Hppsource.jp
Osteoporos IntDOI 10.1007/s00198-011-1528-y
Skeletal mineralization defects in adulthypophosphatasia—a clinical and histological analysis
F. Barvencik & F. Timo Beil & M. Gebauer & B. Busse &T. Koehne & S. Seitz & J. Zustin & P. Pogoda & T. Schinke &M. Amling
Received: 14 April 2010 / Accepted: 3 January 2011
# International Osteoporosis Foundation and National Osteoporosis Foundation 2011
ALPL gene, encoding tissue non-specific alkaline phospha-
Summary Histomorphometry and quantitative backscat-
tase. While it is commonly accepted that the increased fracture
tered electron microscopy of iliac crest biopsies from
risk of the patients is the consequence of osteomalacia, there
patients with adult hypophosphatasia not only confirmed
are only few studies describing a complete histomorphometric
the expected enrichment of non-mineralized osteoid, but
analysis of bone biopsies from affected individuals. There-
also demonstrated an altered trabecular microarchitecture,
fore, we analyzed iliac crest biopsies from eight patients and
an increased number of osteoblasts, and an impaired
set them in direct comparison to biopsies from healthy donors
calcium distribution within the mineralized bone matrix.
or from individuals with other types of osteomalacia.
Introduction Adult hypophosphatasia is an inherited disorder
Methods Histomorphometric analysis was performed on non-
of bone metabolism caused by inactivating mutations of the
decalcified sections stained either after von Kossa/van Giesonor with toluidine blue. Bone mineral density distribution wasquantified by backscattered electron microscopy.
Florian Barvencik and Frank Timo Beil contributed equally to thiswork and therefore share first authorship.
Results Besides the well-documented enrichment of non-mineralized bone matrix in individuals suffering from adult
F. Barvencik F. T. Beil M. Gebauer B. Busse T. Koehne
S. Seitz P. Pogoda T. Schinke M. Amling (*)
hypophosphatasia, our histomorphometric analysis revealed
Department of Osteology and Biomechanics,
alterations of the trabecular microarchitecture and an
University Medical Center Hamburg-Eppendorf,
increased number of osteoblasts compared to healthy
Martinistrasse 52,
controls or to individuals with other types of osteomalacia.
20246, Hamburg, Germanye-mail:
[email protected]
Moreover, the analysis of the mineralized bone matrixrevealed significantly decreased calcium content in patients
F. T. Beil S. Seitz
with adult hypophosphatasia.
Department of Orthopaedics,
Conclusions Taken together, our data show that adult
University Medical Center Hamburg-Eppendorf,Hamburg, Germany
hypophosphatasia does not solely result in an enrichmentof osteoid, but also in a considerable degradation of bone
quality, which might contribute to the increased fracture
Materials Sciences Division, Lawrence Berkeley National
risk of the affected individuals.
Laboratory, University of California,Berkeley, USA
Keywords Alkaline phosphatase . Histomorphometry .
Osteoid . Osteomalacia . qBEI
Institute of Bone Pathology,University Medical Center Hamburg-Eppendorf,Hamburg, Germany
P. PogodaDepartment of Trauma-, Hand- and Reconstructive Surgery,
Hypophosphatasia is an inherited disorder primarily char-
University Medical Center Hamburg-Eppendorf,Hamburg, Germany
acterized by defective mineralization of bones and teeth,
which is caused by inactivating mutations of the gene
since there are no larger studies being performed after the
ALPL, encoding the tissue non-specific alkaline phospha-
standardization of bone histomorphometry by the American
tase Depending on the type of mutation and the
Society for Bone and Mineral Research we decided to
mode of inheritance, the disease is highly variable in its
analyze iliac crest biopsies from eight individuals suffering
clinical expression and can be classified into six major
from adult hypophosphatasia using non-decalcified histology,
forms (perinatal lethal, prenatal benign, infantile, child-
which were compared to biopsies from age-matched indi-
hood, adult, and odontohypophosphatasia) [Given the
viduals without skeletal abnormalities or to biopsies from
severe skeletal hypomineralization, the perinatal form either
individuals with other types of osteomalacia. In addition, we
results in stillbirth or in early postnatal lethality [, ].
have applied quantitative backscattered electron microscopy
The clinical course of the infantile form starts in the first
to determine the calcium distribution within the mineralized
6 months of life and is characterized by rickets, craniosy-
bone matrix.
nostosis, nephrocalcinosis, and premature death ]. Afterthe first year, the childhood form of hypophosphatasia ischaracterized by short stature, bone deformities of the lower
extremities, and premature loss of primary teeth , ].
The adult form of hypophosphatasia is mainly characterized
Patients and histological analysis of iliac crest biopsies
by osteomalacia, pseudofractures, and pathologic fracturesafter minimal trauma, as well as by muscle and joint pain
In this study, we included eight adult hypophosphatasia
patients from whom iliac crest bone biopsies were assessed
The clinical diagnosis of hypophosphatasia is, however,
in the bone pathology department of the University Medical
not only based on radiological findings or bone mineral
Center Hamburg-Eppendorf. All patient records were
density (BMD) measurements, but also on biochemical
screened, and the relevant clinical data were extracted.
assays, such as monitoring the serum activities of alkaline
The group included six women and two men between 24
phosphatase, which are reduced in the affected individuals.
