Transformer in ceratitis
Development 129, 3715-3725 (2002)
Printed in Great Britain The Company of Biologists Limited 2002DEV7952
The transformer gene in Ceratitis capitata provides a genetic basis for
selecting and remembering the sexual fate
Attilio Pane, Marco Salvemini, Pasquale Delli Bovi, Catello Polito and Giuseppe Saccone*
Dipartimento di Genetica, Biologia Generale e Molecolare, Università degli Studi di Napoli ‘Federico II', Via Mezzocannone 8,80134 Napoli, Italy*Author for correspondence (e-mail:
[email protected])
Accepted 30 April 2002
The medfly Ceratitis capitata contains a gene (Cctra) with
expression in XX embryos by RNAi treatment can cause
structural and functional homology to the Drosophila
complete sexual transformation of both germline and soma
in adult flies, resulting in a fertile male XX phenotype. The
Similar to tra in Drosophila, Cctra is regulated by
male pathway seems to result when Cctra autoregulation is
alternative splicing such that only females can encode a
prevented and instead splice variants with truncated open
full-length protein. In contrast to Drosophila, however,
reading frames are produced. We propose that this
where tra is a subordinate target of Sex-lethal (Sxl), Cctra
repression is achieved by the Y-linked male-determining
seems to initiate an autoregulatory mechanism in XX
factor (M).
embryos that provides continuous tra female-specific
function and act as a cellular memory maintaining the
Key words:
Ceratitis capitata, Sex determination,
transformer,
female pathway. Indeed, a transient interference with Cctra
all aspects of somatic sexual dimorphism via a short cascadeof subordinate regulatory genes (Nagoshi et al., 1988). When
A broad variety of genetic cues that determine the sexual fate
the gene is active, it dictates female development; when it is
of a developing individual are known. Even within a minor
inactive, male development follows. Once the gene is activated
taxonomic group, for example, dipteran insects (Marin and
in females, its products initiate a positive autoregulatory
Baker, 1998; Schutt and Nöthiger, 2000), we find male
mechanism that guarantees the continuous production of SXL,
heterogamety with a male-determining Y chromosome
thus forming a cell memory of the sex and maintaining the cells
(
Musca,
Ceratitis) or with a single autosomal factor
on the female pathway throughout development (Bell et al.,
(
Megaselia,
Culex), female heterogamety (
Musca,
1991). In males, however, where
Sxl is not activated, the gene
Chironomus), chromosomal balance systems (
Drosophila,
will remain functionally OFF.
Sxl produces sex-specific
Sciara), and maternal effects (
Chrysomya). This variety raises
mRNAs by alternative splicing: the female-specific mRNAs
the issues of how these different mechanisms have evolved and
encode full-length functional Sxl protein, while the male-
how much they differ at the genetic and molecular level.
specific ones have an additional stop-containing exon and
Comparative analyses of different species can be used to
encode a truncated non-functional Sxl peptide. The ON/OFF
address these. We have chosen the economically important
state of
Sxl activity is set early during embryogenesis by
medfly (Mediterranean fruitfly),
Ceratitis capitata
complex combination of transcriptional and post-
(
Tephritidae). In this species a Y-linked factor,
M, determines
transcriptional gene regulation (Bell et al., 1991; Keyes et al.,
maleness, and absence of
M leads to female development
1992). The initial activation of
Sxl in XX embryos relies on the
(Willhoeft and Franz, 1996). However, how this signal is
use of an alternative XX-embryo-specific promoter that
relayed to genes responsible for expressing dimorphic traits is
responds to the genes signaling the
X:A ratio (Parkhurst et al.,
completely unknown.
1990).
Sxl pre-mRNAs produced from this promoter have such
The genetic cascade regulating sexual development in
a structure that they are spliced in a female-specific mode by
Drosophila is well known down to molecular details (Cline,
the spliceosome independently of additional trans-acting
1993; Cline and Meyer, 1996). In contrast to
Ceratitis, the
factors, such as the Sxl protein itself (Horabin and Schedl,
primary signal in
Drosophila is polygenic and is formed by the
1996; Zhu et al., 1997). The RNA-binding Sxl proteins
ratio of X chromosomes to sets of autosomes, the so-called
translated from these early mRNAs then initiate the
X:A ratio. When this ratio is 1.0 (XX:AA), the gene
Sex-lethal
autoregulatory loop by directing the female-specific processing
(
Sxl) is activated; with a ratio of 0.5 (X:AA),
Sxl remains
of the pre-mRNAs produced from the late
Sxl promoter. The
inactive.
Sxl now acts as the key ON/OFF switch that controls
late pre-mRNAs, in contrast to the early
Sxl pre-mRNAs, can
3716 A. Pane and others
be spliced in the female-specific mode only in the presence of
First-Strand Synthesis System for RT-PCR (Gibco BRL). RT-PCR
Sxl protein.
reported in Fig. 1B was performed with the following primers:
To execute the correct developmental program,
Sxl transmits
164+ (5′-CAGTGGTTCGGTTCGGAAG-3′) located in
Cctra
the determined state to
transformer (
tra) (Boggs et al., 1997),
the next gene in the cascade. At this level,
Sxl regulates the
900– (5′-TCCATGATGTCGATATTGTCC-3′) located in
Cctra
choice between two alternative 3′ splice sites in the pre-mRNA
Cctra male specific cDNA M1 and M2 were amplified by RT-PCR
of
tra (Inoue et al., 1990; Valcárcel et al., 1993). In absence of
using the following oligonucleotides:
SXL, the more proximal site is used resulting in a
tra mRNA
F+ (5′-CATGAACATGAATATTACAAAGGC-3′)
that encodes a truncated inactive protein. When SXL is present,
E– (5′- TCGCGTTCTCTAATCTCGTC-3′)
it will bind to the
tra pre-mRNA and enforce the use of the
These primers were derived from female-specific
CctraF1 cDNA.
distal 3′ splice site to produce an mRNA with a full-length ORF
RT-PCR was performed on RNA from unfertlized eggs, using
(Sosnowski et al., 1989). The state of activity of
tra is then
164+/900- primers. Cycling conditions were denaturation at 94°C for
transmitted to
doublesex (
dsx) (Burtis and Baker, 1989), the last
5 minutes, followed by 35 cycles of 94°C for 1 minutes, annealing at
component of the pathway. In females, TRA, together with
60°C for 1 minute and extension at 72°C for 2.5 minutes, with a final
the constitutively expressed TRA-2, binds to
dsx pre-mRNA
5 minute extension at 72°C. The PCR products were gel-purified,cloned using the Sure Clone Ligation Kit (Amersham Pharmacia
directing its female-specific splicing, such that a mature mRNA
Biotech) and sequenced by T7 Sequencing Kit (Amersham Pharmacia
encoding the DSXF protein is generated (Hoshijima et al.,
Biotech). Y-specific repetitive elements were amplified from genomic
1991; Tian and Maniatis, 1993). In males, absence of TRA
DNA by PCR using the following oligonucleotides:
causes male-specific splicing and the production of a DSXM
Y-spec1 (5′-TACGCTACGAATAACGAATTGG-3′)
protein. The two proteins, DSXF and DSXM, are transcription
Y-spec2 (5′-GCGTTTAAATATACAAATGTGTG-3′)
factors that regulate the activity of sex-specific differentiation
To perform positive control experiment
Cctra-specific primers
genes (Burtis and Baker, 1989).
