Are the adverse effects of glitazones linked to induced testosterone deficiency?
Center for Sexual Medicine
Center for Sexual Medicine Papers
Are the Adverse Effects ofGlitazones Linked to InducedTestosterone Deficiency?
Carruthers, M, TR Trinick, E Jankowska, AM Traish. "Are the adverse effects ofglitazones linked to induced testosterone deficiency?" Cardiovascular Diabetology7:30. (2008)http://hdl.handle.net/2144/2660
Boston University
Hypothesis
Are the adverse effects of glitazones linked to induced testosterone
deficiency?
M Carruthers*1, TR Trinick2, E Jankowska3,4,5 and AM Traish6
Address: 1Centre for Men's Health, 20/20 Harley Street, London, UK, 2Department of Chemical Pathology, The Ulster Hospital, Belfast, (TRT) UK, 3Cardiology Department, Military Hospital, Wroclaw, Poland, 4Institute of Anthropology, Polish Academy of Sciences, Wroclaw, Poland, 5National Heart and Lung Institute, Imperial College, London, (EJ) UK and 6Institute for Sexual Medicine, Boston University School of Medicine, Center for Advanced Biomedical Research, Boston, (AMT) USA
Email: M Carruthers* -
[email protected]; TR Trinick -
[email protected]; E Jankowska -
[email protected]; AM Traish -
[email protected]
* Corresponding author
Published: 15 October 2008
Received: 12 August 2008Accepted: 15 October 2008
Cardiovascular Diabetology 2008,
7:30
2008 Carruthers et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Adverse side-effects of the glitazones have been frequently reported in both clinical and
animal studies, especially with rosiglitazone (RGZ) and pioglitazone (PGZ), including congestive heart
failure, osteoporosis, weight gain, oedema and anaemia. These led to consideration of an evidence-based
hypothesis which would explain these diverse effects, and further suggested novel approaches by which
this hypothesis could be tested.
Presentation of hypothesis: The literature on the clinical, metabolic and endocrine effects of glitazones
in relation to the reported actions of testosterone in diabetes, metabolic syndrome, and cardiovascular
disease is reviewed, and the following unifying hypothesis advanced: "
Glitazones induce androgen deficiency
in patients with Type 2 Diabetes Mellitus resulting in pathophysiological changes in multiple tissues and organs
which may explain their observed clinical adverse effects." This also provides further evidence for the
lipocentric concept of diabetes and its clinical implications.
Testing of the hypothesis: Clinical studies to investigate the endocrine profiles, including
measurements of TT, DHT, SHBG, FT and estradiol, together with LH and FSH, in both men and women
with T2DM before and after RGZ and PGZ treatment in placebo controlled groups, are necessary to
provide data to substantiate this hypothesis. Also, studies on T treatment in diabetic men would further
establish if the adverse effects of glitazones could be reversed or ameliorated by androgen therapy. Basic
sciences investigations on the inhibition of androgen biosynthesis by glitazones are also warranted.
Implications of the hypothesis: Glitazones reduce androgen biosynthesis, increase their binding to
SHBG, and attenuate androgen receptor activation, thus reducing the physiological actions of
testosterone, causing relative and absolute androgen deficiency. This hypothesis explains the adverse
effects of glitazones on the heart and other organs resulting from reversal of the action of androgens in
directing the maturation of stem cells towards muscle, vascular endothelium, erythroid stem cells and
osteoblasts, and away from adipocyte differentiation. The higher incidence of side-effects with RGZ than
PGZ, may be explained by a detailed study of the mechanism by which glitazones down-regulate androgen
biosynthesis and action, resulting in a state of androgen deficiency.
