Oval oklahoma vapors advocacy league

Lessons to Be Learned from
America's War on Vaping
Gregory Conley, J.D., M.BA.
President – American Vaping Association
(609) 947 – 8059 / [email protected]
Twitter: @GregTHR / @AVABoard

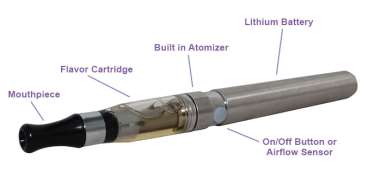
• Smoker for 8 years
• Had previously tried the nicotine gum, patch,
lozenge, cold turkey, & e-cigs
• August 2010 -- bought an "open" vapor product
and finally quit successfully
• 2009 – 2011
• FDA tries to enact a de facto ban on vapor
• Numerous states try and fail to pass bills
banning sales to adults
• 2011 – 2014
• Served as volunteer legislative director for
U.S.' largest consumer advocacy group
• 2014 – Formed the American Vaping Association
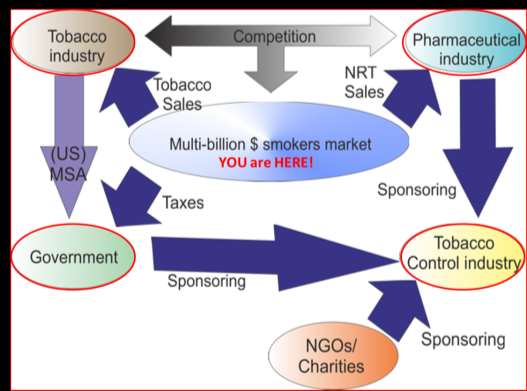
Disruptive Technology
• The Tobacco Companies -
obvious role, sell their product, make
Valorem tax
money, protect themselves from
on the sale of
each pack of
• The Government - biggest
player in the tobacco market. (2000
received around $40 billion annually
from tobacco, against less than $9
Threatens entire established Tobacco Industry
billion in profits received by the
tobacco industry itself)
• Pharmaceutical companies
– profit from repeat sales of largely ineffective smoking cessation products. (NRT, Zyban, Chantix)
• Tobacco Control – works
closely with pharma and gov. selling policy advice to gov., agitating for taxes, bans which benefit both, (includes NGO's, Charities, and many scientists in universities and in government funded knowledge institutes worldwide.)
• Money
• Ideology
• Fear of the unknown
• Prestige
Public Is Getting Mixed Messages About Vaping Risks
• The same 2013 survey found
that among the general public, just 51% believe vaping to be less hazardous than smoking.
• 2015 survey found situation is
worsening -- 44% of general public believe e-cigs are safer
• The U.S.' largest health orgs
should be trying to correct this
misconception, but instead
many are actively endeavoring
to
Tan ASL, Bigman CA. E-cigarette awareness and
mislead adult smokers
perceived harmfulness: prevalence and associations with smoking-cessation outcomes. Am J Prev Med
U.S. Minor Bans
• District of Columbia • Maine • Pennsylvania • Michigan
• Most do not define us as ‘tobacco'
• But states like Rhode Island, Arkansas, etc. are
setting up similar regulatory systems
Taxation
Child-Resistant Packaging
State Legislation: Vapers & Retailers React
Federal Regulation – The Tobacco
Control Act (TCA)
• Signed into law in 2009
• 10 year effort launched by Philip Morris (PM) ("Marlboro Protection
• Result of agreement between Campaign for Tobacco Free Kids & PM
• Gives FDA explicit authority to regulate the content, marketing, and sale of
• (1) cigarettes; (2) cigarette tobacco; (3) RYO; and (4) smokeless
• TCA gives FDA additional power to regulate ‘tobacco products,' defined as
"any product made or derived from tobacco that is intended for human consumption, including any component, part, or accessory of a tobacco product."
"Marlboro Protection Act" Explained
• Nickname given to the bill by Sen. Mike
• Under the TCA, established brands and
products could continue to be marketed without any significant new restrictions, but those looking to compete with new products have to comply with…
• Expensive & burdensome to
requirements to bring products to the market
• Two relevant application types:
• Substantial equivalence (SE) • Premarket review of new tobacco
products (PMTAs)
Substantial Equivalence (SE)
• Virtually ANY change requires an SE application
• Must show that the new / modified product does not
raise different questions of public health than raised by a "predicate product."
• February 15, 2007 & the "predicate product"
• Any product that was commercially available ON this
date can qualify as a predicate product when applying for SE
• Arbitrary date – means nothing, but a huge impact for
Premarket Review of New
Tobacco Products (PMTAs)
• If your product change or new product does not meet SE requirements, you
must file a PMTA.
• Population-level health standard
• Product must be "appropriate for the protection of the public health" of
the population as a whole – both users and nonusers.