and 66 years of age (average age of 47 years, average
In addition, elevated levels of phosphoethanolamine in the
height of 168 cm). Eight age- and sex-matched cases from
urine or of pyridoxal-5-phosphate in the serum are
our iliac crest archive without any bone disease were
supporting the diagnosis of hypophosphatasia, since these
integrated in this study as a control group (six females and
substrates of alkaline phosphatase accumulate in the
two males, average age of 48 years). All of these
absence of the enzyme []. Moreover, the genetic
individuals died in accidents or of acute disease. Reviews
screening methods that are available nowadays have led
of hospital records and autopsy reports were used to
to the identification of more than 221 mutations of the
exclude individuals with cancer, diabetes, glucocorticoid
ALPL gene so far and have helped in the understanding of
medication, or donors on other drugs known to affect
the genetic causes underlying the variability of clinical
calcium metabolism. Moreover, patients with severe liver or
expression [These methods have not only allowed the
kidney disease or periods of longer immobilization before
performance of prenatal diagnostics of the disease, but also
biopsy were excluded. In addition, we have analyzed
helped to confirm the diagnosis of hypophosphatasia ].
biopsies from eight individuals with low circulating 25
However, while there is no doubt about the usefulness of
(OH)-vitamin D levels (8.8±3.3 ng/ml), with an average
genetic diagnosis in the case of hypophosphatasia, the
age of 49 years, and from one patient suffering from X-
availability of these methods certainly explains why
linked hypophosphatemic rickets (male, 45 years old). This
histopathological analyses of bone biopsies from affected
study was carried out according to existing rules and
individuals are not routinely performed anymore.
regulations of the University Medical Center Hamburg-
In fact, the largest histologic study so far, describing
Eppendorf and is in line with the "Hamburg Hospital Law
skeletal pathologies in various forms of hypophosphatasia,
(HmbKHG) April 17th, 1991: Patient Security §12."
was published in 1984 ]. Through the use of non-decalcified sections from iliac crest biopsies, the authors
were able to demonstrate an enrichment of osteoid in mostof the affected individuals, whose degree reflected the
As previously described by Bordier, all samples from the iliac
clinical severity of the disease. From the 17 cases of adult
crest were dissected out 2 cm below and 2 cm behind the crista
hypophosphatasia analyzed in this study, 11 were diagnosed
iliaca superior anterior and fixed overnight at 4°C in 3.7%
with osteomalacia, while five others were characterized by
PBS-buffered formaldehyde []. After dehydration in
decreased bone remodeling. Taken together, these and other
ascending concentrations of ethanol, the samples were
data have helped in the understanding of the skeletal
embedded non-decalcified in methylmethacrylate, and 5-
manifestations of hypophosphatasia [–However,
μm-thick sections were cut using a Microtec rotation
microtome (Techno-Med; Munich, Germany). The sections
with energy dispersive X-ray analysis and qBEI to create a
were stained according to standard protocols after von
calibration curve. A highly linear relationship between
Kossa/van Gieson, Goldner, or with toluidine blue as
backscattered electron imaging gray values and the calcium
described [–].
content (Ca-wt.%) has been reported previously by otherauthors [, The linear dependence (R2=0.98) of
Dual-energy X-ray absorptiometry
the evaluated HA gray values due to the respective calciumconcentration of the HA samples enables the calibration of
BMD was measured by dual-energy X-ray absorptiometry
(DXA) (Lunar Prodigy en Core 2007, GE Healthcare;Madison, WI, USA). Two skeletal areas, the left proximal
Statistical analysis
femur and the lumbar spine (L1–L4), were evaluated byDXA. The patients were scanned according to the manual
All data are presented as means ± SD. Statistical analysis of
supplied by the manufacturer and were placed in the supine
histomorphometric values was compared using unpaired
position. The detected BMD of the projected bone area was
Student's t test. Statistical differences were considered
expressed in grams per square centimeter (g/cm2), and the
significant when p<0.05.
corresponding T-Score was calculated.
Parameters of static histomorphometry were quantified on
Clinical diagnosis of hypophosphatasia
toluidine blue–or von Kossa/van Gieson-stained non-decalcified sections of iliac crest biopsies. Analyses of
Diagnosis of adult hypophosphatasia was based on character-
bone volume (BV/TV), trabecular thickness (Tb.Th),
istic clinical and laboratory findings. Pain and discomfort in
trabecular number (Tb.N), trabecular separation (Tb.Sp),
the thighs and hips were often present, and pseudofractures
osteoid volume (OV/BV), osteoid surface (OS/BS), as well
(Looser Zones), for example of the femur, fibula, or tibia,
as the determination of osteoblast (N.Ob/B.Pm), osteoclast
could be verified on plain X-ray films (Fig. ). Also
number (N.Oc/B.Pm), osteocyte number (Ot.N/B.Ar/mm2)
bone deformities, which occur with this disease, like
and surface indices (Ob.S/BS and Oc.S/BS), mineralized
pathologic alteration of the joint axis (Fig. ) and bowing
bone volume (Md.V/TV), and osteoclasts surface per
of the femur (Fig. ), were documented on radiographs. In
mineralized bone surface (Oc.S/Md.BS) were carried out
addition, we observed pathologic calcium accumulation in
according to the ASBMR standards using the Osteo-
other organs by ultrasound, especially in the kidney (Fig.
Measure histomorphometry system (Osteometrics; Atlanta,
DXA performed in the femur and lumbar spine demonstrated
GA, USA) connected to a Zeiss microscope (Carl Zeiss;
low BMDs in adult hypophosphatasia patients (Fig. ).