(page number not for citation purposes)
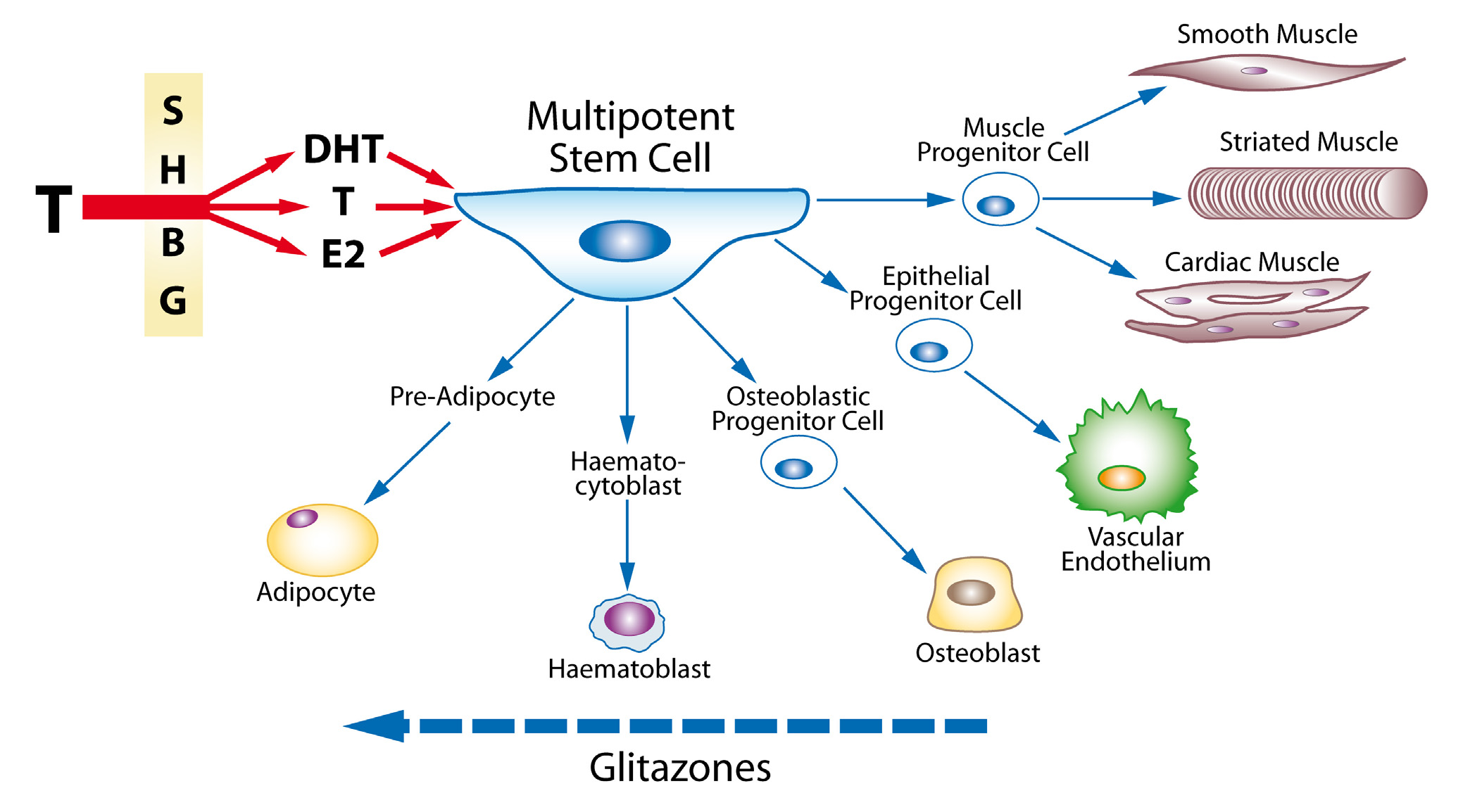
Cardiovascular Diabetology 2008, 7:30
glitazones give rise to a unifying hypothesis based on
Recent clinical studies have raised serious concerns
reduction of testosterone biosynthesis and function
regarding the safety of glitazones, especially rosiglitazone(RGZ) and pioglitazone (PGZ) to regulate hyperglycemia
Presentation of hypothesis
in diabetic patients. A meta-analysis study ] demon-
A Unifying Hypothesis Linking the Adverse Effects of
strated use of RGZ was associated with a "significant
Glitazones to Induced Testosterone Deficiency
increase in the risk of myocardial infarction and with an
We advance the following unifying hypothesis: "Glitazones
increase in the risk from cardiovascular causes that had border-
induce androgen deficiency in patients with Type 2 Diabetes
line significance". These side effects were confirmed by
Mellitus resulting in pathophysiological changes in multiple tis-
other clinical studies[ and meta-analysehough
sues and organs which may explain their observed clinical
some investigators, particularly those reporting the effects
adverse effects
of PGZ treatm reductions in cardiacdeaths.