164+ (described above) and 481– (5′-CTGGAATGGCACTGGTAT-
Previous studies have indicated that control of sexual
TG-3′) were used.
development in the medfly follows a different route. In
RT-PCR experiments to analyze
Ccdsx expression pattern were
particular, the
Ceratitis homolog of
Sxl does not appear to have
performed using a mix of the following
Ccdsx-specific primers:
Non-sex-specific 1400+ (5′-GGCATCAAGGCGTATAGAAGA-3′)
a switch function: the gene is expressed in both sexes,
irrespective of whether the male-determining Y is present or
absent (Saccone et al., 1998), which is inconsistent with a main
For the negative RT-PCR controls reverse transcriptase was not
sex-determining function. However, preliminary data suggest
included in the first strand cDNA synthesis reaction.
that the bottom-most component of the pathway,
dsx, is notonly present in
Ceratitis (
Ccdsx)
, but has conserved a role in
Northern blot analysis
sexual differentiation (Saccone et al., 2000). The pre-mRNA of
We separated 2 µg polyA(+) RNA by formaldehyde gel
this gene is also alternatively spliced giving rise to sex-specific
electrophoresis and transferred RNA onto a Hybond NX membrane
products that show a remarkable structural conservation when
filter (Amersham Pharmacia Biotech). For hybridization, we
compared with the corresponding male and female products in
incubated filters at 42°C overnight in a buffer of 50% formamide,5×SSPE buffer, 5×Denhardt solution and 1% SDS. A
Cctra probe was
Drosophila. Sequence analysis of
Ccdsx revealed the presence
prepared by nick-translation labeling of full-length
CctraF1 cDNA in
of putative TRA/TRA-2-binding sites close to the regulated
the presence of [α32P]dCTP (NEN; 3,000 Ci/mmol).
splice site, suggesting that the underlying mechanism of sex-specific splicing is conserved and under the control of proteins
homologous to TRA and TRA-2 (Saccone et al., 2000; Saccone
Cctra dsRNA was obtained and injected as described for
Drosophila
and Polito, 2002). To extend our comparative analysis, we
(Kennerdel and Carthew, 1998). A
CctraF1 fragment from positions
isolated the
Ceratitis homolog of the
Drosophila transformer
164 to 900 was amplified with primers that introduced a T7 promoter
sequence at each of the product ends. In vitro RNA transcriptions were
In this report, we demonstrate that a homolog of this gene
performed with the Megascript Kit (Ambion). Sense and antisense
(
Cctra), although highly diverged in sequence, is indeed
RNAs were separately obtained and equal amounts of the two ssRNAwere mixed together, ethanol precipitated and resuspended in the
present in the genome of Ceratitis and that, as in
Drosophila,
injection buffer (Rubin and Spradling, 1982). Embryos were collected
Cctra has a female-determining master function. However, in
1 hour AEL (after egg laying), hand dechorionated and microinjected
contrast to the
Drosophila tra,
Cctra plays an essential role in
with either 5 µM or 15 µM dsRNA solutions. We set up 27 cages,
Ceratitis sex determination by maintaining the female sexual
each containing single apparently normal males chosen from the
cell state through a positive feedback loop and by forming an
injected flies and three Benakeion females. Twenty cages produced
epigenetic memory of the sex of the organism (analogous to
bisexual progenies, each consisting of a number of flies ranging from
Sxl in
Drosophila) (Jablonka and Lamb, 1995).
two to 51 individuals. Seven cages gave female-only progenies, eachconsisting of a number of flies ranging from seven to 66 individuals(7, 17, 20, 28, 33, 52 and 66).
MATERIALS AND METHODS
Genomic and cDNA library screening
To identify
Ceratitis l(
3)
73Ah genomic clones, we screened a
PCR and RT-PCR
Ceratitis genomic library in the EMBL3 vector using standard
Total RNA was extracted, as described elsewhere (Andres and
methods. A probe was obtained from a 500 bp RT-PCR product
Thummel, 1994), from adult individuals and from unfertilized eggs.
Oligo-dT-primed cDNA was made from DNaseI-treated total RNA of
and l(3)1581–, 5′-TTGGCCACCAGCTTCTTGAG-3′) corresponding
unfertilized eggs, male and female flies using the SuperScriptTM
to a conserved region of
Drosophila melanogaster l(
3)
73Ah gene
transformer in Ceratitis 3717
(GenBank Accession Number, X84372). Genomic inserts weresubcloned in pBluescript (Stratagene) and sequenced using the T7Sequencing Kit (Amersham Pharmacia Biotech). To clone the female-specific Cctra F1 cDNA we screened an adult female cDNA libraryin Lambda-Zap vector (Stratagene), using a probe obtained from aCctra 400bp HincII genomic fragment corresponding to a region ofthe common exon 2.
Sequence analysis
Protein alignment was performed by MACAW clustalw/) with default settings (NCBI, NIH, Bethesda, USA). The
TRA/TRA-2 binding sites were identified in Cctra, by MACAW
and by DNA Fasta sequence comparison between Ceratitis and
Drosophila sequences.
GenBank Accession Numbers
Ccl(3)73Ah cDNA, AF436077; Cctra F1 cDNA, AF434936; Cctra
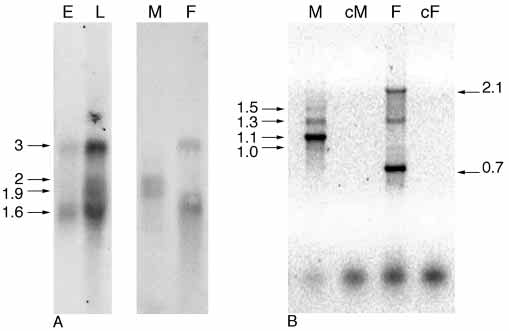
Fig. 1. Analyses of Cctra transcripts. (A) Northern blot analysis on
M1 cDNA, AF434937; Cctra M2 cDNA, AF4349378; Ccdsx F
poly A+ RNA from embryos (E), larvae (L), and adult males (M) and
cDNA, AF 435087; and Ccdsx M, AF434935.
females (F), using as probe the F1 cDNA clone. In males, twopredominant transcripts 1.9 kb and 2 kb long are detected, while twodifferent transcripts 1.6 kb and 3 kb long are present in females. In
embryos, two transcripts are detected and in larvae all fourtranscripts are detected. (B) RT-PCR amplification of Cctra on adultmales (M) and females (F) total mRNA samples. Three main
Isolation of tra in Ceratitis by synteny
products are present in the female lane (F), which are 0.7 kb, 1.3 kb
Given the unusually high degree of sequence divergence
and 2.1 kb long. In the male lane (M) four bands are detectable
among tra homologs in Drosophila (O'Neil and Belote, 1992),
which are 1 kb, 1.1 kb, 1.3 kb and 2.1 kb long. Male (cM) and
we decided to attempt the isolation of the tra gene in the medfly
female (cF) RT-PCR negative controls (reactions without reverse
by exploiting its close linkage in Drosophila to a well-
transcriptase) are shown.