Jena, Germany) [We did not perform dynamic
Biochemical analysis of serum, plasma, and urine demon-
histomorphometry in our study.
strated reduced levels of alkaline phosphatase (AP) (27.8±4.5 U/l; normal range, 35–104 U/l), bone-specific alkaline
Bone mineral density distribution measurements
phosphatase (BAP) (5.0±1.4 μg/l; normal range, 6–26 μg/l)
by quantitative backscattered electron imaging
and elevated levels of pyridoxal phosphate (PLP) (41.2±14.4;normal range, 7.5–18.5 μg/l). In contrast, serum levels of
BMD distribution (BMDD) measurements were performed
phosphate, calcium, 25-OH vitamin D3, intact parathyroid
on non-decalcified, coplanar polished, carbon-coated
hormone (PTH), and creatinine were in the normal range,
methylmethacrylate-embedded bone biopsies. The technical
which ruled out the existence of secondary hyperparathy-
application is based on the work of other groups using
roidism (Fig. ). Pregnancy, anemia, hypothyroidosis,
qBEI and has been reported previously –]. The
anorexia, and malnutrition that can also cause decreased
scanning electron microscope (LEO 435 VP; Cambridge,
alkaline phosphatase levels were ruled out by clinical and
England) was operated at 15 kV and 665 pA at a constant
working distance (BSE Detector, type 202, K.E. Develop-ments Ltd.; Cambridge, England). The pixel size amounts
Histological findings
to 3 μm and lies within the recommendation range ofRoschger et al. []. The standardization of the method was
The light microscopic findings of the iliac crest bone
accomplished by the analysis of synthetic hydroxyapatite
biopsies revealed distinct differences between hypophos-
(HA). Seven HA samples with increasing Ca/P ratios (D.O.
phatasia patients and control individuals. The von Kossa/
T. Medical Solutions; Rostock, Germany) were evaluated
van Gieson- or Goldner-stained specimens of the control
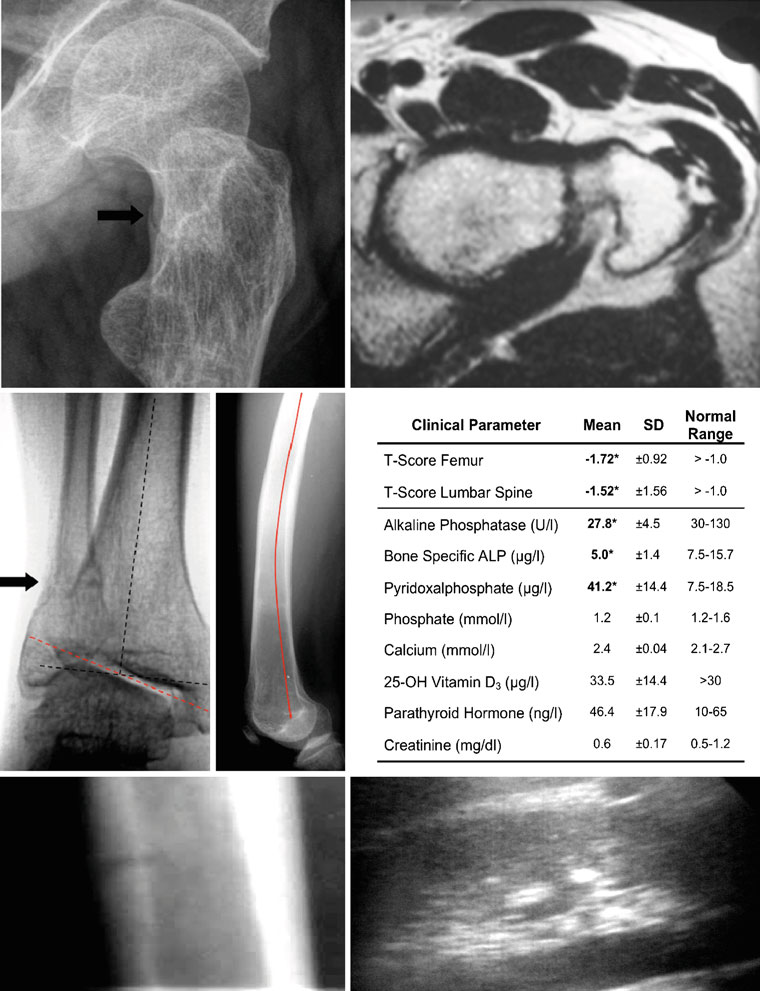
Fig. 1 Typical clinical aspects of adult hypophosphatasia. a X-ray
bowing of the left femur (red line) is clearly visible by X-ray (lateral
(oblique view) and b MRI (axial view) of the left lateral proximal
view). e Results of bone densitometry and biochemical analysis. The
femur of a 32-year-old male patient diagnosed with adult hypophos-
normal ranges of all parameters are given on the right. Pathological
phatasia. A pseudofracture in the femoral neck is indicated by the
abnormalities in the hypophosphatasia patients (n=8) are highlighted
arrow. c X-ray (a.p. view) of the right ankle joint of the same patient
in boldface. f X-ray (lateral view) of the tibia showing a pseudo-
showing also a pseudofracture in the distal fibula. In addition, a
fracture of the tibial shaft (white arrow). g Ultrasound of the kidney
pathologic tilt of the ankle joint axis was observed (dotted red line,
illustrating a calcification spot as an echogenic focus (white arrow)
pathologic axis; dotted black line, anatomic axis). d The pronounced
with posterior acoustic shadowing (black arrow)
group showed a normal orientation and distribution of the
outline of the trabeculae seemed to be regularly formed, but
trabeculae in the cancellous bone, with only thin layers of
the impaired mineralization in hypophosphatasia patients
osteoid (Fig. Compared to the control group, the
resulted in an irregularly formed mineralized part of