It also provides further evidence for Ungar's theory of the'Lipocentric Pathway to Hyperglycemia', and explains the
Because of the widespread use of glitazones, it is of con-
toxic ectopic fat distribution in multiple organs, together
siderable practical importance to understand the potential
with its clinical implications
mechanisms underlying the differing effects of these twothiazolidines on clinical endpoints, in spite of their appar-
Evidence Supporting this Hypothesis
ent similar effectiveness in reducing blood glucose, as well
A. Epidemiological Studies
as their wide range of adverse side-effects, including
There is increasingly considered that low T levels in men
weight gain, anaemia and osteoporosis. These links
play an important role in the causation of T2DM, and are
between the clinical, metabolic and endocrine effects of
associated with reduced insulin sensitivity n men, cir-culating T is inversely related to classical cardiovascular
Unifying hypothesis linkin
g the adverse effects of glitazones to induced testosterone deficiency
Unifying hypothesis linking the adverse effects of glitazones to induced testosterone deficiency. Testosterone,
either directly or by conversion to dihydrotestosterone or oestradiol, all largely regulated by the effect of Sex Hormone Bind-
ing Globulin, acts on the Multipotent Stem Cell to promote differentiation to the progenitor cells for muscle, endothelium,
bone, and red blood cells. By causing androgen deficiency, glitazones may reverse these effects and promote adipocyte produc-
tion and action, with adverse clinical side-effects.
(page number not for citation purposes)
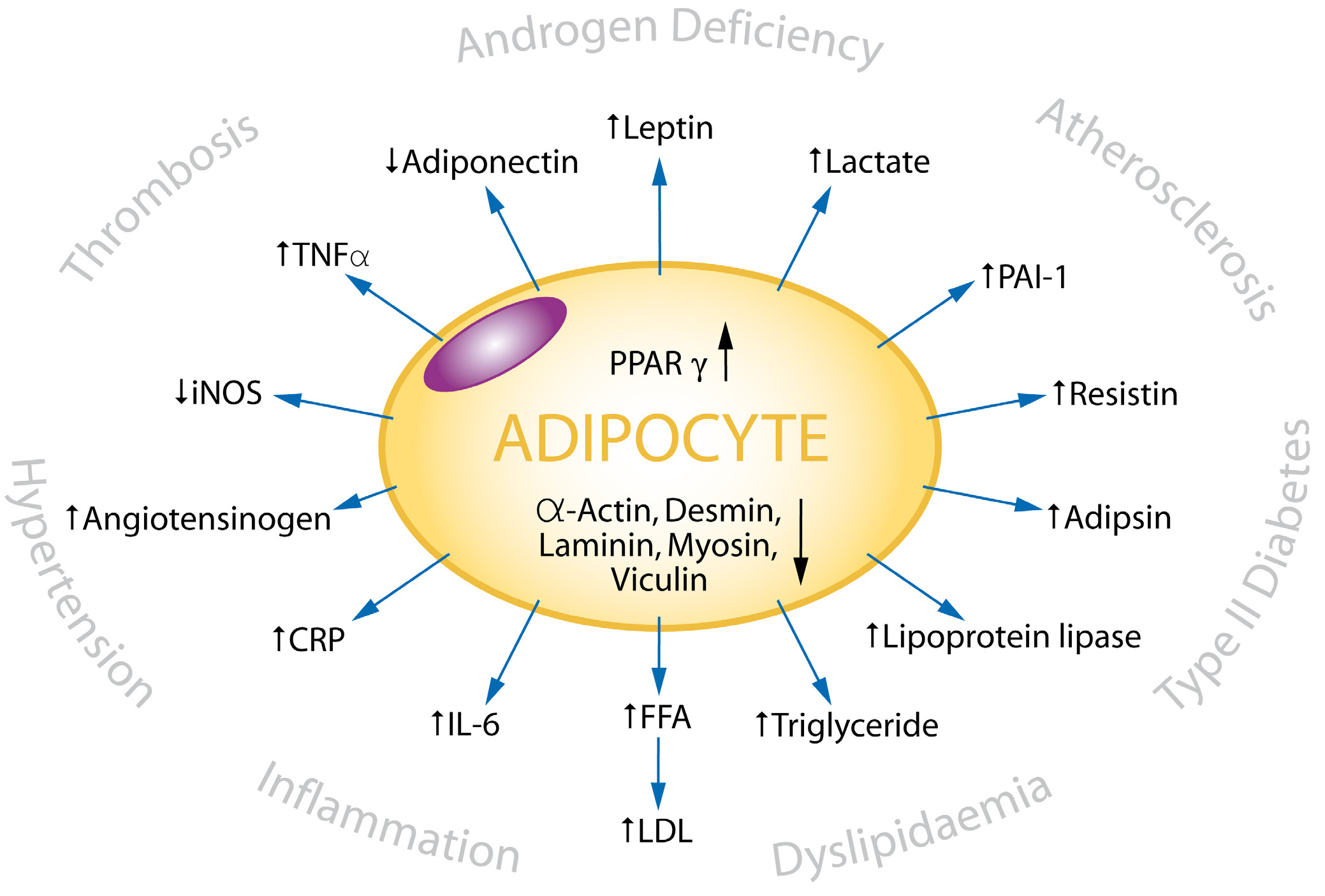
Cardiovascular Diabetology 2008, 7:30
disease (CVD) risk factors, including dyslipidaemia,
C. Treatment in Men
hypertension, pro-thrombotic and pro-inflammatory
Treatment of diabetic men with T has many beneficial
states, insulin resistance, obesity, abdominal fat distribu-
effects, including increasing insulin sensitivity, correcting
tion, endothelial dysfunction, intima-media thickness of
abnormalities in lipid metabolism, especially hypertrig-
the carotid artery and thoracic aorta [ with cor-
lyceridaemia, reducing visceral adiposity, decreasing lep-
onary artery disease (CAD) confirmed by angiography
tin and adiponectin levels, reversing neuropathy, and
have a markedly reduced level of circulating T as com-
improving erectile function. These effects are largely
pared to those with normal coronary ar].
brought about by reducing the adverse metabolic effectsof increased adipose tissue in organs throughout the
B. Suppression Therapy
body, but particularly in abdominal fat, reversing the
Management of prostate cancer via androgen-deprivation
actions of the adipocyte as the 'axis of evil' ) [
therapy with surgical or medical castration rapidly
Beneficial clinical anti-ischaemic effects of T treatment in
induces diabetes in susceptible individuals and is associ-
men with angina pectoris were reported as early as in the
ated with card. Androgen suppres-
1940te T administration reduces exercise-
sion therapy for prostate cancer has been linked to an
induced myocardial ischaemia in men with CAD and low
increased incidence of coronary heart disease and risk fac-
serum testosterone, also prolonging time to ST-segment
tors for atherosclerosis [].
The metabolic and clinical
effects of adipocyte activity
The metabolic and clinical effects of adipocyte activity. The adipocyte as the 'Axis of Evil' – PPARγ agonists such as the
glitazones stimulate the adipocyte to produce adipocytokines and cause insulin resistance, dyslipidaemias, hypertension, and
impaired immunological responses, which together can have the adverse clinical consequences shown.
(page number not for citation purposes)
Cardiovascular Diabetology 2008, 7:30
D. Effects on Muscle and Adipocytes
action on erythropots with diabetes tend
Singh et al [ suggested that androgens regulate the dif-
to be anaemic, especially the elderly, and their low T is
ferentiation of multipotent stem cells into the myogenic
correlated with their reduced haemreatment
lineage and inhibit adipogenesis. They also showed that T
with RGZ and PGZ makes them more anaemic, which is
inhibited adipogenic differentiation of pre-adipocytes by
probably related to lower T levels, not haemodilution
activation of androgen receptor (AR)/beta-catenin interac-
tion and translocation of androgen receptor/beta catenincomplex to the nucleus, thus bypassing canonical Wnt sig-
G. Effects on Bone
nalling. These changes can affect all 3 forms of muscle:
Because osteoblasts and marrow adipocytes are derivedfrom a common mesenchymal progenitor, increased adi-
Smooth muscle
pogenesis may occur at the expense of osteoblasts, leading
Ultrastructural studies by Traish et al have documented
to bone loss. RGZ and PGZ usage were associated with
that trabecular smooth muscle from castrated animals
more than doubling of fractures of the hip and wrist,
appears disorganized, with large number of cytoplasmic
increasing with the dose of either thiazolidin
vacuoles and a decrease in myofilaments. Androgen dep-rivation in the animal model results in accumulation of
Potential Mechanisms of Glitazone-Induced Androgen
adipocytes in penile tissues, particularly in the sub-tunical
re]. T replacement restores normal cavernosal his-
The actions of the glitazones on reductions in both TT and
tological appearance. Recently, Kova] have
DHT have been shown in healthy men roglitazone
shown that treatment of obese diabetic Zucker fa/fa rats
(TGZ) interferes with the activity of the P450 cytochrome
with PGZ produced globular fat-like cells in the corpus
oxidase (CPY) enzymes and was taken off the market in
cavernosum especially at high doses. These observations
the USA because of its hepatotoxicity. It also increases sex
together suggest a link between the function of anti-dia-
hormone binding globulin (SHBG) which redu].
betic agents and interference with T action as shown in
As detailed in T either directly, or by its conversion
to DHT or estradiol, regulates the differentiation ofmultipotent stem cells into smooth, striated and cardiac
Striated Muscle
muscle cells, osteoblastic/osteoclastic balance in bone,
T increases lean body mass and decreases fat mass in
haemopoietic activity, and the formation of cytoskeletal
young men, the magnitude of the changes being corre-
components [iting the differentiation of
lated with T concentrations. Especially in insulin resistant
progenitor cells into adipocytes.
diabetes, impaired muscle strength and mass is likely tobe associated with the reduction in myoglobin associated
The higher incidence of side-effects with RGZ than PGZ,
with low T levels.
may be further explained by a detailed study of the mech-anism by which glitazones down-regulate androgen bio-
Cardiac Muscle
synthe]. Both RGZ and PGZ changed the steroid
Androgen receptors are present in the myocardium (cardi-
profile of human adrenal NCI-H295R cells and inhibited
omyocytes) and vessel walls[]. Their expression is mod-
the activities of P450c17 and 3betaHSDII, key enzymes of
ulated by catecholamines and T itself, as shown by its
androgen biosynthesis. PGZ but not RGZ inhibited the
depletion in hypertrophied and failing hearts, which is
expression of the CYP17 and HSD3B2 genes. Likewise,
accompanied by deranged intracardiac steroid metabo-
PGZ repressed basal and 8-bromo-cAMP-stimulated activ-
liseficiency is related to several changes within
ities of CYP17 and HSD3B2 promoter reporters in NCI-
the myocardium, including impaired contractility of car-
H295R cells. However, PGZ did not change the activity of
diomyocytes []. All these pathologies can be restored to
a cAMP-responsive luciferase reporter, indicating that it
normal on T supplementation.
does not influence cAMP/protein kinase A/cAMPresponse element-binding protein pathway signalling.
E. Effects on Endothelial Progenitor CellsT deficiency is associated with a low number of circulating
There is also evidence that PGZ, to a greater extent than
progenitor cells and endothelial progenitor cells PCs in
RGZ, increases the maturation of small adipocytes to
young men. T treatment induces an increase in these cells
larger ones, promoting a reduction in insulin resist-
through a possible direct effect on the bone marrow
, decreasing lipogenesis in the liver, and increas-ing deposition of fat in the subcutaneous abdominal
F. Effects on Haemopoiesis
tissue, but not visceral fat. PGZ showed an additional ben-
T treatment increases red blood cell production and hence
eficial effect on TG, HDL cholesterol and the levels of
haemoglobin and haematocrit either directly by promot-
small dense LDL compared to RGZ.
ing erythroid stem cell ki], or indirectly by its
(page number not for citation purposes)
Cardiovascular Diabetology 2008, 7:30
Testing of hypothesis
on testosterone and CHF, and Mr Stewart McCrea of The Ulster Hospital,
Clinical studies are needed to investigate the endocrine
Belfast, UK, for the creative artwork in both figures.
profiles, including measurements of TT, DHT, SHBG, FTand oestradiol, together with LH and FSH, in both men
Nissen SE, Wolski K:
and women with T2DM before and after RGZ and PGZ
treatment in double blind, placebo controlled groups.
Engl J Med 2007, 356:2457-2471.
Also, further studies on T treatment in diabetic men
Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones
would further establish if the adverse effects of glitazones
N Engl J Med 2007,
could be ameliorated by androgen therapy. Basic sciences
investigations on the inhibition of androgen biosynthesis
Singh S, Loke YK, Furb JAMA 2007,
by glitazones are also warranted.
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-BenedettiM, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E,
Implications of the hypothesis
Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine
The FDA reports that most PPAR agonists in development
RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T,
are non-thiazolidinediones, and though more than 50
Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, SmithU, Taton J:
Innovative New Drug (IND) applications have been filed
for this group of drugs in the last seven years, most devel-
opment programs have been terminated, all for safety rea-
sons, and none have been approved.
Lincoff AM, Wolski K, Nicholls SJ, Niss JAMA 2007,
This hypothesis explains the adverse effects of glitazones
on the heart and other organs by reducing androgen
action in directing stem cells differentiation into myo-
JAMA 2008, 299:1185-1187.
Kapoor D, Malkin CJ, Channer KS, Jones TH:
cytes, vascular endothelium, erythroid stem cells and oste-
Clin Endocrinol (Oxf)
oblasts, and promoting adipocyte differentiation. The
Choi BG, McLaughlin MA:
adverse clinical effects are directly linked to the metabolic
Endocrinol Metab Clin North Am
actions of these drugs. The higher incidence of side-effects
with RGZ compared with PGZ, may be explained by a
Beld AW van den, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grob-b
detailed study of the mechanism by which glitazones
Am J Epidemiol 2003, 157:25-31.
down-regulate androgen biosynthesis and molecular
Demirbag R, Yilmaz R, Ulucay A, Unlu D:
mechanism of action, resulting in a state of androgen defi-
Endocr Res 2005, 31:335-344.
Muller M, Beld AW van den, Bots ML, Grobbee DE, Lamberts SW,Schouw YT van der: Circulation 2004,
AR: androgen receptor; CHF: congestive heart failure;
Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA
CVD: cardiovascular disease; DHT: dihydrotestosterone;
FT: free testosterone; IL: interleukin; MI: myocardial inf-
Endocrinol Metab 2002, 87:3632-3639.
arction; PCs: progenitor cells; PGZ: pioglitazone; PPAR:
English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS:
Peroxisome Proliferator-Activated Receptor; RGZ: rosigli-
Eur Heart
tazone; SHBG: sex hormone binding globulin; T: testo-
J 2000, 21:890-894.
sterone; TGZ: Troglitazone; TNF-α: tumour necrosis
Phillips GB, Pinkernell BH, Jing TY: Arterioscler
factor-alpha; T2DM: Type 2 diabetes mellitus; TT: total
Thromb 1994, 14:701-706.
Pugh PJ, Channer KS, Parry H, Downes T, Jone TH: Endocr Res
The authors declare that they have no competing interests.
Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS: J Cardiovasc Pharmacol 2002,
MC conceived the unifying hypothesis. All authors con-
Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M,Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P:
tributed to the initial manuscript, and revisions were car-
ried out by MC and AMT. All authors have read and
Circulation 2006,
approved the final manuscript.
Tappler B, Katz M: Clin Endocrinol (Oxf) 1979, 10:219-226.
We thank Prof. Piotr Ponikowski and Prof Waldemar Banasiak of the Car-
Clin Sci (Lond) 2003, 104:195-201.
diology Department, Military Hospital, Wroclaw, Poland, for their advice
(page number not for citation purposes)
Cardiovascular Diabetology 2008, 7:30
D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb
Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR
DS, Joseph D, Tai KH, Malone S, Ludgate C, Steigler A, Kanto
Arch Intern Med 2008,
Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR,
J Clin Oncol 2007, 25:2420-2425.
Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G:
Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Ris-
Diabetes Care
Vierhapper H, Nowotny P, Waldhausl W:
betes Obes Metab 2004, 6:208-215.
Metabolism 2003, 52:230-232.
Diabetes Care 2001, 24:2149-2151.
J Sex Med
Lesser MA: Testosterone propionate therapy in one hundred
cases of angina pectoris. J Clin Endocrinol 1946, 6:549-557.
Kempna P, Hofer G, Mullis PE, Fluck CE
Webb CM, Adamson DL, de ZD, Collins P:
Mol Pharmacol 2007,
Am J Cardiol 1999, 83:437-439.
Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH:
McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C,
Reaven GM, Cushman SW:
Endocrinol Metab 2004, 89:3313-3318.
