catalytic activity of
[37] San-Galli F, Deminière G, Guérin J, Rabaud M. Use of a biodegradable elastin–
serine proteinases. J Cell Biol 1995;131:775–89.
[68] Siedle B, Gustavsson L, Johansson S, Murillo R, Castro V, Bohlin L, et al. The
effect of sesquiterpene lactones on the release of human neutrophil elastase.
[38] Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI. Co-electrospun
Biochem Pharmacol 2003;65:897–903.
poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering
[69] Lamme EN, de Vries HJ, van Veen H, Gabbiani G, Westerhof W, Middelkoop E.
scaffolds. J Biomed Mater Res A 2006;79A:963–73.
Extracellular matrix characterization during healing of full-thickness wounds
A. Vasconcelos et al. / Acta Biomaterialia 8 (2012) 3049–3060
treated with a collagen/elastin dermal substitute shows improved skin
[74] Abramo AC, Viola JC. Heterologous collagen matrix sponge: histologic and
regeneration in pigs. J Histochem Cytochem 1996;44:1311–22.
clinical response to its implantation in third-degree burn injuries. Br J Plast
[70] Lamme EN, van Leeuwen RTJ, Jonker A, van Marle J, Middelkoop E. Living skin
substitutes: survival and function of fibroblasts seeded in a dermal substitute
[75] Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in
in experimental wounds. J Invest Dermatol 1998;111:989–95.
acute and chronic wounds. Wound Repair Regen 1996;4:411–20.
[71] Ferrero C, Massuelle D, Doelker E. Towards elucidation of the drug release
[76] Daamen WF, Veerkamp JH, van Hest JCM, van Kuppevelt TH. Elastin as a
mechanism from compressed hydrophilic matrices made of cellulose ethers. II.
biomaterial for tissue engineering. Biomaterials 2007;28:4378–98.
Evaluation of a possible swelling-controlled drug release mechanism using
[77] Daamen WF, Nillesen STM, Wismans RG, Reinhardt DP, Hafmans T, Veerkamp
dimensionless analysis. J Controlled Release 2010;141:223–33.
JH, et al. A biomaterial composed of collagen and solubilized elastin enhances
[72] Korsmeyer RW, Peppas NA. Effect of the morphology of hydrophilic polymeric
angiogenesis and elastic fiber formation without calcification. Tissue Eng Part
matrices on the diffusion and release of water soluble drugs. J Membr Sci
A 2008;14:349–60.
[78] Ryssel H, Gazyakan E, Germann G, Öhlbauer M. The use of MatriDermÒ in early
[73] Thomsen P, Gretzer C. Macrophage interactions with modified material
excision and simultaneous autologous skin grafting in burns – a pilot study.
surfaces. Curr Opin Solid State Mater Sci 2001;5:163–76.
Source: http://seidentraum.eu/pdf/studie_wundheilung.pdf
4.4 Welche Krankheitsstadien gibt es? Stadium 1: Die Krankheit entwickelt sich aus einem normalen Leistungsniveau. Stadium 2: In der Folge nimmt die/der Betroffene leichte Störungen wahr. Die Merkfähigkeit und das Gedächtnis sind beeinträchtigt. Namen und Termine werden vergessen. Bei manchen Situationen fehlt die Erinnerung und öfters werden Dinge verlegt.
Univ. Sci. 2014, Vol. 19 (1): 11-29 Freely available on line Técnicas analíticas contemporáneas para la identificación de residuos de sulfonamidas, quinolonas y cloranfenicol Y. Verónica Talero-Pérez scar Julio Medina 1, Wilson Rozo-Núñez2 Contemporary analytical techniques to identify residues of sulfonamides,