conserved gene, l(3)73Ah (Irminger-Finger and Nöthiger,1995). Hence, as a first step towards the isolation of tra, weisolated cDNA and genomic Ceratitis sequences that cross-
only in female adults, while two mRNAs, of 1.9 kb and 2 kb
hybridized to a 500 bp Drosophila cDNA fragment of l(3)73Ah
in size, appear only in male adults. In embryos two mRNAs
at reduced stringency. These isolates indeed contained a
are detected having sizes similar to those of the adult female-
structurally well conserved homolog of l(3)73Ah as confirmed
specific transcripts. In RNA sample extracted by larvae of
by sequencing and comparison (Ccl(3)73Ah). We then
mixed sexes all four transcripts can be detected suggesting that,
continued to sequence a 4 kb long genomic region downstream
as in Drosophila, Cctra sex-specific processing may already
of the l(3)73Ah homolog and identified a putative ORF that
operate early in Ceratitis development.
showed by Blast search significant sequence similarity at the
A female-specific cDNA corresponding in size to the 1.6 kb
amino acid level to tra in Drosophila (ranging from 32% to
transcript was isolated and entirely sequenced; a comparison
40% identity scattered over 120 amino acids) and contained
with partial genomic sequences revealed that it is composed of
an arginine-serine-rich domain (SR-rich region) commonly
three exons (Fig. 2). Using RT-PCR with various pairs of
found in splicing regulators (Manley and Tacke, 1996). As in
Cctra-specific primers, sex-specific amplification products
Drosophila, the two genes are transcribed in opposite
were recovered from RNA samples of adult flies (Fig. 1B). The
orientation and sequence analysis of corresponding cDNA
164+/900– pair amplified (only in males) an abundant fragment
clones revealed that they overlap by about 200 bp (data not
of 1.1 kb and three minor bands of 1 (faint), 1.3 and 1.5 kb,
shown). We conclude that this gene arrangement must have
whereas in females, they amplified a prominent 0.7 kb long
already existed in the common ancestor of these fly species.
fragment, and three minor bands of 1 (faint), 1.3 and 2.1 kb
Though the significance of this synteny is unknown, it provided
(Fig. 1B). The size of the female-specific 0.7 kb cDNA product
an ideal entry point to the molecular identification of the tra
corresponds to the one expected on the basis of the F1 cDNA
homolog in Ceratitis (Cctra).
structure. The non-sex-specific fragments of 1 and 1.3 kb, inother RT-PCR experiments, were sometimes undetectable.
Cctra produces sex-specific transcripts
They most probably represent partially spliced and/or unstable
If this tra homologous gene indeed corresponds to the tra
Cctra RNAs. The 2.1 kb female-specific cDNA was isolated
switch gene in Drosophila, we expect it to be regulated sex
and entirely sequenced; a comparison with genomic sequences
specifically. A Northern blot containing poly(A)+ RNA from
revealed that it is an unspliced product (data not shown). The
different developmental stages of the medfly was probed with
size of this cDNA product suggests that it is derived from the
a genomic fragment derived from the Cctra locus. We find that
3 kb female-specific transcript.
Cctra transcripts are continuously present from embryonic
The F+/Z1– pair of primers (Fig. 2A) amplified a male-
stages until adulthood (Fig. 1A). Furthermore, this probe
specific 1.7 kb cDNA product, named CctraM1 (data not
detects sex-specific transcripts in samples from adult flies (Fig.
shown). The nucleotide sequence alignment of CctraM1 and
1A). The Ceratitis tra locus expresses four different mRNA
CctraF1 revealed that they are colinear with the exception of
variants: two products, of 1.6 kb and 3 kb in size, are found
two additional exons present in the male-specific cDNA. The
3718 A. Pane and others
Tra/Tra2 putativebinding sites
TC C ATCAACA Drosophila
Tra/Tra2 binding sites
Fig. 2. Genomic organization of the Ceratitis capitata tra gene. (A) The top line represents the genomic DNA encompassing the Cctra locus.
The positions of exons in the Cctra mRNAs are shown above the line, with Ex1, Ex2 and Ex3 representing exons in common between the male
and the female mRNAs, the blue boxes representing male-specific exons, the yellow box indicating a male-specific exon in the M1 mRNA, and
the red box representing a male-specific exon included in the M2 mRNA. Numbered green ovals indicate TRA/TRA-2-binding sites (see B).
Introns are represented by solid lines. Open boxes represent the ORF of the female-specific 1.6 kb long mRNA (Female F1) encoding the
putative 429 amino acid TRA protein (see Fig. 3). Gray boxes indicate 5′ and 3′ untranslated regions. Arrows above the first line represent the
positions of the oligonucleotides used in the RT-PCR experiments. The bar indicates the scale of the figure. (B) Sequence alignment of eight
putative TRA/TRA-2 binding sites found in the Cctra genomic sequence (see A). Conserved positions between Ceratitis and Drosophila are
indicated in bold.
male-specific exons are located between the first and the
prematurely the protein translation. Indeed partially different
second exon of CctraF1 and they are 40 bp (ME1a) and 203
intronic sequences are retained in the M1 and M2 cDNA
bp (ME1b) in length (Fig. 2A). Another pair of primers, F+/E–
clones, adding stop codons in different positions (Fig. 2A).
(Fig. 2A), amplified a male-specific fragment of 0.9 kb (data
This finding suggests that a functional full-length TRA is only
not shown), named CctraM2, that was cloned and sequenced,
encoded by the female-specific transcripts. This mode of sex-
showing with respect of CctraF1 two additional exon
specific regulation at the level of splicing is well documented
sequences of 210 bp (ME2a) and 176 bp (ME2b). ME2a is an
for the tra gene in Drosophila (Boggs et al., 1997). Different
alternative exon including the previously described exons 1 and
from Drosophila, however, where sex-specific regulation is
ME1a, plus the intervening intronic region (Fig. 2A). This
based on the alternative use of two 3′ splice acceptor sites, sex-
‘composed' new exon is produced by skipping the first 5′ splice
specific regulation in Ceratitis appears more complex and is
donor site. ME2b has an identical sequence to ME1b but it
achieved by a combination of exon skipping and differential
lacks the first 27 bp because of the usage of a downstream 3′
use of 5′ donor and 3′ acceptor sites.
alternative splice site (Fig. 2A).