• Extremely complex and burdensome
• Intentionally so – designed to keep new products off the market
• With the exception of allowing minor changes to Swedish Match's General
Snus products, FDA has yet to accept a single PMTA for the products it
currently regulates
• 4 have been rejected • 2 have been refused • 1 withdrawn by the manufacturer
The Deeming Regulation
• TCA gives CTP the authority to propose new regulations
• IF "such regulation would be appropriate for the protection of public
• BUT "public health" must be measured at the population level
• April 25, 2011
• FDA announces in Stakeholder Letter that it intends to issue a
regulation "deeming" e-cigarettes to be "tobacco products" under the TCA
• April 24, 2014
• Proposed deeming regulation released
The Deeming Regulation – In Short
The Deeming Regulation -- Detailed
• FDA proposes to treat vapor products the same as tobacco
• Some aspects are perfectly reasonable
• Ban on sales to minors • Ban on sales in vending machines • Adulterated and misbranded product provisions
• Others would decimate most companies in these industries
• Two BIG Problems for vapor
• (1) Inability to file Substantial Equivalence (SE) applications
• (2) Costs associated with Premarket Tobacco Applications
SE Applications – The Problem
• E-cigarettes were barely available in 2007
• Those products that did exist are technologically inferior
• FDA recognizes this:
• "*F+or some products, there may not be predicate products that
were on the markets as of February 15, 2007, to which to claim [SE]. This may be particularly true for e-cigarettes and similar novel products."
• FDA's solution: "For this reason, we are proposing that these
manufacturers who cannot use the SE pathway submit PMTAs to FDA no later than 24 months following the effective date of the final rule."
• For many companies, this is merely a delay in their execution
PMTAs – The Problem
• A PMTA will be required for every single covered vaping product on the market
today and in the future
• Bare minimum: $1 - $1.5 million per product
• But evidence is pointing to true cost being $5-$20 million
• Many e-liquid companies have hundreds or thousands of SKUs
• FDA estimate: 5,000 man hours per application
• Including 4,800 hours of clinical and nonclinical studies
• Even for those who can afford to file a PMTA, their fate is uncertain
• FDA is sending strong signals that it believes any non-tobacco, non-menthol
product must be kept off the market.
• FDA's position: Congress gave us the statute and we must follow it
• Position of much of the industry: Several examples of FDA exercising regulatory
flexibility in tobacco and medicine regulations.
The Grim Future Post-Deeming
• FDA knows this regulation will ban 99.9% of vapor products
• Agency only expects 25 PMTA applications per year
• FDA: "Products that do not have sufficient sales to justify incurring the
costs of complying with the proposed rule would exit."
• FDA also expects less products to be offered from those that stay in
• Who benefits if no significant changes are made?
• Big Tobacco
• A select couple Wall Street-backed e-cigarette companies
• Not small- and medium-sized businesses or consumers
• Some may move to nicotine-free market, but that will come with
• Not public health
• Smokers need more options to quit, not less
Achilles Heel of FDA Deeming Regulation
Hands Vapor Industry to Big Tobacco
How to Win … Or At Least Survive
• Create advocates
• Form national and local trade organizations
• Cooperation is key
• Engage with the government now, not later
• Don't endorse anti-competitive regulations
• Know your enemy
Questions?
Source: http://www.smileexpo.ru/public/upload/node/gregory_conley_14633943767958_userfile_1.pdf
MENTAL HEALTH LAW CENTRE (WA) Inc. ABN 40 306 626 287 Our Ref: SB NCW 572 6 March 2012 Mental Health Commission GPO Box X2299, Perth Business Centre WA 6847 DRAFT MENTAL HEALTH BILL 16 DECEMBER 2011 Mental Health Law Centre (WA) Inc. SUBMISSION PHYSICAL AND DENTAL HEALTH OF INVOLUNTARY PATIENTS AND PRISONERS We take this opportunity to make a submission on the Draft Mental Health Bill released for public comment on 16 December 2012. General Position The Bill authorises a single "authorised mental health practitioner" (not necessarily a doctor and perhaps simply an enrol ed mental health nurse with two days training in making referrals) to assess/ examine, refer, detain and transport a person against their wil and without their consent, to a psychiatrist (sometimes thousands of kilometres away from the patient's home) for an examination to decide whether or not that person requires detention (involuntary admission to an authorised psychiatric hospital or hostel) and/or involuntary treatment; and authorises the examining psychiatrist (who may or may not have trained in Australia) to make an involuntary detention and/or treatment order sometimes on the basis of a hurried five minute examination. Mistakes and oversights happen in a busy, under-resourced system whose priority and objective is treating a person's mental illness. Physical and dental il health may be the underlying cause of mental il -health – such as urinary tract infection in elderly people causing psychosis, may impede recovery from mental illness and may be low on the agenda of mental health
Poder Judicial de la Nación Causa Nro. 1799-2012 "C. B. J. s/ medida de seguridad" Interlocutoria Sala VI Juzgado de Instrucción N° 20 ///n la ciudad de Buenos Aires, a los 28 días del mes de noviembre de 2012, se reúnen los integrantes de esta Sala VI y el Secretario autorizante, para tratar la apelación interpuesta a fs. 59/62vta. por la defensa de J. C. B.