iliac crest biopsies of the hypophosphatasia patients were
trabeculae lying underneath the thick osteoid layer
remarkably different with increased osteoid volume
(Fig. In fact, the interface between mineralized bone
(Fig. In the toluidine blue-stained sections, the
and unmineralized osteoid was irregularly shaped in
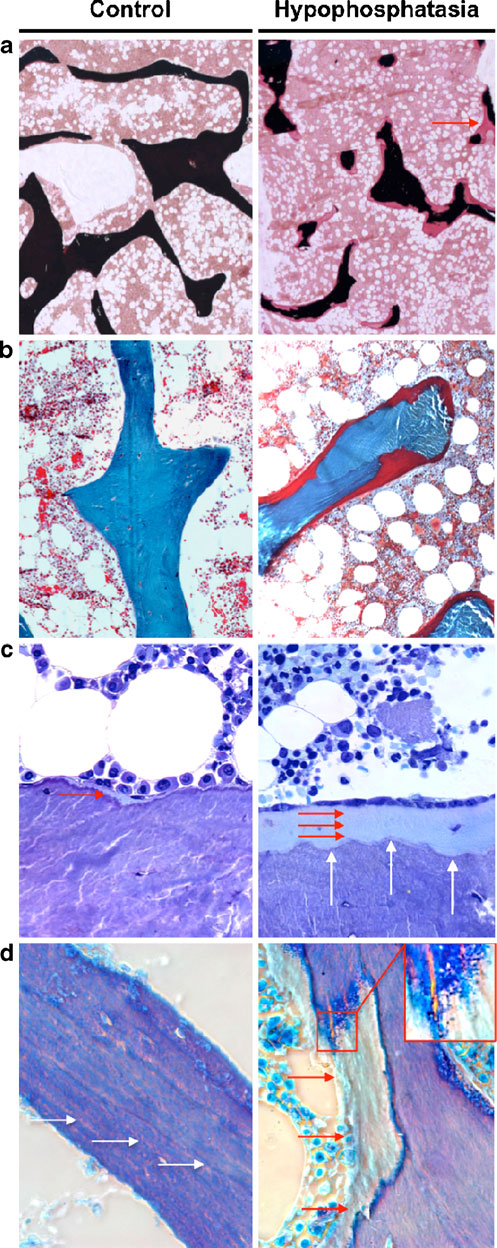
Fig. 2 Non-decalcified histology of iliac crest biopsies from healthyb
individuals and adult hypophosphatasia patients. a von Kossa/vanGieson staining (×25 magnification) and b Goldner staining (×100magnification) showing an accumulation of osteoid (stained in red) inhypophosphatasia patients. The red arrow indicates a site where acomplete trabecule is bridged by osteoid. c Toluidine blue staining(×400 magnification) confirms the existence of thick osteoid layers(red arrows) in hypophosphatasia patients and demonstrated anincreased number of osteoblasts covering these surfaces. The whitearrows indicate scalloped appearance of the cement lines, which werecharacteristic for the hypophosphatasia cases. d Polarized brightfieldmicroscopy reveals an accumulation of osteoid (red arrows) but alsodemonstrates that the lamellar structures observed in control biopsies(white arrows) are impaired in the hypophosphatasia cases. The insertshows basophilic pellets accumulating on the osteoid, which was alsocharacteristic for the sections of the hypophosphatasia patients
sections from hypophosphatasia patients, which resemblesthe findings reported by Balena et al., who has introducedthe term "scalloped cement lines" [
At the cellular level, both osteoclasts and osteoblasts
appeared morphologically similar in the two groups.
However, toluidine-blue staining revealed an increasednumber of osteoblasts in the hypophosphatasia patients,which were orientated in line on the thick layer ofosteoid (Fig. ). Moreover, a polarized microscopic viewrevealed striking differences between the two groups. Infact, the regular structure of bone layers is disrupted inhypophosphatasia patients by areas of unmineralizedosteoid that seem to be randomly distributed (Fig. ).
At the interface of osteoid and mineralized bone, wefurther observed basophilic pellets, which may representclusters of calcium complexes (Fig. Interestingly,these structures did not progress uniformly outward fromthe cement line, thus implying that the deficiency of ALPLrather affects the initiation of mineralization, rather thanits continuation.
To quantify the observed structural changes, we performedhistomorphometry according to the guidelines of theAmerican Society for Bone and Mineral Research (Table ).
We first determined the trabecular bone volume (BV/TV)and found a non-significant increase in the biopsies derivedfrom the hypophosphatasia patients compared to the controlgroup. In addition, we observed a significant increase of thetrabecular number (Tb.N) and a significant decrease oftrabecular separation (Tb.Sp) and thickness (Tb.Th) insections from hypophosphatasia patients. As expected, thebiopsies from hypophosphatasia patients also showed asignificant increase in osteoid volume (OV/BV) and osteoidsurface (OS/BS) compared to biopsies taken from thecontrol group. Therefore, when we determined the miner-alized bone volume per tissue volume (Md.V/TV), therewas no increase in the hypophosphatasia cases.
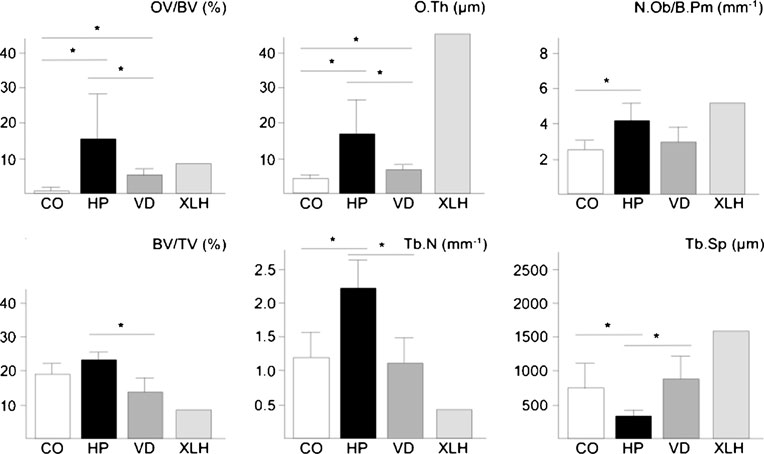
Table 1 Histomorphometric
parameters of the iliac crestbiopsies derived from healthy
donors (control) and adulthypophosphatasia patients (HP)
N.Ob/B.Pm (mm−1)
N.Oc/B.Pm (mm−1)
Shown are the mean values and
standard deviation (SD)
* P values below 0.05 were
N.Oc/Md.BS (mm−1)
considered statistically significant
We next quantified the numbers of osteoblasts, osteo-
To address the question, whether the increased
clasts, and osteocytes. Here we found an increase of
osteoblast number and the structural changes of trabec-
osteoblast number (N.Ob/B.Pm) and surface (Ob.S/BS) in
ular bone are generally observed in cases of osteomala-
the hypophosphatasia patients, although their morphology
cia, we further performed histomorphometry in biopsies
appeared to be normal. In contrast, the number (N.Oc/B.
derived from eight individuals with low circulating 25
Pm) and surface of osteoclasts (Oc.S/BS) were not
(OH)-vitamin D levels and from one patient suffering
significantly different between the two groups, and the
from X-linked hypophosphatemic rickets (Fig. In both
same was the case for the osteocyte number (Ot.N/B.Ar/
cases, we found the expected pathological increases of
mm2). Given the large increase of the osteoid surface in the
osteoid volume and thickness, albeit both parameters were
cases of adult hypophosphatasia, we further quantified the
significantly lower in the cases of vitamin D deficiency
osteoclast surface per mineralized bone surface (Oc.S/MS).
compared to adult hypophosphatasia. Most importantly,
Here we observed a non-significant increase compared to
however, the number of osteoblasts was only elevated in
the control cases, which may explain, at least in part, the
individuals with adult hypophosphatasia, but not in
scalloped pattern of cement lines described above.
individuals with vitamin D deficiency, and the same was
Fig. 3 Histomorphometricanalysis of iliac crest biopsiesderived from control individuals(CO) or from patients with adulthypophosphatasia (HP), vitaminD deficiency (VD) or X-linkedhypophosphatemic rickets(XLH). Bars represent means ±SD, and asterisks indicatestatistically significantdifferences (p<0.05) betweentwo groups (n=8)
the case for the changes in trabecular number and
separation (Fig. ).
Bone mineral density distribution measurementsby quantitative backscattered electron imaging
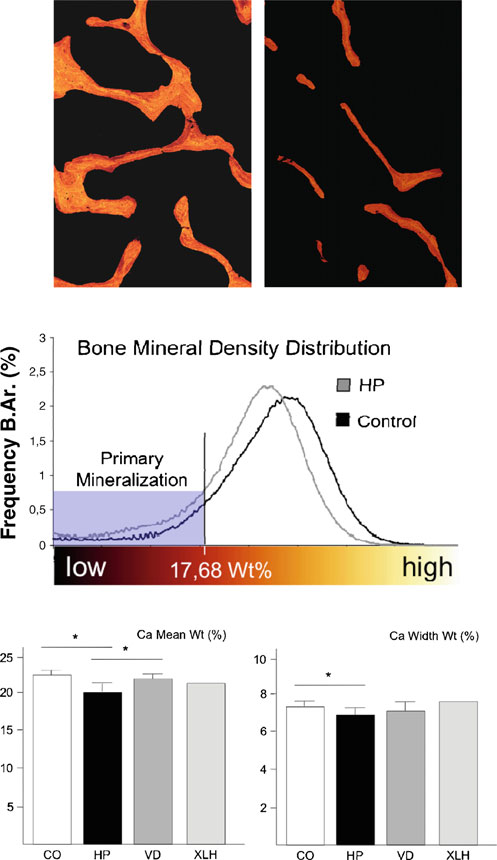
Since the histomorphometric analysis clearly confirmed thatthe major skeletal abnormality associated with an ALPLinactivation is a pathological impairment of matrix miner-alization, we finally addressed the question whether themineralized bone matrix in hypophosphatasia patientscontains the same amount and distribution of calcium,when compared to the control biopsies. This was achievedby measuring the BMDD using quantitative backscattered
electron microscopy. Here we found that the mineraldistribution was indeed markedly impaired in sections fromhypophosphatasia patients, with an increased amount ofbone packets in a low mineralized state (Fig. In thehypophosphatasia sections, we further observed a reductionof bright pixels and a decrease of the mean gray valuecompared to the control cases, which is reflected by asignificantly decreased overall calcium content (Ca meanwt). The significantly lower calcium width (Ca width wt)reflects a less heterogenic structure due to the absence ofhighly mineralized bone packages (Fig. ). Again, weperformed the same measurements for the cases of vitamin
D deficiency, but here we failed to detect a statisticallysignificant difference compared to the control group.
Interestingly, however, the overall calcium content (Camean wt) was decreased in the one case of X-linkedhypophosphatemic rickets, representing the influence ofPHEX inactivation on BMD, which needs to be confirmedin a larger number of affected individuals.
Fig. 4 Measurement of BMDD in non-decalcified bone biopsies fromhypophosphatasia patients and control individuals. a Quantitative
Taken together, our study demonstrates that individuals
backscattered electron images expressed by pseudo-colors (×25magnification). Highly mineralized bone is represented by brightly
suffering from adult hypophosphatasia display specific
colored pixels, whereas lower mineralized bone areas are predominant
skeletal abnormalities, in addition to the previously estab-
in darker colors. Completely unmineralized tissue, such as the bone
lished osteomalacia. These include increased trabecular
marrow, remains black. b The BMDD evaluated by the appropriate
number, decreased trabecular separation, as well as
gray levels characterizes the mineralization profile of the hypophos-phatasia patients (gray graph) and the control cases (black graph).
increased osteoblast number and surface compared to age-
The evaluated histogram revealed an increase of mineralized bone
matched control individuals and compared to individuals
underlying primary mineralization in hypophosphatasia patients
with osteomalacia due to low circulating vitamin D levels.
(mineralized bone beneath 17.68 wt.% Ca). c Quantification of the
Moreover, we were able to show that the calcium content
overall calcium content (Ca mean wt) and calcium width (Ca widthwt) for control individuals (CO) or for patients with adult hypophos-
within the mineralized phase was significantly lower in the
phatasia (HP), vitamin D deficiency (VD), or X-linked hypophospha-
cases of hypophosphatasia, an aspect of the phenotype,
temic rickets (XLH). Bars represent means ± SD, and asterisks
which has not been addressed before. Although we can
indicate statistically significant differences (p<0.05) between two
only speculate whether these previously unrecognized
skeletal abnormalities contribute to the increased fracturerate observed in hypophophatasia patients, we believe thatour data are an important contribution to our understanding
of this disease, especially since there are only few
control and vitamin D deficiency cases by qBEI measurement.
histomorphometric studies published so far , ].
Moreover, both the increased and decreased trabecular
The largest of these studies, involving 17 patients with
separations were specifically observed in the cases of adult
adult hypophosphatasia, has been reported in 1984 ],
hypophosphatasia, and there was also no increased number of
which was 3 years before the standardization of histomor-
osteoblasts in the cases of vitamin D deficiency. Taken
phometric parameters by the American Society for Bone
together, our findings have revealed some previously unrec-
and Mineral Research [However, although there are
ognized skeletal alterations in adult hypophosphatasia
some structural histomorphometric parameters missing in
patients, which are not generally observed in disorders with
this study, the authors have clearly demonstrated an
impaired skeletal mineralization.
accumulation of osteoid as the major abnormality. Thisosteomalacia was especially pronounced in the six individualswith a history of fractures (mean osteoid volume of 27.5%)
The authors thank Ms. Olga Winter for excellent
and less evident in individuals only displaying altered serum
technical assistance in preparing the samples for qualitative and
parameters, which were mostly first-degree relatives of the
above-mentioned individuals (mean osteoid volume of 4.5%).
Conflicts of interest
While there was no consistent change in the number ofosteoblasts observed in this collective, it was interesting thatthe five cases, where no enrichment of osteoid has been
observed, displayed histological features of low bone turn-over, including a decrease of fluorescent labeling following
1. Mornet E (2008) Hypophosphatasia. Best Pract Res Clin
Rheumatol 22:113–127
In this regard, we would like to point out that one major
2. Greenberg CR, Evans JA, McKendry-Smith S, Redekopp S,
Haworth JC, Mulivor R, Chodirker BN (1990) Infantile
weakness of our study is that the patients did not receive
hypophosphatasia: localization within chromosome region
tetracycline, thus excluding the possibility of dynamic
1p36.1-34 and prenatal diagnosis using linked DNA markers.
histomorphometry. However, as in our study, all hypophos-
Am J Hum Genet 46:286–292
phatasia cases were characterized by a pathological accu-
3. Henthorn PS, Raducha M, Fedde KN, Lafferty MA, Whyte MP
(1992) Different missense mutations at the tissue-nonspecific
mulation of osteoid; we believe that it would have been
alkaline phosphatase gene locus in autosomal recessively inherited
difficult to demonstrate low bone turnover here, since in the
forms of mild and severe hypophosphatasia. Proc Natl Acad Sci
case of osteomalacia, one can only observe diffuse
USA 89:9924–9928
tetracycline labeling, which cannot be utilized to determine
4. Henthorn PS, Whyte MP (1992) Missense mutations of the tissue-
nonspecific alkaline phosphatase gene in hypophosphatasia. Clin
the mineral apposition rate. Thus, it is probably most
Chem 38:2501–2505
important that, besides the osteomalacia, we have found
5. Moore CA, Ward JC, Rivas ML, Magill HL, Whyte MP (1990)
altered parameters of trabecular architecture, as well as
Infantile hypophosphatasia: autosomal recessive transmission to
increased numbers of osteoblasts, both of which have not
two related sibships. Am J Med Genet 36:15–22
6. Orimo H, Goseki-Sone M, Sato S, Shimada T (1997) Detection of
been reported for the patients analyzed by Fallon et al.
deletion 1154–1156 hypophosphatasia mutation using TNSALP
However, in one case of infantile hypophosphatasia, similar
exon amplification. Genomics 42:364–366
observations have been made [In addition, our study
7. Orimo H, Hayashi Z, Watanabe A, Hirayama T, Hirayama T,
has demonstrated for the first time that adult hypophospha-
Shimada T (1994) Novel missense and frameshift mutations in thetissue-nonspecific alkaline phosphatase gene in a Japanese patient
tasia is not only characterized by an enrichment of non-
with hypophosphatasia. Hum Mol Genet 3:1683–1684
mineralized osteoid, but also by impaired mineralization of
8. Fauvert D, Brun-Heath I, Lia-Baldini AS, Bellazi L, Taillandier A,
non-osteoid areas, which may contribute to the detrimental
Serre JL, de Mazancourt P, Mornet E (2009) Mild forms of
effects of ALPL inactivation on skeletal stability.
hypophosphatasia mostly result from dominant negative effect ofsevere alleles or from compound heterozygosity for severe and
Albeit interesting, however, our results certainly raise the
moderate alleles. BMC Med Genet 10:51
question whether the observed abnormalities are unique to
9. Whyte MP, Wenkert D, McAlister WH, Mughal MZ, Freemont
hypophosphatasia, or if they are also found in other forms
AJ, Whitehouse R, Baildam EM, Coburn SP, Ryan LM, Mumm
of osteomalacia, such as vitamin D deficiency –or
S (2009) Chronic recurrent multifocal osteomyelitis mimickedin childhood hypophosphatasia. J Bone Miner Res 24:1493–
hypophosphatemic rickets , In an attempt to address
this question, we have so far performed a histomorpho-
10. Whyte MP (1990) Heritable metabolic and dysplastic bone
metric analysis of iliac crest biopsies from eight individuals
diseases. Endocrinol Metab Clin North Am 19:133–173
with low circulating levels of 25(OH)-vitamin D and from
11. Brun-Heath I, Chabrol E, Fox M, Drexler K, Petit C, Taillandier
A, De Mazancourt P, Serre JL, Mornet E (2008) A case of lethal
one individual suffering from X-linked hypophosphatemic
hypophosphatasia providing new insights into the perinatal benign
rickets. While we did observe a low BMDD in the latter
form of hypophosphatasia and expression of the ALPL gene. Clin
case, we found no significant differences between the
Genet 73:245–250
12. Smilari P, Romeo DM, Palazzo P, Meli C, Sorge G (2005)
microarchitecture of the spine, the iliac crest, the femur, and the
Neonatal hypophosphatasia and seizures. A case report. Minerva
calcaneus. J Bone Miner Res 11:36–45
Pediatr 57:319–323
32. Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron
13. Whyte MP (1995) Hypophosphatasia. In: Scriver CR, Beaudet
R, Demay MB (1999) Rescue of the skeletal phenotype of vitamin
AL, Sly WS, Valle D (eds) The metabolic and molecular bases of
D receptor-ablated mice in the setting of normal mineral ion
inherited disease. McGraw-Hill, New York, pp 4095–4112
homeostasis: formal histomorphometric and biomechanical anal-
14. Whyte MP (1994) Hypophosphatasia and the role of alkaline
yses. Endocrinology 140:4982–4987
phosphatase in skeletal mineralization. Endocr Rev 15:439–461
33. Jones SJ, Glorieux FH, Travers R, Boyde A (1999) The
15. Coe JD, Murphy WA, Whyte MP (1986) Management of femoral
microscopic structure of bone in normal children and patients
fractures and pseudofractures in adult hypophosphatasia. J Bone
with osteogenesis imperfecta: a survey using backscattered
Joint Surg Am 68:981–990
electron imaging. Calcif Tissue Int 64:8–17
16. Barvencik F, Gebauer M, Schinke T, Amling M (2008) Case
34. Roschger P, Plenk HJ, Klaushofer K, Eschberger J (1995) A new
report: multiple fractures in a patient with mutations of TWIST1
scanning electron microscopy approach to the quantification of
and TNSALP. Clin Orthop Relat Res 466:990–996
bone mineral distribution: backscattered electron image grey-
17. Reibel A, Maniere MC, Clauss F, Droz D, Alembik Y, Mornet E,
levels correlated to calcium K alpha-line intensities. Scan Microsc
Bloch-Zupan A (2009) Orodental phenotype and genotype
findings in all subtypes of hypophosphatasia. Orphanet J Rare
35. Roschger P, Paschalis EP, Fratzl P, Klaushofer K (2008) Bone
mineralization density distribution in health and disease. Bone
18. Whyte MP (2009) Atypical femoral fractures, bisphosphonates,
and adult hypophosphatasia. J Bone Miner Res 24:1132–1134
36. Skedros JG, Bloebaum RD, Bachus KN, Boyce TM, Constantz B
19. Mornet E (2007) Hypophosphatasia. Orphanet J Rare Dis 2:40
(1993) Influence of mineral content and composition on grayle-