The long ORF in the female-specific CctraF1 encodes a
putative protein of 429 amino acids. The CcTRA protein
Cctra female-specific transcript encodes a SR-rich
exhibits a low degree of similarity to TRA proteins in
Drosophila species and it is significantly larger in size in both
An alignment of CctraF1, CctraM1 and CctraM2 cDNA
N and C termini. Sequence processing tools of MACAW led
sequences with the genomic sequence exposes the organization
to the identification of five small blocks of sequence similarity
of tra in Ceratitis (Fig. 2A). The gene is composed of five
dispersed throughout the longest ORF of the female-specific
exons. The first, fourth and fifth exons are included in the
transcripts (Fig. 3). The regions with highest similarity
mature transcripts of both sexes, while the second and the third
(identified also by FastA analysis) are located between CcTRA
exons are male specific. The most important finding is that the
positions 150-230, 286-292 and 332-342 (Fig. 3). The SR-rich
female-specific transcript has a long open reading frame, while
region in Ceratitis TRA and possibly the other conserved
the male-specific mRNAs contain stop codons that abort
domains may confer specific RNA binding and protein-protein
transformer in Ceratitis 3719
interactions consistent with a proposed role in splicing
suggested to us that this gene had an essential role in female
regulation (Manley and Tacke, 1996). The male-specific
development of the medfly. To test its function, we employed
truncated protein isoforms lack the conserved boxes, the SR-
the RNAi technique that permits functional studies of genes in
rich region and do not show significant similarity with other
genetically less amenable organisms (Kennerdel and Carthew,
1998; Hunter, 1999). A 900 bp fragment of CctraF1 was usedas a template to produce dsRNA that was then injected as a 15
tra is essential for female development in C. capitata
µM solution into either the anterior or the posterior poles of
The confinement of transcripts with a long ORF to females
embryos of two different laboratory strains (Benakeion and
Fig. 3. Multiple sequence alignment of TRA proteins. Ceratitis capitata (Cc), D. melanogaster (Dm), D. erecta (De), D. simulans (Ds), D.
virilis (Dv) and D. hydei (Dh). Asterisks indicate amino acid identity in all species. Intron/exon boundaries are indicated by vertical arrows.
Amino acid residues occurring in the conserved regions are indicated by capital letters.
3720 A. Pane and others
Fig. 4. Phenotypic analysis of RNAi intersexes. (A) Wild-
type female has long pigmented bristles on the femur
pointing towards the coxa of the foreleg (arrow in E) and
the ovopositor (A,I). (B) Wild-type male exhibits two
spatulated bristles on the head (B), a row of non-pigmented
bristles on the ventral part of the femur towards the coxa of
the foreleg, short pigmented bristles grouped on the dorsal
part of the femur (arrow in F) close to the coxa of the
foreleg (F) and male genitalia (B,O). (C,D) Intersexes
obtained by dsRNA injection into the anterior pole of the
embryos exhibit male-specific spatulated bristles on the
head (arrow in C), male-specific bristles (upper arrow in H)
and female-specific bristles (arrow in G; lower arrow in H)
mixed together on the femur of the foreleg (G,H) and
female genitalia (C,D). Some intersexes show various
degrees of abnormal gonadal development exhibiting bent
(arrow in D), deformed (L-N) or completely absent (arrow
in P) genitalia. Scale bar in D applies at A-D; scale bar in
H applies to E-H; scale bar in I applies to I; scale bar in L
applies to L; and scale bar in P applies to M-P.
anterior pole resulted in the formation of male-specific spatulated bristles on the head of intersexes(Fig. 4C,D), male-specific blue eye reflections (datanot shown), male-like bristles mixed with female-likebristles on the femur toward the coxa of the foreleg(Fig. 4G,H), but the genitalia at the posteriorremained female-like (Fig. 4C). Conversely, injectioninto the posterior pole gave rise to mosaic adults withmale genitalia but with female bristles on the head andfemale-specific green eye reflections (data notshown). The intersexes showed also various degreesof abnormal gonadal development, with abnormallybent (Fig. 4D) or deformed ovopositor (Fig. 4L) andwith mixed male-like and female-like tissues (Fig.
4M,N). A few intersexes apparently lacked genitalia(Fig. 4P).
Karyotypic analyses of RNAi-treated adults
To assess the sexual karyotype of affected flies, we
performed a PCR amplification of genomic DNA
using Ceratitis Y-specific primers (Anleitner and
Haymer, 1992). No products were detected in single
preparations of 10 randomly chosen intersexes (data
not shown) and six out of 10 phenotypic males did
not reveal the presence of a Y chromosome by this
test, indicating that all these animals have a female
XX karyotype (Fig. 5). These results are in agreement
with the expected loss of female-promoting activity
when tra function is impaired by RNAi. On the
contrary, male development of XY flies seems not to
be affected by RNAi of tra, suggesting that the gene,
white-eye). From a total of 900 injected embryos, 272 adult
as in Drosophila, is dispensable in this sex. The occurrence of
flies were recovered and grouped by their sexual phenotype. A
intersexes and of few females is most likely due to incomplete
strong sex ratio bias was observed in favor of males. Out of
penetrance of the RNAi effect. Indeed, when a lower
272, 231 flies (84.9%) showed a normal male morphology, 37
concentration of dsRNA (5 µM versus 15 µM) was injected
flies (13.6%) exhibited various degrees of intersexuality (Fig.
into the anterior embryonic region, we obtained 64 intersexes,
4) and the remaining four (1.4%) were the only flies recovered
76 males and four females out of 144 adult flies. Therefore the
with a normal female phenotype. All of the 37 intersexes
percentage of intersexes increased from 14% to 44%, while the
exhibited an anteroposterior pattern of intersexuality. More
percentage of males decreased from 84% to 52%, suggesting
tellingly, the position of male tissues correlated exactly with
that XX individuals were only partially masculinized. From
the initial injection site in the embryo: injection into the
these results, we conclude that tra is required for female
transformer in Ceratitis 3721
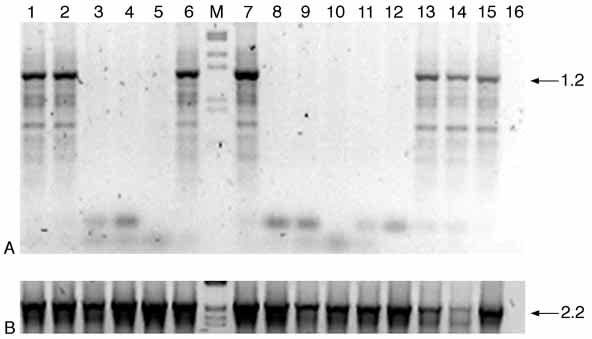
Fig. 5. Karyotypic analysis of RNAi treated individuals.
(A) PCR with Y-specific oligonucleotides carried on medfly
genomic DNA. From lane 1 to 10, PCR on single males
developed from dsRNA-injected embryos; lanes 11 and 12,
PCR on single wild-type females; lanes 13 and 14, PCR on
single wild-type males. PCR on flies of mixed sexes and a
negative control are shown, respectively, in lanes 15 and 16.