Diabetologia 2007,
Pugh PJ, Jones TH, Channer KS:
Eur Heart J
Rizzo M, Christ ER, Rini GB, Spinas GA, Berneis K:
Malkin CJ, Jones TH, Channer KS:
Expert Opin
Eur J Heart Fail
Piepoli MF, Kaczmarek A, Francis DP, Davies LC, Rauchhaus M,
Jankowska EA, Anker SD, Capucci A, Banasiak W, Ponikowski P:
Circulation 2006,
114:126-134.
Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid
NF, Bhasin S:Endocrinology 2006, 147:141-154.
Traish AM, Kim N: Aging Male 2005, 8:141-146.
Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-
BJU Int
2006, 98:116-124.
Schock HW, Herbert Z, Sigusch H, Figulla HR, Jirikowski GF, Lotze
U: Horm Metab Res 2006, 38:225-229.
Burtea C, David A, Rom J Endocrinol 1993, 31:41-48.
Golden KL, Marsh JD, Jiang Y, Brown T, Moulden J: Am J Physiol Endocrinol Metab 2003, 285(3):E449-E453.
Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Ferlin A, Garolla
A: J Clin Endocrinol Metab 2006,
91:4599-4602.
Peschle C, Magli MC, Cillo C, Lettieri F, Pizzella F, Migliaccio G, Mas-
scientist can read your work free of charge
Blood Cells 1978, 4:233-252.
DeLong M, Logan JL, Yong KC, Lien YH
"BioMed Central will be the most significant development for
disseminating the results of biomedical researc h in our lifetime."
Sir Paul Nurse, Cancer Research UK
Nephrol Dial Transplant 2005, 20:585-590.
Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P
Your research papers will be:
available free of charge to the entire biomedical community
peer reviewed and published immediately upon acceptance
Berria R, Glass L, Mahankali A, Miyazaki Y, Monroy A, De Filippis E,
cited in PubMed and archived on PubMed Central
Cusi K, Cersosimo E, Defronzo RA, Gastaldelli A:
yours — you keep the copyright
ClinPharmacol Ther 2007.
Submit your manuscript here:
(page number not for citation purposes)
Source: http://dcommon.bu.edu/bitstream/handle/2144/2660/1475-2840-7-30.pdf?sequence=1&isAllowed=y
Research and the Approval Process An agent sequentially collects information to obtain a principal's approval, such as a pharmaceutical company seeking FDA approval to introduce a new drug. To capture suchenvironments, we study strategic versions of the optimal stopping time problem …rst proposedby Wald (1945). Our ‡exible model allows us to consider di¤erent types of rules and commit-ments by the principal as well as strategic withholding of information by the agent. We shedlight on current regulation and proposed reforms of the drug approval process. The modelalso captures situations such as a …rm seeking antitrust approval to merge with a competitor,a manager proposing a project to the …rm's headquarters or an author submitting a paper toan editor.
Fall 2012 Edition Website Updates Personalizing Tamoxifen Therapy in London The Lawson Translational Cancer Research Team (LTCRT) based in London has been facilitating the development and adoption of personalized cancer medicine using pharmacogenomics. Dr. Richard Kim, a recipient of the Cancer Care Ontario (CCO) Research Chair (Tier-1) Award in 2010 and leader of a program of Personalized Medicine at the London Health Sciences Centre, has been working closely with a team of oncologists and the LTCRT to ensure a group of breast cancer patients experience the best outcomes possible. Tamoxifen is an important drug for the treatment and prevention of certain breast cancers, and is metabolized in the liver by a drug metabolizing enzyme called CYP2D6. This enzyme is responsible for converting tamoxifen to its active form, endoxifen. The CYP2D6 enyzme occurs in different forms in human beings (called "polymorphisms") which may metabolize the same drugs differently. There is compelling research that shows that women with less active variants of CYP2D6 are at greater risk for breast cancer recurrence compared to those without such polymorphisms when treated with tamoxifen. In March of 2010, Dr. Kim started a personalized medicine clinic for breast cancer patients on tamoxifen therapy using the research funds available through his CCO Chair award. Patients referred to his clinic have their CYP2D6 genotyped as well as tamoxifen and endoxifen blood levels assessed using state-of-the-art genotyping and drug level analysis technologies. Dr. Kim then provides a detailed report to the referring oncologist in terms of the patient's ability to metabolize tamoxifen. He now has data from over 200 patients that show not only are CYP2D6 polymorphisms important to tamoxifen bioactivation to endoxifen, but so are polymorphisms in a another enzyme (CYP3A4) and the patient's vitamin D level.