20. Mornet E (2010) The tissue nonspecific alkaline phosphatase gene
vels in backscattered electron images of bone. J Biomed Mater
mutations database. At
. Accessed 12 August 2010
37. Boyde A, Maconnachie E, Reid SA, Delling G, Mundy GR
21. Fallon MD, Teitelbaum SL, Weinstein RS, Goldfischer S, Brown
(1986) Scanning electron microscopy in bone pathology: review
DM, Whyte MP (1984) Hypophosphatasia: clinicopathologic
of methods, potential and applications. Scan Electron Microsc
comparison of the infantile, childhood, and adult forms. Medicine
(Baltimore) 63:12–24
38. Boyde A, Travers R, Glorieux FH, Jones SJ (1999) The
22. Ramage IJ, Howatson AJ, Beattie TJ (1996) Hypophosphatasia. J
mineralization density of iliac crest bone from children with
Clin Pathol 49:682–684
osteogenesis imperfecta. Calcif Tissue Int 64:185–190
23. Ornoy A, Adomian GE, Rimoin DL (1985) Histologic and
39. Roschger P, Fratzl P, Eschberger J, Klaushofer K (1998)
ultrastructural studies on the mineralization process in hypophos-
Validation of quantitative backscattered electron imaging for the
phatasia. Am J Med Genet 22:743–758
measurement of mineral density distribution in human bone
24. Wolff C, Zabransky S (1982) Hypophosphatasia congenita letalis.
biopsies. Bone 23:319–326
Eur J Pediatr 138:197–199
40. Balena R, Shih MS, Parfitt AM (1992) Bone resorption and
25. Anderson HC, Hsu HH, Morris DC, Fedde KN, Whyte MP (1997)
formation on the periosteal envelope of the ilium: a histomor-
Matrix vesicles in osteomalacic hypophosphatasia bone contain
phometric study in healthy women. J Bone Miner Res 7:1475–
apatite-like mineral crystals. Am J Pathol 151:1555–1561
26. Whyte MP (2002) Hypophosphatasia. In: Bilezikian JP, Raisz LG,
41. Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier
Roda GA (eds) Principles of bone biology, 2nd edn. Academic,
S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling
San Diego, pp 1129–1248
M (2010) Bone mineralization defects and vitamin D deficiency:
27. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H,
histomorphometric analysis of iliac crest bone biopsies and
Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry:
circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner
standardization of nomenclature, symbols, and units. Report of the
ASBMR Histomorphometry Nomenclature Committee. J Bone
42. Liberman UA (2007) Vitamin D-resistant diseases. J Bone Miner
Miner Res 2:595–610
Res Suppl 2:105–107
28. Bordier P (1972) Quantitative histology of metabolic bone
43. Koren R (2006) Vitamin D receptor defects: the story of
disease. J Clin Endocrinol Metab 1:197–215
hereditary resistance to vitamin D. Pediatr Endocrinol Rev Suppl
29. Amling M, Hahn M, Wening VJ, Grote HJ, Delling G (1994) The
microarchitecture of the axis as the predisposing factor for fracture
44. Beck-Nielsen SS, Brusgaard K, Rasmussen LM, Brixen K, Brock-
of the base of the odontoid process. A histomorphometric analysis
Jacobsen B, Poulsen MR, Vestergaard P, Ralston SH, Albagha
of twenty-two autopsy specimens. J Bone Joint Surg Am
OM, Poulsen S, Haubek D, Gjørup H, Hintze H, Andersen MG,
Heickendorff L, Hjelmborg J, Gram J (2010) Phenotype presen-
30. Amling M, Grote HJ, Posl M, Hahn M, Delling G (1994)
tation of hypophosphatemic rickets in adults. Calcif Tissue Int
Polyostotic heterogeneity of the spine in osteoporosis. Quantita-
tive analysis and three-dimensional morphology. Bone Miner
45. Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ (2010)
Treatment of X-linked hypophosphatemia with calcitriol and
31. Amling M, Herden S, Posl M, Hahn M, Ritzel H, Delling G
phosphate increases circulating fibroblast growth factor 23
(1996) Heterogeneity of the skeleton: comparison of the trabecular
concentrations. J Clin Endocrinol Metab 95:1846–1850
Source: http://hppsource.jp/assets/Barvencik_2011.pdf
Methods of inducing 2.1 Sweeping (stripping) the membranes 3.1 Amniotomy used alone 3.2 Amniotomy with oxytocic drugs versus amniotomy 3.3 Amniotomy with oxytocic drugs versus oxytocic 4.1 Routes and methods of administration 4.2 Hazards of oxytocin administration 5.2 Prostaglandin E2 versus prostaglandin F 5.3 Routes and methods of administration 5.4 Hazards of prostaglandin E2 administration
Einstufungstest (B2): 1. Leseverstehen Lesen Sie zuerst die 10 Überschriften. Lesen Sie dann die fünf Texte und entscheiden Sie, welcher Text (1–5) am besten zu welcher Überschrift (a–j) passt. Tragen Sie Ihre Lösungen in den Antwortbogen bei den Aufgaben 1–5 ein. Neue Richtlinie für Zertifizierung von Medikamenten für Kinder