The PCR amplification patterns in lanes 1,2,6 and 7
correspond to those of wild-type males, indicating that the
analysed adults have an XY karyotype. By contrast, no
bands are detected in lanes 3-5,8-10 indicating that these
males lack a Y chromosome and therefore are XX sexually
transformed males. (B) Positive PCR control with Cctra
specific primers (Cctra164+ and Cctra481–) showing that
medfly genomic DNA is present in all samples. Lane M
(A,B) presents the molecular weight marker.
development in Ceratitis. Moreover, it is conceivable that
male fertility, as previously suggested by others (Willhoeft and
absence of tra activity constitutes a signal that triggers the male
fate. Thus, as in Drosophila, Ceratitis tra may act as a geneticswitch between female (when functionally ON) and male
Activity of tra is maintained by autoregulation
(when functionally OFF) development. The male-specific short
Next, we wanted to investigate the mechanisms which control
peptides encoded by the alternatively spliced male-specific
the activity of tra in Ceratitis. In Drosophila, regulation of tra
transcripts seem to be non-functional, at least at early
activity is achieved at the post-transcriptional level based on 3′
embryonic stages, because the RNAi has no evident effects on
splice site selection (Boggs et al., 1997). When SXL protein is
the development of XY males. We cannot determine, however,
present, it prevents the use of a distal acceptor site, thereby
whether they play a function at later stages, when the RNAi
promoting the use of the next downstream available 3′ splice
starts to lose its efficiency.
site, and it shifts about 50% of the pre-mRNA molecules froma non-sex-specific splicing to a productive female-specific
Adult XX males developed from RNAi-injected
mRNA (Sosnowski et al., 1989). It has been shown that this
embryos are fertile
regulation requires the direct binding of SXL to a poly (U)8
To investigate the fertility of the RNAi-treated adults, 27 males
stretch upstream of the regulated splice site (Kanaar et al.,
obtained from embryos injected with 15 µM dsRNA solution
1995). Several findings argue against a similar mechanism for
were individually crossed with wild-type females. We
conferring sex-specific splicing of tra in Ceratitis (Saccone et
predicted that if XX males are fertile than they should give a
al., 1998). First, Cctra splicing is based on a combination of
female-only progeny when crossed with wild-type virgin
exon skipping and 5′ and 3′ splice site regulation, rather than
females. Indeed out of 27, seven crosses gave a unisexual
on 3′ splice site selection. Second, CcSXL protein is present
female-only progeny. The karyotype of these seven males was
in both sexes of Ceratitis. However, upon close inspection of
then analyzed by PCR, as previously described, confirming
the Cctra sequence, we made an important discovery: within
that they were XX fertile males. As expected, PCR karyotypic
the two male-specific exons and the male-specifically retained
analyses of those males giving a bisexual progeny revealed that
intron, eight repeats were found by DNA sequence comparison
they were XY males (data not shown). Our data demonstrate
that are structurally related to the TRA/TRA-2 binding sites
that the Y-chromosome does not carry genes necessary for
(13 nucleotides long) in the dsx gene of Drosophila (Tian and
Fig. 6. Analysis of Cctra and Ccdsx splicing
patterns in adult individuals. (A) RT-PCR with
Cctra specific primers Cctra164+ and Cctra900–
on XY and XX males from dsRNA-injected
embryos (lanes 1 and 2) and on wild-type males
(lane 3) and females (lane 4). Lanes c1-c4 show RT-
PCR negative controls. The dsRNA injection in XX
embryos induces a permanent shift in the splicing
pattern of Cctra that turns from a female to a male
mode. (B) RT-PCR with Ccdsx-specific primers
(Ccdsx1400+, Ccdsx1130– and Ccdsx2000–) on the
same cDNA samples used in A. The 0.6 kb
fragment corresponds to a region of Ccdsx female-
specific transcript, while the 0.3 kb fragment
represents a region of Ccdsx male-specific
transcript. A consequence of the Cctra-specific RNAi is a persistent change in Ccdsx regulation that turns from a female-specific to a male-specific splicing mode. A molecular weight marker is also shown in lane M (A,B).
3722 A. Pane and others
Maniatis, 1993) (Fig. 2A,B). Similar repeats are alsodetected in the female-specific exon of the dsx
homolog in Ceratitis (Saccone et al., 2000). Their highsequence similarity to Drosophila
binding sites (Fig. 2B) and peculiar localization withinthe Cctra gene led us to believe that these sequences
are involved in the sex-specific splicing regulation ofCctra itself. In Drosophila, dsx and fru genes these
cis-elements act as, respectively, 3′ and 5′ splice
enhancers by recruiting the TRA/TRA-2 complex topromote the use of the regulated splice site (Tian andManiatis, 1993; Heinrichs et al., 1998). The presence
of potential TRA/TRA-2-binding sites in and aroundthe male-specific exons suggests that the female-specific CcTRA could inhibit their usage and led us toinvestigate whether an autoregulatory function of
Cctra is involved in the process of sex-specificsplicing.
If female-specific splicing of tra pre-mRNA indeed
depends on tra activity, we reasoned that a transientdepletion of tra activity should no longer be able tosustain the female mode of splicing. To test thissupposition, we analysed sex-reversed XX males
Fig. 7. Model for sex determination in Ceratitis capitata. (A) In XX
recovered from Cctra dsRNA injections. By RT-PCR
embryos, a maternal Cctra mRNA provides full-length CcTRA protein thatinitiates a positive feedback regulation. This protein drives a female-specific
analysis, only male-specific tra products were
splicing of the zygotically transcribed Cctra pre-mRNA so that new CcTRA
detected in adult tissues of injected XX and XY
protein can be produced. The newly synthesized protein controls the
individuals, but no female-specific products (Fig. 6A).
maintenance of Cctra autoregulation and the female-specific splicing of
In addition, the same males contained predominantly
Ccdsx pre-mRNA. Therefore a CcDSXF protein is produced that induces, at
male-specific splice variants of dsx, a probable
least in part, female development. (B) In XY embryos, Cctra autoregulation
downstream target of tra also in Ceratitis (Fig. 6B).
is impaired by the male determining M factor. The M factor could prevent
We infer from these results that early application of
the translation of the maternal Cctra transcript (1) or inhibit the function of
RNAi transiently eliminates Cctra mRNAs and, thus,
the protein that is produced by this mRNA (2). It is also conceivable that the
prevents continued production of TRA protein. Once
M could interact with the spliceosome or repress Cctra transcription
tra pre-mRNA production is resumed at a later stage
initiation in the zygote (3). In any case, the result is always that a full-lengthCcTRA protein is not produced in XY embryos and, thus, the autoregulatory
in development, the unproductive male mode of tra
loop can not initiate. In absence of CcTRA protein, Ccdsx is expressed by
splicing is launched because of the absence of
default to produce the CcDSXM isoform, which induces, in turn, male
functional TRA. Likewise, absence of TRA causes its
direct target dsx to be spliced in the male mode. Theseresults are compatible with our postulate that Cctrasustains the productive mode of its splicing by anautoregulatory feedback loop and mediates female
processing its own mRNA, thus initiating an autoregulatory
differentiation, at least in part, by the control of its target gene
mechanism to continuously produce a full-length protein.
dsx. The initiation of the autoregulatory loop in XX embryos
Interference at this level, for example, by injection of Cctra
could be based on maternal Cctra mRNAs that have been
dsRNA, leads to a breakdown of the regulatory loop and to the
detected in unfertilized eggs by RT-PCR experiments (data not
production of male-specific mRNAs encoding truncated
shown). These mRNAs are spliced in the female mode and
peptides. Thus, Cctra can be regarded as (1) an early binary
hence could provide a source of CcTRA activity that allows
switch in the sex-determining pathway of Ceratitis: when ON,
female-specific splicing of zygotic Cctra pre-mRNA.
female development ensues, when OFF, male developmentfollows; (2) a key gene controlling an epigenetic cell ‘memory'system of Ceratitis sex determination with evident analogies
DISCUSSION
with the Drosophila Sxl gene.
