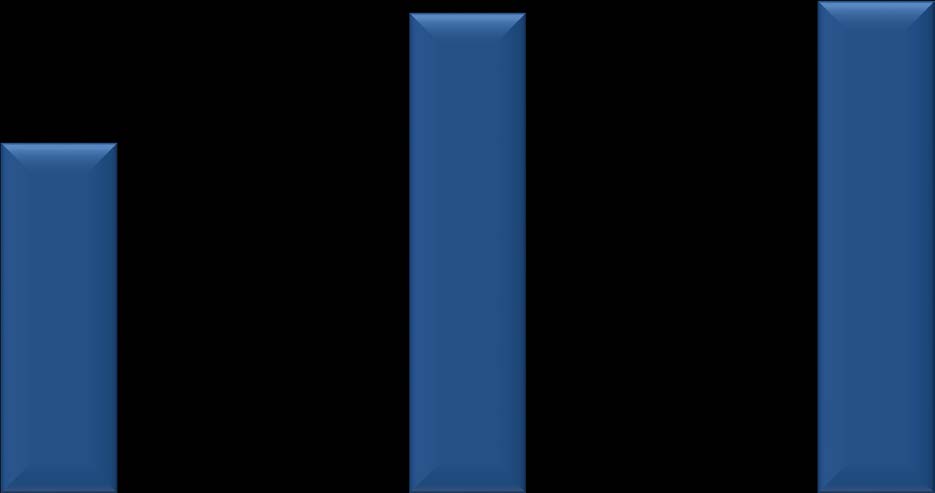Powerpoint-präsentation


EMSP Annual Congress
Brussels, 12. Mai 2011
New treatments for spasticity and other symptoms
Norbert Goebels, MD
Department of Neurology
Düsseldorf, Germany
Multiple Sclerosis Therapy
Immunmodulation-> Treatment of the underlying "autoimmune disease":
-> Investment into the future
Symptomatic Therapy
Therapy of the symptoms of the disease:
->"immediate (?) relief from symptoms"
Multiple Sclerosis: Scope of Symptoms
• Blurred vision
• Concentration
• Reduced colour
• Visual field deficit
• Articulation
• Articulation
• Eye movements
•Tremor, vertigo
• Sensory symptoms • Spasticity
• Palsy • Sphincter dysfunction • Sexual dysfunction
And many others, often nonspecific symptoms!
Ten most common MS Symptoms
Sherrat et al. 1980
Aminopyridine-MOA
Korenke et al., 2008
Early Studies II
Stefoski et al. 1987
P-Kinetics of unsustained Formulation
Stefoski et al. 1987
P-Kinetics of unsustained Formulation
Bever et al. 1990
Dalfampridine (Biogen-Idec / Acorda)
• 4-aminopyridine, sustained release (SR)
• Modifies axonal function
-> Duration of action potential increases
• 10 mg Dalfampridine 2x/d (N=119) vs. Placebo (N=120)
• Timed 25 ft walk test
Andrew D. Goodman, MD, Annals of Neurology 2010
Phase III Trial: Extended Release Oral
Dalfampridine in Multiple Sclerosis (MSF204)
Andrew D. Goodman, MD et al, Annals of Neurology 2010
Primary Outcome: Percentage of
Timed-Walk Responders (Pooled)
Across trials, approximately 38% of patients treated with PR-fampridine showed a
consistent increase in walking speed (timed-walk responders)
MS-F203 Goodman et al. Lancet 2009; MS-F204 Goodman et al. Ann Neurol 2010. Biogen Idec data on file.