We have isolated a gene, Cctra, which is an ortholog of
A comparison between Ceratitis tra gene and its
Drosophila tra and acts as key regulator in sex determination
homolog in Drosophila: parallels and differences
of the medfly Ceratitis capitata. Cctra is regulated, as in
Our results show that Ceratitis and Drosophila sex-
Drosophila, by sex-specific splicing and encodes a protein
determining cascades share a conserved tra>dsx genetic
showing, as expected, low sequence conservation, when
module to control sex determination and sexual differentiation
compared with TRA proteins of Drosophila species (O'Neil,
as well as that tra sex-specific splicing regulation differs in the
and Belote, 1992). We present evidence that female
two species. In Drosophila, TRA protein, together with TRA-
development depends on an active Cctra that, in XX
2, binds to the TRA/TRA-2 recognition sequences on the
individuals, seems to promote the productive mode of
Drosophila dsx pre-mRNA and promotes the use of a nearby
transformer in Ceratitis 3723
female-specific acceptor site. We show that Cctra is needed to
As zygotes that carry a Y chromosome do not activate Cctra
impose the female-specific splicing of Ccdsx, most probably
female-specific splicing and autoregulation, we propose that
by a similar mechanism as in Drosophila, invoking the
the Y-linked male-determining M factor prevents this activation
existence of a Cctra2 homolog (Saccone et al., 2000; Saccone
(Fig. 7). It is conceivable that Cctra is a direct target of the M
and Polito, 2002). This hypothesis is also supported by the
factor. Presence of this M factor in the zygote may prevent the
finding of TRA/TRA-2 recognition sequences located in close
production of CcTRA protein. The Cctra positive feedback
vicinity to the female-specific acceptor site in Ccdsx pre-
loop is a probable target for regulation, because of its
mRNA (Saccone et al., 2000).
sensitivity (already shown by RNAi). An important question to
In Drosophila, tra female-specific splicing is promoted by
be addressed is how autoregulation of Cctra is initiated in XX
SXL, which blocks the use of the non-sex-specific splice site
embryos of C. capitata and how this is prevented in XY
present in the tra pre-mRNA. In Ceratitis, the presence of
embryos. A possible explanation is suggested by the Cctra
multiple TRA/TRA-2-binding elements within the Cctra
female-specific mRNAs encoding the full-length protein,
male-specific exonic sequences strongly suggests that CcTRA
which have been detected in unfertilized eggs. Depositing these
and a hypothetical CcTRA-2 proteins could bind to them
Cctra transcripts in eggs may provide a source of activity that
mediating a direct autoregulation. The unusually strong
can be used later for ‘female-specific' processing when Cctra
phenotypic effects of the RNAi against this gene also support
is zygotically transcribed (Fig. 7). Once zygotically activated
this model of Cctra regulation. The localization of the putative
in XX embryos, Cctra promotes its own female-specific
regulatory elements within the Cctra gene indicates a
splicing maintaining the female sex determination and the
repression mode by which CcTRA in females prevents the
female-specific splicing of the downstream Ccdsx gene. Taken
recognition of male-specific splice sites. The mechanism by
together, these events induce the female differentiation (Fig.
which Cctra seems to promote the female mode of processing
7A). In our model for sex determination of medfly, the M factor
of its own pre-mRNA by TRA/TRA-2-binding elements
is directly involved in the Cctra sex-specific regulation (Fig.
appears to be different also from the female-specific splicing
7B). Thus, in the presence of M Cctra, autoregulation is
of dsx. Rather than activating a splice site nearby the regulated
blocked and the gene produces male-specific transcripts
exon, as in the case of dsx, inclusion of male-specific Cctra
encoding short and possibly non-functional CcTRA peptides.
sequences is suppressed when CcTRA is present. Although
The absence of CcTRA leads Ccdsx to produce male-specific
this would be a novelty with the respect to known Drosophila
transcripts by default, promoting male differentiation (Fig.
TRA/TRA-2 activities, it has been previously shown that the
7B). The control of the M factor upon Cctra expression could
‘behavior' of these cis elements is context dependent and that
be exerted at different levels. The male determiner M could,
changing the location of splicing enhancers can transform
for example, act at the pre-translational level blocking the
them into negative regulatory elements (Kanopka et al., 1996;
production of CcTRA protein from the maternal transcripts. M
Lopez, 1998).
could act at the post-translational level antagonising theformation of protein complexes necessary for the female
A model for sex determination in Ceratitis capitata
splicing mode. Or M could act as a transient transcriptional
In Drosophila, the presence of the Y chromosome is necessary
repressor of Cctra to reduce the amount of active CcTRA
for male fertility but not for male development (Hardy et al.,
below a threshold needed to maintain the feedback loop. The
1981). By contrast, RNAi-treated Ceratitis embryos with a
proposed autoregulatory model of Cctra may also explain the
female XX karyotype can develop into fertile males, which
remarkable efficiency of sex reversal by Cctra RNAi: a
indicates that transient repression of Cctra by RNAi is
transient silencing of Cctra by injecting dsRNA is sufficient to
sufficient to implement fully normal male development. The
let the loop collapse. Furthermore, the sensitivity of this
cases of complete sexual transformation of genetic Ceratitis
positive autoregulation could be an evolutionary widely
females (XX) into fertile males by RNAi demonstrate that the
conserved pre-requisite to permit a ‘faster' recruitment/
Y chromosome, except for the dominant male determiner M,
replacement of different upstream regulators and to easily
does not supply any other contribution to both somatic and
evolve different sex determining primary signals, as observed
germline male development, as suggested by previous Y-
in dipteran species.
chromosome deletion analysis (Willhoeft and Franz, 1996).
Sex can even be determined by a maternal effect in dipteran
Other dipteran species, such as Musca domestica (Hilfiker-
species such as Sciara coprophila (Crouse, 1960) and
Kleiner et al., 1994) and Chrysomya rufifacies (Ullerich, 1984)
Chrysomya rufifacies (Ullerich, 1984). Our hypothesis of a
show a female and male germline sex determination that is
Cctra maternal contribution to the activation of the zygotic
completely dependent on the sexual fate of the soma. However,
Cctra gene has similarities to the model of sex determination
in Drosophila, the XX and XY germ cells seem to respond
proposed for Musca domestica (Dübendorfer and Hediger,
differently to sex determining somatic cues (Waterbury et al.,
1998). In the common housefly, the maternal product of the
2000; Steinmann-Zwicky et al., 1989). Indeed the XY germ
key switch gene F is needed to activate the zygotic function
cells have also an autonomous stage-specific sex determination
of F in females. Musca male development results whenever
mechanism that probably integrates the somatic signal (Janzer
F cannot become active in the zygote. This happens when the
and Steinmann-Zwicky, 2001). In Ceratitis, Cctra could be
male-determining M is present in the zygotic genome, or
required in XX somatic cells to let them induce the XX germ
when maternal F is not functional because of either the
cells to differentiate as oogenic cells. Alternatively, Cctra
presence of M or the mutational loss of function of F (Fman)
could be required in XX germ cells to ‘feminize' them. This
in the germline (Dübendorfer et al., 2002). More
case would be a novelty with the respect of the known
interestingly, embryonic RNAi against the Musca tra-2
Drosophila transformer gene functions.
homolog caused sex reversion of Musca XX adults into
3724 A. Pane and others
intersexes and fertile males, although this gene is not sex-
specifically expressed (Dübendorfer et al., 2002). Theserecent data in Musca and our results in Ceratitis support
Andres, A. J. and Thummel, C. S. (1994). Drosophila melanogaster:
the idea that F of Musca functionally corresponds to the
Practical Uses in Cell and Molecular Biology, pp. 570-573. London:Academic Press.
Ceratitis tra gene, that seems to autoregulate and maternally
Anleitner, J. E. and Haymer, D. S. (1992). Y enriched and Y specific DNA
contribute to its own activation, rather than to the Drosophila
sequences from the genome of the Mediterranean fruit fly, Ceratitis capitata.
tra gene.
Chromosoma, 101, 271-278.
Bell, L. R., Horabin, J. I., Schedl, P. and Cline, T. W. (1991). Positive
autoregulation of Sex-lethal by alternative splicing maintains the female
Evolution of sex determining cascades
determined state in Drosophila. Cell, 65, 229-239.
Our data show that a basic structure of sex determination is
Beverley, S. M. and Wilson, A. C. (1984). Molecular evolution in Drosophila
conserved in the two dipteran species, namely the flow of
and higher diptera. II. A time scale for fly evolution. J. Mol. Evol. 21, 1-13.
Boelens, W. C., Jansen, E. J., van Venrooij, W. J., Stripecke, R., Mattaj,
‘instructions' from tra to dsx. This confirms the model of
I. W. and Gunderson, S. I. (1993). The human U1 snRNP-specific U1A
‘bottom-up' evolution (Wilkins, 1995), suggesting that during
protein inhibits polyadenylation of its own pre-mRNA. Cell 72, 881-892.
evolution developmental cascades are built from bottom up and
Boggs, R. T., Gregor, P., Idriss, S., Belote, J. M. and McKeown, M. (1997).
that the genes at the bottom are widely conserved, while further
Regulation of sexual differentiation in D. melanogaster via alternative
upstream new regulatory elements may be recruited. Our
splicing of RNA from the transformer gene. Cell 50, 739-747.
Burtis, K. C. and Baker, B. S. (1989). Drosophila doublesex gene controls
results show that Ceratitis and Drosophila sex-determining
somatic sexual differentiation by producing alternatively spliced mRNAs
cascade differ at the level of transformer as well as upstream
encoding related sex-specific polypeptides. Cell 56, 997-1010.
of it. Indeed the gene has conserved its function during
Chabot, B., Blanchette, M., Lapierre, I. and la Branche, H. (1997). An
evolution, but it has female-specific positive autoregulation in
intron element modulating 5′ splice site selection in the hnRNP A1 pre-
mRNA interacts with hnRNP A1. Mol. Cell. Biol. 17, 1776-1786.
Ceratitis, while in Drosophila it needs Sxl as upstream
Cline, T. W. (1993). The Drosophila sex determination signal: how do flies
regulator to express its female determining function. More
count to two? Trends Genet. 9, 385-390.
likely the sex-determining function of Sxl was co-opted after
Cline, T. W. and Meyer, B. J. (1996). Vive la difference: males vs females in
Drosophila and Ceratitis had separated more than 100 Myr
flies vs worms. Annu. Rev. Genet. 30, 637-702.
ago (Saccone et al., 1998; Beverley and Wilson, 1984).
Crouse, H. V. (1960). The nature of the influence of X-translocations on sex
of progeny in Sciara coprophila. Chromosoma 11, 146-166.
Furthermore, it is conceivable that the autoregulatory
Dübendorfer, A. and Hediger, M. (1998). The female-determining gene F of
mechanism of Sxl could have been selected to overcome a
the housefly, Musca domestica, acts maternally to regulate its own zygotic
mutation impairing the tra autoregulation. Hence, in both
activity. Genetics 150, 221-226.
species the female pathway is maintained by a single gene
Dübendorfer, A., Hediger, M., Burghardt, G. and Bopp, D. (2002). Musca
domestica, a window on the evolution of sex-determining mechanisms in
positive-feedback mechanism through sex-specific alternative
insects. Int. J. Dev. Biol. 46, 75-79.
splicing. Single gene autoregulation by alternative splicing
Hardy, R. W., Tokuyasu, K. T. and Lindsley, D. L. (1981). Analysis of
seems not to be infrequent in nature, especially in those genes
spermatogenesis in Drosophila melanogaster bearing deletions for Y-
encoding splicing regulators. Indeed, other genes encoding
chromosome fertility genes. Chromosoma 83, 593-617.
RNA-binding proteins are thought to autoregulate their
Hasty, J., McMillen, D., Isaacs, F. and Collins, J. J. (2001). Computational
studies of gene regulatory networks: in numero molecular biology. Nat. Rev.
expression by controlling the processing of their own pre-
Genet. 2, 268-279.
mRNAs (Mattox and Baker, 1991; Boelens et al., 1993; Chabot
Heinrichs, V., Ryner, L. C. and Baker, B. S. (1998). Regulation of sex-
et al., 1997; Jumaa and Nielsen, 1997). Such a single-gene
specific selection of fruitless 5′ splice sites by transformer and transformer-
network with positive regulation is capable of bistability (Hasty
2. Mol. Cell. Biol. 18, 450-458.
Hilfiker-Kleiner, D., Dubendorfer, A., Hilfiker, A. and Nöthiger, R. (1994).
et al., 2001). This suggests that the emergence of analogous
Genetic control of sex determination in the germline and soma of the
positive autoregulation in different genes such as Drosophila
housefly, Musca domestica. Development 120, 2531-2538.
Sxl and Ceratitis tra genes would have been selected, during
Horabin, J. I. and Schedl, P. (1996). Splicing of the Drosophila Sex-lethal
evolution, to guarantee a similar ON/OFF-female/male bistable
early transcripts involves exon skipping that is independent of Sex-lethal
protein. RNA 2, 1-10.
Hoshijima, K., Inoue, K., Higuchi, I., Sakamoto, H. and Shimura, Y.
As Ceratitis capitata is a major agricultural pest in many
(1991). Control of doublesex alternative splicing by transformer and
areas of the world, the isolation of a key sex-determining gene
transformer-2 in Drosophila. Science 252, 833-836.
such as Cctra will substantially aid the development of new
Hunter, C. P. (1999). Genetics: a touch of elegance with RNAi. Curr. Biol.
strategies to optimize the efficacy of currently used male
17, 440-442.
Inoue, K., Hoshijima, K., Sakamoto, H. and Shimura, Y. (1990). Binding
sterile techniques for pest control (Saccone et al., 2000;
of the Drosophila Sex-lethal gene product to the alternative splice site of
Robinson et al., 1999). We expect that tra is also a key sex-
transformer primary transcript. Nature 344, 461-463.
determining gene in many other insect species. Hence, the
Irminger-Finger, I. and Nöthiger, R. (1995). The Drosophila melanogaster
isolation of corresponding tra genes will open new means to
gene lethal(3)73Ah encodes a ring finger protein homologous to the
control not only agricultural pests but also medically relevant
oncoproteins MEL-18 and BMI-1. Gene 163, 203-208.