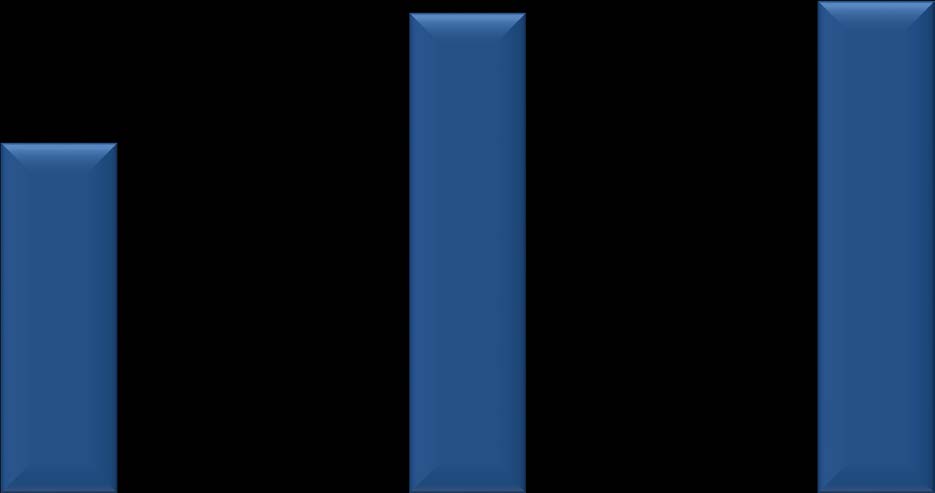


PR-Fampridine Shows Consistent Effects
Regardless of MS Type
Treatment Group by Disease Type
mpridine
PRMS=progressive-relapsing MS.
MS-F203 Goodman et al. Lancet 2009; MS-F204 Goodman et al. Ann Neurol 2010. Biogen Idec data on file.
Phase 3 Studies: Most Frequent Adverse Events (AEs)
Urinary tract infection
Insomnia
Dizziness
Headache
Asthenia
Upper respiratory tract infection
Back pain
Balance disorder
Studies MS-F203 and MS-F204: all AEs seen in >5% of PR-fampridine–treated patients.
MS-F203 Goodman et al. Lancet 2009; MS-F204 Goodman et al. Ann Neurol 2010. Biogen Idec data on file.
Quantitative Benefit-risk Profile for PR-Fampridine
Favoring
Favoring
PR-Fampridine 10 mg
Benefits
T25FW responders
Endpoint
Improvement in
MSWS-12 overall score
Improvement in LEMMT
Secondary
Endpoints
Improvement in Ashworth
score (spasticity)
≥20% Improvement in
T25 walking speed
CGI Score ≤3
at end DB period
Endpoints
Average SGI score
over DB period ≥6
CNS excitation
Urinary tract infection
Seizures
–150 –100 –50
Risk Difference and 95% CI (of 1000 Patients)
DB=double-blind; CNS=central nervous system.
Biogen Idec, data on file.
€ Fampridine $
Retail-Price Dalfampridine (AMPYRA ®) in the USA:
12.672,- USD / year (= 7,2 g)
7,2 g 4-Aminopyridin: 22,83 € (30,50 $)
Ten most common MS Symptoms
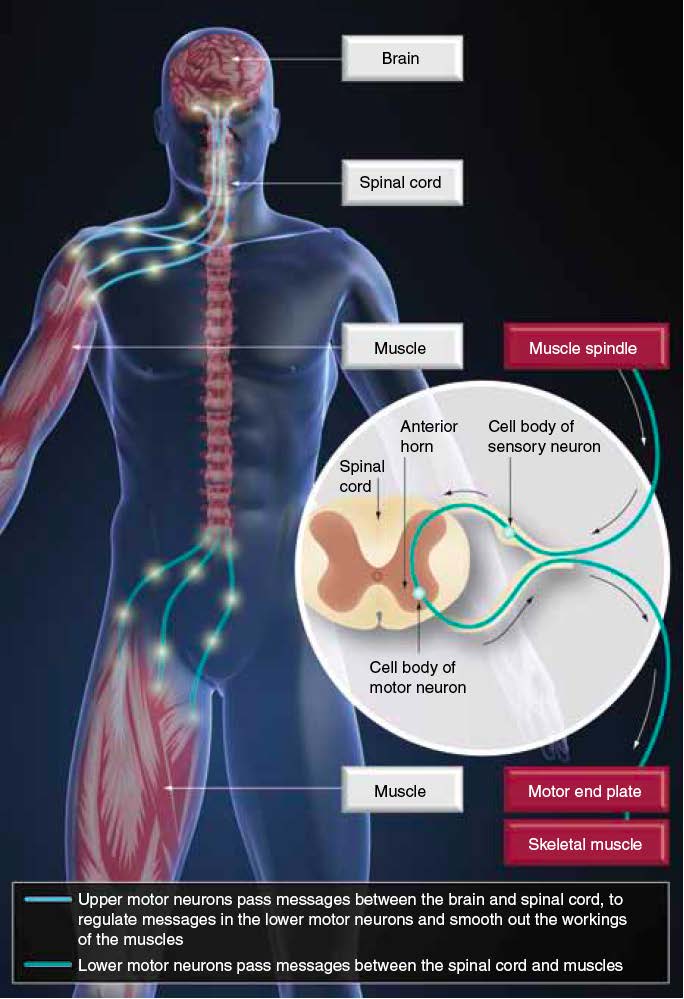
The stretch-reflex arc
in MS spasticity
"disordered
sensorimotor
control resulting from an upper motor
neuron lesion, presenting as inter-
sustained
involuntary
activation of muscles."
Stevenson VL. Clin Rehab 2010
description:
tightening of muscles that feels like leg
stiffness, jumping of legs, a repetitive
bouncing of the foot, muscle cramping in
the legs or arms, legs going out tight and
straight or drawing up".
Rizzo et al. Mult Scler 2004
MS spasticity: causes
• Result of myelin and nerve fibre degradation
• MS plaques inhibit supraspinal control of reflex
• ->Impairment of functional movements of muscles of
the extremities and of the trunk.
• Progressive damage -> loss of inhibition and a
disruption of the stretch-reflex arc
Bavikatte & Gaber Br J Med Pract 2009; 2: 29-34;
Springhouse. Handbook of signs and symptoms. LWW, 2005.
MS spasticity severity
(% from US survey of > 20000 patients)
Rizzo et al. Mult Scler 2004; 10: 589-95.
MS spasticity: symptom rating scales
The most frequently used have been:
• Ashworth Scale/Ashworth (modified) Scale
• Numerical Rating Scale (NRS)
• Multiple Sclerosis Spasticity Scale (MSSS)
• Daily mean spasm score
• Tardieu Scale (rarely used today)
MS spasticity: conclusions
• Spasticity is one of the most disabling symptoms
associated with MS.
• Like all MS symptoms, spasticity occurs as a result of
myelin and nerve fibre degradation.
• The Ashworth scale is the most widely used rating scale
for assessing the degree of spasticity.
• The NRS is a valid and sensitive diagnostic tool for
determining the severity of spasticity.
Management of spasticity in MS patients
Symptomatic Therapy for Spasticity (1)
Treatment of contributing factors
(Urinary tract) Infections
(Infected) decubitus ulcer
Beta-Interferon associated increase of spasticity
Symptomatic Therapy for Spasticity (2)
• Multimodal rehabilitation including intense physiotherapy to
reduce the extent of motor deficits
• Passive movement of major joints (motordriven bicycle)
• If possible: Aerobic Fitnesstraining
• Important: Sufficient Intensity and frequency
Medication:
Substance (Drugname)
Side effects
Baclofen (e.g. Lioresal)
Fatigue, nausea, confusion, ataxia
Tizanidin (e.g. Sirdalud)
Hypotonia, dry mouth, nausea
Gabapentin (e.g. Neurontin)
300 – 2400 (3600) mg/d
Vertigo, fatigue, weakness
Symptomatic Therapy for Spasticity (3)
• Oral anti-spasticity agents (z.B. Baclofen/Lioresal®,
Tizanidin/Sirdalud®)
• cave: weakness
• Increase dosage slowly (start with e.g. 3x2.5 mg Lioresal, 3x1 mg
Sirdalud), -> maximum dosage according to effect, combine if necessary
• Botulinumtoxin A (z.B. Botox®)
• Intrathecal Baclofen ("Lioresal pump")
• Intrathecal Triamcinolon (Volon A)
• Cannabis
Medical use of cannabis
• Cannabis has a long-history of use as both a
medicine and as a recreational drug.
• Medically, street cannabis has been used to utilise
it's antispastic, muscle relaxant and pain relief
• In a UK survey of persons using cannabis medically
(mostly smokers) between 1998 and 2002, almost
75% indicated that it was better or somewhat
better than their previous treatment for MS or
various pain states.
Ware et al. Int J Clin Pract 2005;59: 291-95.
Street cannabis: concerns/limitations
• Legal issues. • Street cannabis lacks standardization and purity • In
Tetrahydrocanabinol (THC, psychoactive cannabinoid) and low levels of Canabidiol CBD (antipsychotic
cannabinoid) were reported .
• Largely smoked and this increases the risk of lung cancer,
heart disease, etc.
• Smoked cannabis has variable pharmacokinetics, causing
very high THC peaks, which lead to psychoactivity and
other adverse events.
Chong et al. Mult Scler 2006; 12: 646-51.; Wade et al. Mult Scler 2006; q12: 639-45.;
Aldington et al. Eur Resp J 2008; 31: 280-86.; Potter et al. J Forensic Sci 2008; 53: 90-4.
The endocanabinoid system
• 1990 breakthrough in the field of canabinoid research:
CB1 receptor was discovered by Matsuda et al.
• 1992 Discovery of anandamide (endocanabinoid) by
Devane et al.
• 1993 Discovery of CB2 receptor by Munro et al.
• During the last 10 years the antispastic and analgesic
effects of cannabinoids were investigated
New target for the regulation of physiological functions Experimental studies showed, that the endocanabinoid
system significantly changes in processes of spasticity.
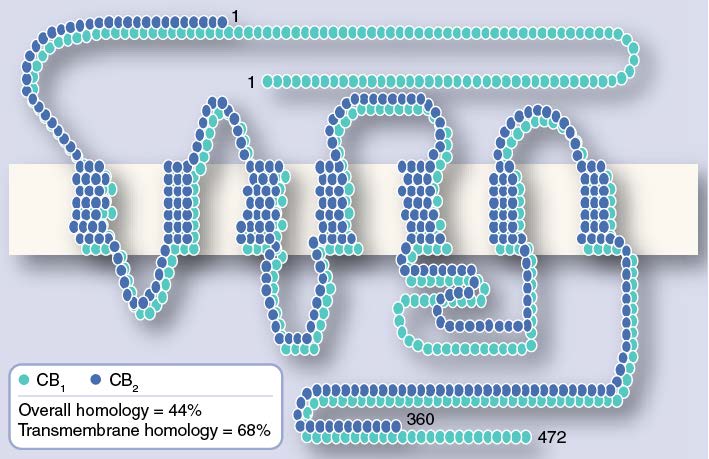
The protein sequences of CB1 and CB2 receptors
CB1 receptors: hippocampus, basal ganglia, cortex, cerebellum,hypothalamus,
pituitary, limbicstructure and gastrointestinal tract.
CB2 receptors: immune cells and tissues and bone.
Endocannabinoids act as retrograde neuromodulators
CB: Cannabinoid; EC: Endocannabinoid; NT: Neurotransmitter
Cannabinoids: mechanism of action
1. A nerve impulse reaching the synapse
stimulates the release of neurotransmitters
(the yellow molecules). These cross the
synapse and bind to receptors on the post-
synaptic cell, initiating a series of events.
2. One of these events is the release of
endocannabinoids (the red molecules) which
are released locally, crossing the synapse in
the opposite direction of the nerve impulse.
3. The endocannabinoids bind to pre-synaptic
CB1 receptors (the light blue receptors)
neurotransmitters,
neurotransmitters are inhibitory (e.g., GABA)
or excitatory (e.g., glutamate). This is an
example of negative feedback system.
4. Phytocannabinoids mimic the action of these
endocannabinoids. In this way, they are able to
augment the effect that endocannabinoids
have in regulating the transmission of impulses
from one nerve to another.
CNS forum. Cannabinoid receptors 2009.
Biozzi mice with chronic relapsing EAE
CB1 agonists ameliorate spasticity
*p < 0.001 compared with baseline
Collin et al. 2007
Rationale for the development of the
standardised fix combination THC/CBD
• To produce a standardised medicinal product based
upon the main active constituents of Cannabis sativa,
tetrahydrocannabinol (THC) and cannabidiol (CBD).
• Formulated to ensure purity and stability. • To administer in a way (oromucosal) which provides
a satisfactory pharmacokinetic profile avoiding the
high plasma levels and risks associated with
• To benefit from the synergistic interaction between
CBD and THC, with a reduction in psychoactivity and
enhanced cannabinoid-mediated clinical effects.
Perez Drugs of Today 2006; 42: 495-501; Potter et al. J Forensic Sci 2008; 53: 90-4.
Standardized fix combination of THC/CBD
• Oromucosal spray contains two canabinoids, which act
synergistically:
• 1) Tetrahydrocanabinol (THC)
• 2) Canabidiol (CBD)
• THC is a CB1- and CB2-receptor agonist
• CBD is a CB1- receptor antagonist and prevent the
psychoactive effects of THC
THC and CBD: synergy
(complementary effects)
Russo & Guy Med Hypotheses 2006; 66: 234-46.
Standardised fix combination THC/CBD:
Composition and production
• First-in-class endocannabinoid system modulator
comprising THC + CBD.
• Cannabinoid-based medicine derived from Cannabis
• Prepared from 2 cloned chemovars of C. sativa to
ensure standardisation and quality.
• 10ml amber vials with a pump for oromucosal
Standardised fix combination THC/CBD
manufacturing: sophisticated cultivation
Maximum plasma THC levels with the standardised fix
combination THC/CBD and Street Cannabis (smoked)
Standardised fix
Standardised fix
combination THC/CBD
combination THC/CBD
steady state dose
Guy & Stott In Parnham et al. (eds) Milestones in drug therapy:
cannabinoids as therapeutics, 2005.
Clinical Overview (Phase III)
Author/publ. year Patients n =
Primary endpoint Results
Score of five MS-
1. endpoint: not sign.;
symptoms: spasticity,
2. endpoint: spasticity
spasms, bladder,
alone: significant
tremor, pain (VAS)
189 / 12 centers
spasticity (NRS)
statistically significant
Pivotal study II
spasticity (NRS)
spasticity (NRS)
statistically significant
Time to failure of
favour of THC/CBD
Incidence of AEs
comparable to short term studies
Severity of worst
Dosage & severity
constant -> longterm efficacy
Standardized fix combination THC/CBD:
third pivotal clinical trial
Published (abstract available and full text pending)
EU (multicentre)
A 2-phase study: Phase A- single- blind response assessment and Phase B- a randomised,
placebo-controlled, double-blind, paral el group study
To assess the efficacy and safety of the standardised fix combination THC/CBD vs. placebo
in patients with MS spasticity
• N = 572 MS adult patients
• MS with spasticity and an inadequate response to drug therapy
• Single-blind the standardised fix combination THC/CBD for 4 week, with initial responders
(improving 20% or more from baseline NRS score) randomised to the standardised fix
combination THC/CBD or placebo for 12 more weeks
• Participants continued with current therapies throughout the study
•14 day follow-up after controlled period of 12 weeks
Change in Spasticity numerical rating scale (NRS) score
outcome Secondary
• Improvement in NRS responses of 30% or more and 50% or more
• Modified Ashworth scale of spasticity
• Timed 10-metre walk and motricity index
• Spasm frequency and sleep disruption
• Barthel ADL index
• Carer's global impression of change (CGIC)
• Quality of Life
Novotna et al. Eur J of Neurology 2011
Standardised fix combination THC/CBD*: third pivotal
clinical trial: two-phase study design
Phase A (n=572)
Phase B (n=241)
12 weeks THC/CBD*
12 weeks Placebo
THC/CBD*
Novotna et al. Eur J of Neurology 2011
Standardized fix combination THC/CBD*: third pivotal clinical
trial results: NRS resolution from phase A responders
Novotna et al. Eur J of Neurology 2011
Standardised fix combination THC/CBD third pivotal
clinical trial: Well-being and quality of life (QoL)
• Barthel activities of daily living (ADL) (p = 0.0067).
• Physician, carer and patient global impression of change
(p = 0.0045, p = 0.0053 and p = 0.0234, respectively).
• Sleep disruption NRS (p <0.0001).
• Spasm frequency (p = 0.0046).
• QoL EQ-5D (0.48 to 0.57; +19%).
• QoL SF-36 Role Physical 0-100 (35.1 to 48.1; +37%).
Ambler et al. Mult Scler 2009; 15: S258; Montalbán & Wright Mult Scler 2009; 15: S272.
Standardised fix combination THC/CBD:
adverse events (AEs)
• During the first 4 weeks of exposure dizziness (14-32%)
and fatigue (12-25%) were the most common AEs.
• Usually mild to moderate and resolved quickly. • When the recommended gradual "up titration" schedule
was introduced the incidence of AEs was reduced.
• In clinical trials the rates of withdrawal due to AEs was
• The standardised fix combination THC/CBD does not
exhibit the side effects typically associated with
recreational cannabis use.
Wade et al. Mult Scler 2004; 10: 434-41; Wade et al. Mult Scler 2006; 12: 639-45;
Collin et al. Eur J Neurol 2007; 14:290-96. Collin et al. Mult Scler 2007; 13: S129;
Ambler et al. Mult Scler 2009; 15: S258.
Standardised fix combination THC/CBD:
AEs listed in the SmPC
MeDRa System Organ Class disorders
Very common
≥1/100 to <1/10
Infections and infestations Metabolism and nutrition
Anorexia (including ↓appetite), ↑ appetite
Depression, disorientation, dissociation,
Amnesia, balance disorder, attention
problems, memory impairment, somnolence,
dysarthria, dysgeusia, lethargy
Ear and labyrinth
Cardiac Vascular Respiratory, thoracic, mediastinal Gastrointestinal
Constipation, diarrhoea, nausea, dry mouth,
glossodynia, vomiting, mouth ulcers, oral
discomfort/pain,
General disorders and admin site
Application site pain, asthenia, feeling
abnormal/drunk, malaise
Injury. Poisoning and procedural
Treatment-related neurological AEs
Standardised fix combination
Placebo (n = 853)
THC/CBD (n = 921)
Disturbance in attention
Memory impairment
Coordination abnormal
Cognitive disorder
Depressed consciousness
[From the standardised fix combination THC/CBD integrated safety analysis (Sept 1, 2007) from
non-cancer studies.]
NB. These data do not include results from the third pivotal clinical trial which used the "up-titration"
schedule and was associated with a significantly lower incidence of AEs.
Cognitive and Neuropsychiatric Effects
• Cognitive impairment occurs with the standardised
fix combination THC/CBD, but in the majority of
instances the symptoms were mild-to-moderate.
• Psychiatric AEs were also reported for the
standardised fix combination THC/CBD , but they
were mostly of mild-to-moderate severity.
• There is no evidence from RCTs that the
standardised fix combination THC/CBD poses any
long-term or irreversible neuropsychiatric or
cognitive risk to patients
[From the standardised fix combination THC/CBD integrated safety analysis (May 11, 2007)]
Potential for abuse
• The standardised fix combination THC/CBD does not
exhibit the psychostimulant effects typically associated
with recreational cannabis use.
• Intoxication was reported to be very low during the
course of short- and long-term studies.
• No association with signs of drug tolerance and in a
long-term trial the mean dosage decreased slightly.
• No consistent withdrawal syndrome has been observed,
and there is no evidence of drug misuse or abuse.
• Lower abuse potential than equivalent doses of
dronabinol, which itself is considered to have minimal
abuse potential, in 23 abuse-prone recreational
marijuana users.
Wade et al. Mult Scler 2006;12: 639-45; Collin et al. Eur J Neurol 2007; 14:290-96; Schoedel et al. 2010.
Indication of standardized fix combination THC/CBD
Add-on treatment, for symptom improvement in patients
with moderate to severe spasticity due to multiple
sclerosis (MS), who have not responded adequately to
other anti-spasticity medication
Standardised fix combination THC/CBD
clinical efficacy: conclusions
• Results from well-controlled RCTs provide conclusive
evidence of the efficacy of the standardised fix
combination THC/CBD in MS-related spasticity.
• Randomized withdrawal of the standardised fix
combination THC/CBD treatment provided definitive
proof of long-term efficacy.
• The standardised fix combination THC/CBD not only
reduced the symptoms associated with MS-spasticity,
it also increased the ability of the patient to perform
certain tasks and improved the perception of patients
and their carers regarding functional status.
Standardized fix combination THC/CBD
Approval expected in:
• Standardised fix combination THC/CBD well tolerated • Dizziness and fatigue are the most common AEs • Most AEs are mild to moderate • Only few withdrawals due to unwanted effects. • Fix combination THC/CBD does not appear to pose
risks of long-term or irreversible neuropsychiatric or
cognitive impairment
• Famprine increases mobility in 1/3 of patients,
currently not approved in EU, expensive
Source: http://www.emsp.org/wp-content/uploads/2015/08/New-Treatments-for-Spasticity-and-other-symptoms-Norbert-Goebels.pdf
Biochemical Journal Immediate Publication. Published on 12 Apr 2006 as manuscript BJ20060409 The life-extending gene Indy encodes an exchanger for Krebs-cycle Felix Knauf1,2, Nilufar Mohebbi1, Carsten Teichert1, Diana Herold1, Blanka Rogina3, Stephen Helfand3, Maik Gollasch1, Friedrich C. Luft1 and Peter S. Aronson2* 1Franz Volhard Clinic at the Max Delbruck Center, HELIOS Kliniken – Berlin, Medical Faculty of the Charité, Humboldt University, D-13125 Berlin, Germany
Why aircraft disinsection?Norman G. Gratz,1 Robert Steffen,2 & William Cocksedge3 A serious problem is posed by the inadvertent transport of live mosquitoes aboard aircraft arriving from tropicalcountries where vector-borne diseases are endemic. Surveys at international airports have found many instances oflive insects, particularly mosquitoes, aboard aircraft arriving from countries where malaria and arboviruses areendemic. In some instances mosquito species have been established in countries in which they have not previouslybeen reported. A serious consequence of the transport of infected mosquitoes aboard aircraft has been thenumerous cases of ‘‘airport malaria'' reported from Europe, North America and elsewhere. There is an importanton-going need for the disinsection of aircraft coming from airports in tropical disease endemic areas intononendemic areas. The methods and materials available for use in aircraft disinsection and the WHOrecommendations for their use are described.