Jablonka, E. and Lamb, M. J. (1995). Epigenetic Inheritance and Evolution:
vectors of diseases such as Glossina palpalis and Anopheles
The Lamarckian Dimension. Oxford: Oxford University Press.
Janzer, B. and Steinmann-Zwicky, M. (2001). Cell-autonomous and somatic
signals control sex-specific gene expression in XY germ cells of Drosophila.
We thank Daniel Bopp, Rolf Nöthiger, Lucas Sanchez, Adam
Mech. Dev. 100, 3-13.
Wilkins, Geoffrey Nette and Nicolas Carels for critical review of the
Jumaa, H. and Nielsen, P. J. (1997). The splicing factor SRp20 modifies
splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO
manuscript and for very useful suggestions. We also thank Rosaria
J. 16, 5077-5085.
Terracciano, Giovanni Manno and Giuseppe Falcone for their
Kanaar, R., Lee, A. L., Rudner, D. Z., Wemmer, D. E. and Rio, D. C.
technical assistance. This work was supported by Ministero della
(1995). Interaction of the Sex-lethal RNA binding domains with RNA.
Ricerca Scientifica e Tecnologica (PRIN 2000).
EMBO J. 14, 4530-4539.
transformer in Ceratitis 3725
Kanopka, A., Muhlemann, O. and Akusjarvi, G. (1996). Inhibition by SR
C. (1998). The Ceratitis capitata homologue of the Drosophila sex-
proteins of splicing of a regulated adenovirus pre-mRNA. Nature 381, 535-
determining gene Sex-lethal is structurally conserved but not sex-
specifically regulated. Development 125, 1495-1500.
Kennerdel, J. R. and Carthew, R. W. (1998). Use of dsRNA-mediated
Saccone, G., Pane, A., Testa, G., Santoro, M., de Martino, G., di Paola, F.,
genetic interference to demonstrate that frizzled and frizzled 2 act in the
Louis, C. and Polito, L. C. (2000). Sex determination in medfly: a
wingless pathway. Cell 95, 1017-1026.
molecular approach. Area-wide control of fruitflies and other pest insects
Keyes, L. N., Cline, T. W. and Schedl, P. (1992). The primary sex
(ed. K.-H. Tan), pp. 491-496. Penang: Penerbit USM.
determination signal of Drosophila acts at the level of transcription. Cell 68,
Saccone, G. and Polito, L. C. (2002). Sex determination in flies, fruitflies and
butterflies. Genetica (in press).
Lopez, A. J. (1998). Alternative splicing of pre-mRNA: developmental
Schutt, C. and Nothiger, R. (2000). Structure, function and evolution
consequences and mechanisms of regulation. Annu. Rev. Genet. 32, 279-
of sex determining systems in Dipteran insects. Development 127, 667-
Loukeris, T. G., Livadaras, I., Arcà, B., Zabalou, S. and Savakis, C. (1995).
Sosnowski, B. A., Belote, J. M. and McKeown, M. (1989). Sex-specific
Gene transfer into the medfly, Ceratitis capitata with a Drosophila hydei
alternative splicing of RNA from the transformer gene results from
transposable element. Science 270, 2002-2005.
sequence-dependent splice site blockage. Cell 58, 449-459.
Manley, J. L. and Tacke, R. (1996). SR proteins and splicing control. Genes
Steinmann-Zwicky, M., Schmid, H. and Nöthiger, R. (1989). Cell-
Dev. 10, 1569-1579.
autonomous and inductive signals can determine the sex of the germline of
Marin, I. and Baker, S. B. (1998). The evolutionary dynamics of sex
Drosophila by regulating the gene Sxl. Cell 57, 157-166.
determination. Science 281, 1990-1995.
Tian, M. and Maniatis, T. (1993). A splicing enhancer complex controls
Mattox, W. and Baker, B. S. (1991). Autoregulation of the splicing of
alternative splicing of doublesex pre-mRNA. Cell 16, 105-114.
transcripts from the transformer-2 gene of Drosophila. Genes Dev. 5, 786-
Ullerich, F. H. (1984). Analysis of sex determination in the monogenic blowfly
Chrysomya rufifacies by pole cell transplantation. Mol. Gen. Genet. 193,
Nagoshi, R. N., McKeown, M., Burtis, K. C., Belote, J. M. and Baker, B.
(1988). The control of alternative splicing at genes regulating sexual
Valcárcel, J., Singh, R., Zamore, P. D. and Green, M. R. (1993). The protein
differentiation in D. melanogaster. Cell 53, 229-236.
Sex-lethal antagonizes the splicing factor U2AF to regulate alternative
O'Neil, M. T. and Belote, J. M. (1992). Interspecific comparison of the
splicing of transformer pre-mRNA. Nature 362, 171-175.
transformer gene of Drosophila reveals an unsually high degree of
Waterbury, J. A., Horabin, J. I., Bopp, D. and Schedl, P. (2000). Sex
evolutionary divergence. Genetics 131, 113-128.
determination in the Drosophila germline is dictated by the sexual identity
Parkhurst, S. M., Bopp, D. and Ish-Horowicz, D. (1990). X:A ratio, the
of the surrounding soma. Genetics 155, 1741-1756.
primary sex-determining signal in Drosophila, is transduced by helix-loop-
Willhoeft, U. and Franz, G. (1996). Identification of the sex-determining
helix proteins. Cell 63, 1179-1191.
region of the Ceratitis capitata Y chromosome by deletion mapping.
Robinson, A. S., Franz, G. and Fisher, K. (1999). Genetic sexing strains in
Genetics 144, 737-745.
the medfly, Ceratitis capitata: development, mass rearing and field
Wilkins, A. S. (1995). Moving up the hierarchy: a hypothesis on the evolution
application. Trends Entomol. 2, 81-104.
of a genetic sex determination pathway. BioEssays 17, 71-77.
Rubin, G. M. and Spradling, A. C. (1982). Genetic transformation of
Zhu, C., Urano, J. and Bell, L. R. (1997). The Sex-lethal early splicing
Drosophila with transposable element vectors. Science 218, 348-353.
pattern uses a default mechanism dependent on the alternative 5′ splice sites.
Saccone, G., Peluso, I., Artiaco, D., Giordano, E., Bopp, D. and Polito, L.
Mol. Cell. Biol. 17, 1674-1681.
Source: http://star.evosexdevo.eu/pdf/14-pane_%202002.pdf
CUSTOMER SOLUTIONS NEXIUM CUSTOMER SOLUTIONS "El Centro Virtual de Experiencias de Internacionalización" (The On-line Centre for International Business Cases) is the result of the collaboration of the Spanish Institute for Foreign Trade ICEX and AEEDE, the Spanish Association of Business Schools , which includes eleven leading Business Schools. The aim of this project is to promote the internationalisation of Spanish SMEs,
Journal of Learning Disabilities Executive Impairment Determines ADHD Medication Response: Implications for Academic Achievement James B. Hale, Linda A. Reddy, Margaret Semrud-Clikeman, Lisa A. Hain, James Whitaker, Jessica Morley, Kyle Lawrence, Alex Smith and Nicole Jones J Learn Disabil The online version of this article can be found at: can be found at:


