Microsoft word - manual of operations v1 9.doc
MANUAL OF OPERATIONS
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
Table of Contents
Topic . Section number
Introduction .I
Key SPS3 Contacts . II
Study Overview . III
Screening and Recruitment . IV
Eligibility . V
MRI in SPS3 . VI
Intracranial Arterial Imaging . VII
Measuring Blood Pressure . VIII
Characterization of Hypertension . IX
Study Entry/Randomization . X
Management of Blood Pressures . XI
Study Follow-Up . XII
Event Documentation and Processing . XIII
Premature Study Termination . XIV
Common Antihypertensive Medications . XV
Management Guidelines for Other Cerebrovascular Risk Factors . XVI
Sample Consent Form and Participant Materials . XVII
SPS3 Study Medications . XVIII
Completing SPS3 Forms . XIX
SPS3 Data Forms and Line-by-lines . XX
Data Transfer and Monitoring . XXI
Site Visits . XXII
Site payments . XXIII
Additional Training Materials .
A. How-to-get started . XXIV B. Neuropsych Examiners: Cognitive Battery Administration/Instructions . XXV C. Randomization and Data Transfer System . XXVI D. Use of Colin Automated BP Device . XXVII E. Use of Ambulatory BP Device . XXVIII F. Sending Imaging files on CD . XXIX G. Substudies . XXX
Table of Contents- 03/17/11
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
Current Date of Each Section
Section Number
Section Date
II. Key SPS3 Contacts
III. Study Overview
IV. Screening and Recruitment
VII. Intracranial Arterial Imaging
VIII. Measuring Blood Pressure
IX. Characterization of Hypertension
X. Study Entry/Randomization
XI. Management of Blood Pressures
XII. Study Follow-up
XIII. Event Documentation and Processing
XIV. Premature Study Termination
XV. Common Antihypertensive Medications
XVI. Management Guidelines for Cerebrovascular Risk
Factors XVII. Sample Consent Form and Participant Materials
XVIII. SPS3 Study Medications
XIX. Completing SPS3 Forms
See Table in Section
20 for Form/Line-by-
XX. SPS3 Data Forms and Instructions
Line dates and versions
XXI. Data Transfer and Monitoring
XXII. Site Visits
XXIII. Site Payments
XXIV. Getting Started
XXV. Cognitive Battery/Administration
XXVI. Randomization and Data Transfer System
XXVII. Use of Colin Electronic BP Device
XXVIII. Use of Ambulatory BP Device
XXIX. Sending Imaging Files in CD
XXXI. End of Study
Section Dates- 03/17/11
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
I. INTRODUCTION
This is the Secondary Prevention of Small Subcortical Strokes (SPS3)
Manual of
Operations. It contains detailed "how-to-do-it" information, including where to look for
eligible patients, how to determine eligibility, what laboratory and other data are needed
for enrollment and at each follow-up, how to randomize patients, when and how to fill
out the data forms, and how to screen for stroke and study events. It is intended for use
as a handbook of how-to advice on the routine (and sometimes not so routine) aspects
of study operation.
If the answers to your questions are not clear in this manual or the protocol, call or email
us (email inquires will be answered on the same working day). Don't guess about how
to do it, contact us. It is important to do it right, and if you have an unanswered
question, probably others have the same question and will need the answer too.
SPS3 MANUAL OF OPERATIONS MOTTO:
Don't Guess - - Ask
Get it right!
ManOp section I - 04/15/03
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
II. KEY SPS3 CONTACTS
SPS3 Coordinating Center
SPS3 Statistical Center
S169 2211 Wesbrook Mall
1530 3rd Avenue South
Vancouver, BC, V6T 2B5
Phone: (604) 822-1789
Birmingham, AL 35294-0022
Toll free: (877) 633-3612 Phone:
Toll free: (866) 304-5010
WEB site: www.sps3.org
Events Coordinator:
Medical Safety Monitor:
Barney Stern, MD
Axio Research Corporation
University of Maryland
2601 - 4th Avenue, Ste 200
Seattle WA 98121
Phone: (410) 328-3372
Phone: (206) 577-0215
E-mail:
[email protected]
E-mail:
[email protected]
Fax: (410) 328-5899
For questions about
patient eligibility, follow-up, events, data form completion,
patient management issues, drug supplies, etc:
Marie-France Benavente, RN, BScN, North American SPS3 Sites Study Coordinator (UBC)
Voice: (604) 822-1789 or (877) 633-3612
FAX: (604) 822-6698
Ana Roldan, MD, Latin American SPS3 Sites Study Coordinator (UBC)
Voice: (604) 822-1789 or (877) 633-3612
[email protected]
FAX: (604) 822-6698
Oscar Benavente, MD (UBC)
Voice: (604) 822-1789 or (877) 633-3612
[email protected]
Robert Hart, MD (UTHSCSA)
Voice: (210) 450-0530 or (877) 633-3612
[email protected]
For questions about
budget, IRB issues, administrative matters:
Camelia Robu, SPS3 Administrator (UBC)
Voice: (604) 822-1789 or (877) 633-3612
[email protected]
For questions about
payment invoices, SPS3 equipment (Colin electronic BP
monitor, Spaclebs ambulatory monitor), Eduardo Martinez, SPS3 Data Manager (UTHSCSA)
Voice: (210) 450-0528 or (877) 633-3612
ManOp section II – 02/01/10
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
For
physician consultation about hypertension management:
Pablo Pergola, MD, Coordinating Center Hypertension Expert
Voice:
[email protected]
For questions about
drugs, drug interactions, side-effects:
Robert Talbert, PharmD
Voice: (210) 567-8318
[email protected]
For questions about
drug shipments:
Dave Hunt, Robert Ringer, VA Cooperative Studies Central Pharmacy
Voice:
For questions about
sending neuroimaging files, CDs or MRI reprints:
Ana Roldan (UBC)
Voice: (604) 822-1789 or (877) 633-3612
[email protected]
For questions about
event materials:
Elaine Nasco, SPS3 Events Coordinator
Voice:
For technical questions about
entering data forms and the database:
Lisa Irby, Assistant Data Manager (UAB)
Voice:
[email protected];
[email protected]
For
patient unblinding: (contact Benavente, 778-938-0093
/Hart 210-235-0972 first, -> DDC)
Dave Hunt or Robert Ringer /DDC: (505) 248-3203/ after hours follow the instructions in the message.
For questions about
neuropsych testing:
Lesly
Voice: (604) 822-1789 or (877) 633-3612
[email protected]
For any other problem which is not satisfactorily resolved:
Oscar Benavente, MD (UBC) or Robert Hart, MD (UTHSCSA)
Voice: (604) 822-1789 or (877) 633-3612
[email protected];
[email protected]
ManOp section II – 02/01/10
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
III. STUDY OVERVIEW
Small subcortical (a.k.a. lacunar) strokes comprise about 25% of brain infarcts, occur at
a younger mean age than other common stroke types, and are harbingers of vascular
dementia. Most are due to intrinsic disease of the small, penetrating cerebral arteries.
Despite their frequency and importance, little is known about the natural history and
secondary prevention relative to other stroke subtypes (see background, SPS3 Study
Protocol for more information).
The overall aim of the Secondary Prevention of Small Subcortical Strokes (SPS3)
project is to learn how to reduce recurrent stroke, other major vascular events and
cognitive decline among survivors of S3/subcortical stroke. Randomized comparisons
of two antiplatelet regimens (clopidogrel plus aspirin vs. aspirin alone) and of "usual" vs.
"intensive" blood pressure lowering will be combined with efforts to identify high-risk
groups. This project also focuses on subcortical vascular dementia and ethnic
The main objectives of SPS3 are:
1. To compare the value of clopidogrel plus aspirin vs. aspirin alone for prevention of
recurrent stroke and cognitive decline.
2. To compare the effects of "usual" vs. "intensive" blood pressure lowering in patients
with recent S3 on recurrent stroke, cognitive decline, and quality of life.
3. To identify and characterize risk factors for stroke recurrence and cognitive decline
in patients with recent S3, including ethnicity, and nocturnal/diurnal blood pressure
To be eligible for SPS3, patients must be at least 30 years of age and have a
clinical
diagnosis of a small subcortical infarct or subcortical TIA with corresponding lesion on
DWI occurring within 6 months of randomization. Clinical diagnosis must be confirmed
by MRI, demonstrating a corresponding small subcortical stroke (see section VI), or
ManOp section III – 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
multiple hemispheric S3 in the absence of a cortical infarct, and absence of large
cortical and subcortical stroke. Patients whose clinical presentation is a sub-cortical TIA
must have a corresponding lesion on DWI. In order to collect participants likely to have
cerebral small artery disease as the underlying cerebrovascular disorder, in addition to
these MRI features, patients with cervical carotid atherosclerosis causing >50%
stenosis or with a major cardioembolic risk source are not eligible.
The rationale for including those with TIA with a corresponding lesion on DWI is that this
group is felt to have risk of stroke that is similar to that of patients with minor stroke. A
population-based prospective cohort study found the early risk of stroke after TIA at 30
and 90 days was 12% and 17% respectively. For patients with minor stroke, the risk
during of the corresponding time intervals was 15% and 18%. In addition, the presence
of abnormalities on DWI for TIA patients was found to be an independent predictor of
The study consists of two randomized trials: All participants will be randomized to
receive antiplatelet therapy and all participants will be randomized to a target level of
blood pressure control. Participants will be randomized using a computer-generated
sequence that is blocked from previewing, with participants having an equal chance to
receive each antiplatelet therapy and to be assigned to each target level of blood
pressure control.
The study will be carried out at approximately 70 clinical sites across the US, Canada,
Spain, Mexico, and South America. Following completion of the enrollment phase of
SPS3 (no later than April, 2011), all patients will be followed for an additional one year
to a common end-study date (no later than April, 2012). Those participants who reach
the originally planned 4.5 years of follow-up (this was the original projected follow-up
and was stated as such on most clinical site consent forms) will be invited to re-consent
to be followed to the common end-study date.
ManOp section III – 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
An MRI and intracranial arterial imaging are required at study entry, and reprints/image
files on CD sent to the Coordinating Center (CC). If an MRI or imaging is not done as
part of routine care and the individual meets all other entry criteria, contact the CC to
discuss an SPS3-funded MRI and MRA. Other MRIs will be performed if a participant
suffers a stroke or TIA or meets the criteria for major cognitive decline.
Neuropsychological testing will be performed at study entry and annual follow-ups.
Participants will be seen in-clinic at least monthly for the first 3 months and then every
three months during the course of the trial to encourage compliance, to assess for side-
effects, to measure blood pressure, and to detect events. Patients will be seen for
additional in-clinic visits as needed to achieve and maintain blood pressure targets.
Randomization of eligible patients into SPS3 and entry of SPS3 data forms from each
clinical site will be via a web-based data management system developed by the
Statistical Center at the University of Alabama (Birmingham). Reprints/image files on
CD of MRI imaging will be sent to the Coordinating Center. Medications for SPS3
participants – the antiplatelet agents
and antihypertensive meds - will be
SPS3 Organizational Overview
distributed by the VA Cooperative
Clinical Sites (CS)
Studies Pharmacy (Albuquerque).
Center (CC)
The SPS3 Coordinating Center will
take a very hands-on role in day-to-
Statistical
Center (SC)
day management and is located at
the University of British Columbia in
Vancouver, Canada. In addition to all
D SM B Committee
Medical Safety
Steer ing Com mittee
Publications Com mittee
protocol matters and participant-
related issues, administrative, contractual, and budgetary aspects are handled at the
Coordinating Center.
As we begin to recruit and enroll patients, you should keep in mind three key points
about the design of the study:
ManOp section III – 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
1. Once a patient has been randomized, he or she should be kept on the assigned
therapy if at all possible, considering patient safety.
2. Every living randomized patient, whether they are receiving active therapy or not,
must be followed for the duration of the study.
3. It is worse to enter a patient who drops out or who is non-compliant than never to
have entered the patient at all.
A general outline of activities is diagrammed on the next page. The remainder of this
manual describes "how to do it" in detail.
ManOp section III – 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
ManOp section III – 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
IV. SCREENING AND RECRUITMENT
A. Where to Find Patients
Recruitment is always much harder than expected. What works best will be different at
each clinical center. Based on estimated numbers of stroke patients at SPS3 clinical
sites, there should be at least 50 eligible patients per year at most SPS3 sites (average
is 80). You must set up a system to find them, and this will vary from site-to-site.
Further, remember that SPS3 is targeting recruitment of
Hispanic Americans, and this
should be kept in mind as you search for participants.
Potential sources of patients include:
• Regular review of hospital admissions with stroke. • Screening of CT/MRIs for S3/subcortical TIA of recent onset (not asymptomatic
• Screening of outpatient consultations of large neurology groups. • Soliciting referrals from non-study affiliated physicians, especially neurologists. • Media
From experience in the SPS3 pilot study, identifying patients admitted to the hospital for
acute stroke was by far the best source of participants. You cannot simply put up a sign
and wait to be called (physician referrals are notoriously poor source of patients), but
rather you need to take an active role in screening. At some hospitals, patients with
non-disabling S3/subcortical TIA, particularly if sensory, may not be routinely admitted
to the hospital, and so screening emergency center logbooks may be important.
Because SPS3 participants will be followed closely with good blood pressure
management, non-SPS3 affiliated neurologists may be quite willing to refer their
patients to the study, but you can't count on them to call you: after obtaining permission,
go to their offices and screen their recent consultations, then get permission to
approach possible candidates.
Given the large numbers of patients with S3, you should be able to achieve the
recruitment goal with sufficient organization and effort and with the support of local
ManOp section IV - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
neurologists. This latter aspect may require the SPS3 physicians to present the study to
colleagues, to convince them to be supportive.
The Health Insurance Portability and Accountability Act (HIPAA) may impact on the
process by which American sites recruit patients to participate in SPS3. In preparation
for screening patients for SPS3, it is important that you inform yourself of the HIPAA
regulations and any implications these regulations have on screening and recruiting
procedures at your individual site. Sites in Canada, Mexico, South America, and Spain
should similarly ensure that they are compliant with both federal legislation and where
applicable, provincial legislation. The governing act in Canada is the Personal
Information Protection and Electronic Documents Act – PIPEDA.
B. Efficient Screening
Screening begins at the time of initial identification of the potential subject and ends as
soon as an exclusion criterion is identified or the patient is deemed eligible for the study.
It makes sense, therefore, to examine the most readily available criteria first and, if the
patient is still eligible, to then move on to assess for the other exclusion criteria. If a
physician or nurse refers a potential patient, he/she may be able to provide information
immediately that will indicate an excluding condition. For example, the patient may be
known to have had a recent subcortical stroke and have atrial fibrillation. In such a
circumstance, you should simply complete the Screening - 01 Form, checking "major
cardioembolic source of stroke" and any other known medical exclusion, and go on to
the next potential patient.
Record all medical reasons for exclusion known to you at the
time of the first medical exclusion – but don't waste energy tracking down more reasons
for exclusion once you are aware of at least one.
All patients excluded from SPS3 require only the completion of the Screening Form.
Again, record whatever you know at the time when it is clear that the patient will be
ManOp section IV - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
C. Who to Screen
All patients with a clinical diagnosis of a subcortical stroke within the past 6 months with
neuroimaging data (CT or MRI) available should be screened systematically and a
screening form completed. Additionally all patients with sub-cortical TIA in the past 6
months and corresponding lesion on DWI should also be screened for eligibility. As part
of usual clinical care (i.e., not paid by the SPS3 study), transthoracic or
transesophageal echocardiogram, cervical carotid imaging, EKG, CBC, BUN,
creatinine, INR, lipids, glucose, and HbA1C (if diabetic) should have been done. Based
on patient's history, neurological examination and supplementary tests, eligibility will
initially be assessed. If the patient fulfills all inclusion criteria with no apparent exclusion
criteria, he/she will be invited to participate in the study. If the individual agrees to
participation, the CT does not show exclusion criteria, and an MRI was not done as part
of routine stroke care, contact the CC to discuss an SPS3-funded MRI. If the clinical
syndrome is compatible with a subcortical stroke but the neuroimaging does not show
subcortical strokes compatible with SPS3 entry, still complete the screening form and
D. General Principles of Recruitment
Patients who are initially considered to be eligible will be interviewed by a study
investigator and the nature and purpose of the study explained. A patient information
booklet will be provided to supplement the verbal explanation. Patients will be required
to grant informed consent for further participation. The patient information booklet is
designed to explain the study and potential adverse reactions to study medications in
Informed consent should be obtained only when study investigator is reasonably sure
that there are no medical exclusions and that the patient is likely to be compliant with
study procedures. There is a major difference between obtaining informed consent and
randomization: all
randomized patients will be followed and counted in our results. A
patient is not in the study until the point of randomization, which will occur some time
ManOp section IV - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
after informed consent is obtained. A minority of participants will not have had a clinical
MRI prior to SPS3. In these patients, informed consent will be obtained and after
approval from the principal investigator at the CC, an SPS3-funded MRI will be
obtained. If the SPS3-funded MRI reveals an unexpected cortical infarct (an exclusion
criteria) or other reason for exclusion, then the patient will not be randomized (please
send images to CC for payment).
Never stop or change any therapy in a patient who is about to enter the SPS3 study
without first seeking permission of the patient's personal physician (typically for poorly
controlled blood pressure). When a potential participant has been given aspirin,
clopidogrel, dipyridamole, or other antiplatelet agent for secondary stroke prevention,
your SPS3 neurology PI will discuss the situation with the patient and primary care
physician. Documentation that the patient's personal physician agrees should appear in
the medical record.
Education of non-study physicians at your medical institution and in the community
about the SPS3 study is of paramount importance for recruiting. The literature indicates
that non-study physicians are a notoriously poor source of patient referrals for research
studies; furthermore, these physicians can block your recruiting efforts if they are not
supportive of the study. There is no substitute for the time and energy of the research
nurse and the commitment of physician investigators.
E. Approaching a Patient about Joining SPS3 and Obtaining Informed Consent
Once you have established that a patient had a clinical S3 or sub-cortical TIA with
corresponding lesion on DWI within 6 months, screen to make sure that the patient
does not have any of the medical exclusions. As described above, at any point in the
screening process, if you find that the patient is medically ineligible, you need only
check that exclusion criteria on the Screening Form, enter the form, and you are
finished with this particular patient. The exclusion criteria are not prioritized. Use any
readily available information to complete this form, but record all reasons for exclusion
ManOp section IV - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
that you know. This screening form will be periodically analyzed to help us consider
protocol modifications and to monitor the vigor of screening activity at your center.
If there are no obvious medical exclusions, the next step is to contact the patient.
Patients may be contacted directly at the hospital before they are discharged. You can
give them information about the SPS3 study (verbal information, brochure, etc,) and
make an appointment for subsequent outpatient evaluation and blood pressure
measurement. A brief interview with the patient may be enough to determine the
patient's eligibility. At this point, you are actively seeking medical reasons for exclusion.
The more personal (and personable) the initial contact, the more likely you are to enroll
eligible patients. Be sure to identify yourself and why you are there (or calling) clearly
and immediately. As you explain the study and its goals to the patient, encourage him or
her to ask questions and express concerns (and then make sure you answer them).
Many patients or their families will have notions about the legitimacy of medical
research. If they feel that your primary focus is simply to get patients into the study
rather than genuine concern about that particular patient's health and well-being, even if
you manage to enroll the patient, he or she is not likely to stay in the study. Be
prepared to answer questions such as "Why should I be in your study?" and "If it was
your mother/father, what would you do?" If you are not too pushy, don't overwhelm the
patient with too much information at first, and allow the patient and family time and
opportunity to resolve doubts and concerns, you will establish relationships which will
strengthen the patient's commitment to the study. If a family member or friend is
important in the decision to join SPS3, identify this person early.
Face to face contact with patients is the most desirable setting for recruitment, even if it
is just a quick visit to the hospital room or clinic to introduce yourself. Ask them if they
would like to hear more about the study, and drop off a patient manual. If a visit or
phone contact is not possible, you may write to patients inquiring about their potential
interest. In these cases a prompt reply to their response is essential.
ManOp section IV - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
At the same time that we are encouraging patients to join the study, we must avoid
entering patients who may initially be enthusiastic but who will later withdraw for
nonmedical reasons. Things that are helpful in this regard include:
1. Education and generation of enthusiasm in the patient's family or friends who
are influential in health advice.
2. Permission and support of the patient's personal physician is crucial.
3. Having the patient and spouse/family come to at least two clinic visits before
randomization, to demonstrate their sustained interest and commitment.
It is critical to choose our patients carefully, walking the fine line between successful
recruitment and avoidance of withdrawals.
Remember to choose your patients wisely… It is worse to enter a patient who
drops out or who is non-compliant than never to have entered the patient at all.
F. Informed Consent
The process of obtaining informed consent begins the moment you start explaining the
study to the patient. Before a patient ever sees the consent form he or she should be
thoroughly familiar with the study goals, what will be expected, and what risks are
involved. If you are open and straightforward from the start, the consent form should
hold no surprises. While it may be only the patient who ultimately signs the form,
remember that in many ways "consent" also comes from the patient's family members
and caregivers. Take the time to discuss the study with them and encourage them to
be present when the patient signs the form.
The informed consent document is
intimidating: You should review it carefully with your patients and explain it using
language that you have already used in discussing the study.
Once the consent form is signed, don't assume that you no longer need to be
concerned with informed consent. "Informed Consent" is really "informed consenting"
and is an ongoing process throughout the course of the study. Your patients may
ManOp section IV - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
withdraw consent at any time and you MUST honor their request. Establishing good
rapport with your patients from the outset and working to maintain positive relationships
can head off many of these withdrawals. If your patients trust you, they will be much
more willing to come to you with new or old concerns which may surface and give you a
chance to talk with them.
An SPS3 physician must be present and participate in
obtaining informed consent.
ManOp section IV - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
V. ELIGIBILITY
A. Inclusion Criteria - based on TOAST criteria supplemented by required MRI data.
All of the following criteria must be met, and the patient must be randomized within 6
months of the qualifying stroke/TIA with corresponding lesion on DWI.
1. One of the following lacunar clinical syndromes (adapted from Fisher):
a. Pure motor hemiparesis (PMH)
g. PMH with facial sparing
b. Pure sensory stroke
h. PMH with horizontal gaze palsy
i. PMH with contralateral III palsy
j. PMH with contralateral VI palsy
e. Dysarthria-clumsy hand syndrome
k. Cerebellar ataxia with contralateral III palsy
2. Absence of signs or symptoms of cortical dysfunction such as aphasia, apraxia,
agnosia, agraphia, homonymous visual field defect, etc.
3. No ipsilateral cervical carotid stenosis (≥50%) by a reliable imaging modality done in
an approved laboratory within 6 mo of qualifying event, if hemispheric.
4. No major-risk cardioembolic sources requiring anticoagulation or other specific
therapy. Minor-risk cardioembolic sources will be permitted if anticoagulation is not
prescribed by the patient's primary care physician.
Major risk sourcesa:
Minor risk sourcesc:
Mitral valve prolapse + myxomatous changes
Mitral annular calcification
Prosthetic cardiac valves
Patent foramen ovale
d
Recent (< 3 mos transmural MI
LV thrombus, especially if mobile or protruding LV wall abnormalities (without thrombus) Atrial
Calcific aortic stenosis
Non-ischemic dilating cardiomyopathies
b Marantic
aAssociated with a substantial absolute risk of stroke, firmly linked to an embolic mechanism
bRisk varies with the type and severity
cAssociated with a low or uncertain absolute risk of initial or recurrent stroke or are incompletely established as direct embolic source
dMajor risk if associated with pulmonary HTN with sustained right-to-left shunting and venous source of emboli
ManOp section V - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
5. MRI evidence of S3, specifically both A and B:
A. S3 on MRI: at least one of the following four specific criteria
i. Diffusion-weighted imaging lesion < 2.0 cm in size at largest dimension
(including rostro-caudal extent), confirmed by the ADC image, and
corresponding to the clinical syndrome. This criterion must be met when the
qualifying event is a TIA.
ii. Well delineated focal hyperintensity < 2.0 cm in size at largest dimension
(including rostro-caudal extent) on FLAIR or T2 and clearly corresponding to
the clinical syndrome. If other focal hyperintensities are present, contact the
Coordinating Center prior to randomization to discuss the case. The date of
imaging must be within 2 months of date of qualifying stroke. If diffusion-
weighted imaging is negative, this criterion alone is not sufficient for study
iii. Multiple (at least 2) hypointense lesions of size 0.3-1.5 cm at largest
dimension (including rostro-caudal extent) only in the cerebral hemispheres
on FLAIR or T1 in patients whose qualifying event is clinically hemispheric. If
qualifying event is clinically brainstem or cerebellar, this criterion alone is not
sufficient for study entry.
iv. Well delineated hypointense lesion < 1.5 cm in size at largest dimension
(including rostro-caudal extent) on FLAIR or T1 corresponding to the clinical
syndrome. MRI must be done at least 1 month after the qualifying stroke.
B. Absence of cortical stroke and large (> 1.5 cm) subcortical stroke, recent or
Note: "Microbleeds" < 2 mm in diameter detected by diffusion tensor imaging or
other special sequences do not exclude the patient.
ManOp section V - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
B. Exclusion Criteria
To be eligible for entry into the study, the patient must not meet any of the criteria listed
1. Disabling stroke (Modified Rankin Scale ≥4)
2. Previous intracranial hemorrhage (excluding traumatic) or hemorrhagic stroke
3. Age under 30 years
4. High risk of bleeding (e.g. recurrent GI or GU bleeding, active peptic ulcer disease, etc)
5. Anticipated requirement for long-term use of anticoagulants (e.g. recurrent DVT) or
other antiplatelets
6. Prior cortical or retinal stroke (diagnosed either clinically or by neuroimaging), or
prior cortical or retinal TIA
7. Prior ipsilateral carotid endarterectomy or ipsilateral carotid stent
8. Impaired renal function: estimated GFR < 40
9. Intolerance or contraindications to aspirin or clopidogrel (including
thrombocytopenia, prolonged INR)
10. Score on the Folstein MMSE < 24, adjusted for age and education
11. Medical contraindication to MRI
12. Pregnancy or women of child-bearing potential who are not following an effective
method of contraception
13. Geographic or social factors making study participation impractical
14. Unable or unwilling to provide informed consent
15. Unlikely to be compliant with therapy / unwilling to return for frequent clinic visits
16. Concurrent participation in another study with an investigational drug or device that
is likely to interfere with SPS3 participation and/or outcomes.
17. Other likely specific cause of stroke (e.g. dissection, vasculitis, prothrombotic
diathesis, drug abuse)
ManOp section V - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
C. Pre-Randomization Procedures: 5 Requirements
1. Brain MRI - SPS3 MRI criteria for study inclusion are two-fold:
∗ Absence of recent or remote evidence of cortical or large subcortical stroke ∗ Presence of an S3 likely corresponding to the qualifying event in the judgment
of the local neurology principal investigator (required for brainstem qualifying
events and when the qualifying event is a subcortical TIA) or multiple
hemispheric S3 on T-1 or FLAIR images
2. Imaging of the cervical carotid arteries (duplex ultrasonography, MRA, spiral CT,
catheter arteriography) - all patients, not just those with hemispheric S3
3. Electrocardiography and either transthoracic or transesophageal echocardiography
- Either a transthoracic or transesophageal echocardiogram, along with an
electrocardiogram is required to exclude candidates with major-risk cardiogenic
embolic sources. If a patient has a completely normal ECG and no history
suggestive of a cardiac source of stroke, and neither a TTE nor TEE has been done
as part of clinical care, contact Benavente/Hart to discuss eligibility.
4. Laboratory blood work - to assess for potential bleeding diathesis, neutropenia,
and/or thrombocytopenia; to assess baseline renal function and changes influenced
by lowering of BP and antihypertensive medications; and to assess vascular risk
profile. Specific tests include: CBC, electrolytes, glucose, creatinine, BUN, and INR,
fasting lipid profile, and HbA1C (diabetics only).
5. SPS3 Measured Blood Pressures - The SPS3 BP at entry will be the average
reading from two consecutive outpatient visits, the first at least one week after stroke
and at least one week prior to the second. Blood pressure will be measured 3X at
each clinic visit in a standardized manner using the automated Colin electronic BP
D. Intracranial Arterial Imaging
Intracranial arterial imaging is required for all SPS3 participants, but it may be done up
to 1 mo post-randomization (or within 3 mo pre-randomization). If not done as part of
routine clinical care (MRA, spiral CT, or angiogram), SPS3 will fund a non-contrast MRA
ManOp section V - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
of the intracranial arteries (with pre-approval by the CC). Visualization of intracranial
arterial circulation is important to understand and stratify prognosis and response to
treatment.
Identification of intracranial atherosclerotic stenosis does not exclude SPS3
participation.
ManOp section V - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
VI. MRI IN SPS3
A brain MRI is required in the following three situations:
• Prior to randomization to assess eligibility criteria • After a stroke or TIA during the course of the trial • Following a major cognitive decline event during the course of the trial
Most MRIs will be those obtained as part of routine clinical evaluation done for acute
stroke and not paid-for by the SPS3 study. Consequently, the types of available MRI
images will vary between sites, depending on those used for routine clinical imaging.
The "ideal" MRI sequence for SPS3 includes T1, T2, FLAIR, and diffusion-weighted
images. While eligibility will be based on demonstration of S3, the presence, extent and
distribution of white matter abnormalities on FLAIR images (or T2, if FLAIR is not
available) will be assessed centrally as potentially important prognostic variables. The
availability of diffusion-weighted images will be important for meeting MRI eligibility
criteria in some patients (see below).
If required to assess eligibility, the SPS3 study will pay for an MRI that is not justified by
routine clinical care. Discussion with and pre-approval by the Coordinating Center are
required: The SPS3 Neurologist should contact Dr. Benavente or Dr. Hart to discuss
the case. SPS3 can only afford to pay $500 for such studies, well below market rates.
Thus, it will be necessary for you to negotiate a special rate for SPS3-sponsored MRIs.
These MRIs do not need to be interpreted by your local radiologist (saving the cost of
interpretation) and need only consist of T1, T2 and FLAIR sequences (but diffusion-
weighted sequences would be great!). Private, free-standing MRI units sometimes offer
discount rates if done after hours.
Image files on CD (preferable and less costly) or reprints of the essential films (T1, T2,
FLAIR and diffusion-weighted images if available) should be sent by traceable mail to
the Coordinating Center. CDs/reprints need to be sent at least monthly as receipt is
ManOp section VI- 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
part of the criteria for site reimbursement (see section XXIII). In most centers, it will be
much less costly to send image files on a CD, and this is the preferred method. If this is
not possible, it is worthwhile making an arrangement with your imaging center to obtain
high-quality MRI copies for SPS3 participants.
A. SPS3 MRI Criteria for an S3
Criteria for an S3 on MRI are:
A black "hole" between 3-15 mm in its greatest dimension on either T1 or FLAIR images
or
an area of brightness < 20 mm in greatest dimension on diffusion-weighted images
located in the subcortical white matter, basal ganglia, internal capsule, thalamus,
brainstem or subcortical cerebellar hemispheres. Black holes on the lamina cribrosa
cut(s), where Virchow-Robin spaces are typically prominent in elderly, hypertensive
patients, do not count as S3. Consider the rostral-caudal dimension and make sure it
does not exceed the maximal dimension based on number of sequential slices and skip
Focal hyperintensities on T2/proton-weighted images by themselves qualify as S3 if all
of the following criteria are met:
• Corresponds to the clinical event • DWI does not conflict (i.e. not done) • Well-delineated and not one of multiple hyperdense patches
For participants with brainstem/cerebellar S3, a new lesion corresponding to the
qualifying clinical event must be visible on MRI. In this situation, MRI criteria for a new
lesion include any of the following:
• Positive diffusion-weighted image • Enhancement of a lesion on T1/FLAIR imaging • New black hole on T1/FLAIR since prior MRI • Bright lesion on T2/FLAIR that is well-delineated and clearly corresponds to
ManOp section VI- 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
the clinical deficit if diffusion imaging is not available
• Evolution of the lesion on serial MRIs in the weeks after the clinical event.
For participants entering with a subcortical TIA as the qualifying event, a new lesion
corresponding to the qualifying clinical event must be visible on diffusion weighted
B. Entry MRI
Interpretation by the local SPS3-affiliated Neurologist will be used to determine SPS3
eligibility. MRIs will be re-interpreted centrally at the Coordinating Center for secondary
analyses and to give feedback to the local neurologists, but the original interpretation by
the on-site SPS3 Neurologist will stand for the purpose of eligibility. So it's crucial to get
A special problem: size of the MRI lesion is 16 - 20 mm when imaged acutely - Based
on pathological studies, lacunes are traditionally defined as cavitated areas of infarction
of ≤ 1.5 cm in size. It is well known to all who interpret MRIs in acute stroke that the
size of the lesion on diffusion-images, enhanced T1 images, and T1/FLAIR may not
reflect its ultimate size months later. It is felt that the size usually diminishes. There is,
however, limited data to support this hypothesis.
SPS3 participants with acute lesions on MRI measuring between 1.5 and 2.0 cm in
maximum diameter have been undergoing a repeat MRI in 3 to 6 months. The rationale
for having a second MRI was to assess if the acute lesions change in size over time into
smaller cavitated lesions. We performed an analysis on the first 42 repeat MRIs. In
81% of the repeat MRIs the lesion was reduced to ≤1.5 cm and in the remainder, all
were ≤2.0 cm. Based on the results of this analysis, the protocol has been modified to
include participants with lesions up to 2.0 cm in maximum dimensions on MRIs
ManOp section VI- 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
performed during the acute phase without the need for a repeat MRI in 3 to 6 months
after study entry.
For participants with brainstem/cerebellar S3, the protocol states that a new lesion
corresponding to the qualifying clinical event must be visible on MRI. In this situation,
MRI criteria for a new lesion include any of the following:
∗ Positive diffusion-weighted image ∗ Enhancement on T1/FLAIR imaging ∗ New black hole on T1/FLAIR since prior MRI ∗ Bright lesion on T2/FLAIR that is well-delineated and clearly corresponds
to the clinical deficit if diffusion-weighted imaging is not available
∗ Evolution of the lesion on serial MRIs in the weeks after the clinical event.
If you have a potential participant who doesn't fulfill these criteria, call the Coordinating
Center to discuss the case and options for documenting MRI entry criteria.
Particularly during the initial months of SPS3 screening and recruitment, there is
concern that varying interpretation of MRIs by 70+ SPS3 Neurologists may result in the
inclusion of participants who may not meet MRI inclusion criteria when centrally
reinterpreted by neuroradiologists at the Coordinating Center. To minimize this
problem, sites are required to undergo central review of the MRIs of their initial 5
patients prior to enrolling the patient. If all 5 MRIs meet criteria, this step is
discontinued. Secondly, active sites where minor protocol deviations have been
detected on the MRI are also required to send all MRIs for review prior to patient entry
until they have demonstrated knowledge of the criteria (i.e., they have delivered 5 MRIs
that meet criteria).
Irrespective of these steps, please contact the Coordinating
Center regarding any "if-y" MRIs before randomizing the patient.
C. T2 Gradient Imaging Sequences
There is much current interest in detecting old "microbleeds" using special T2 gradient
ManOp section VI- 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
imaging. SPS3 offers the opportunity to correlate their presence with prognosis
(particularly symptomatic intracerebral hemorrhage during antiplatelet therapy) and
response to therapy. However, these pulse sequences are not widely available at
present and not done as part of routine clinical evaluation. If such sequences are
available or can be obtained, it would be great to collect these data in a subset of SPS3
participants. Consideration will be given to a supplement grant proposal is there is
general interest by the SPS3 Investigators.
ManOp section VI- 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
VII. INTRACRANIAL ARTERIAL IMAGING IN SPS3
Visualization of intracranial arterial circulation proximate to study entry is required for
SPS3 participants; however identification of intracranial atherosclerotic stenosis will not
exclude SPS3 participation. SPS3 seeks to include a broad spectrum of patients with
lacunar stroke, for whom there are no established therapies for secondary prevention
beyond antiplatelet agents. Despite the recognized limitations of intracranial MRA, it is
crucial to obtain intracranial imaging on SPS3 participants in order to understand and
stratify prognosis and response to treatment.
Acceptable types of imaging include spiral CT, MRA, and catheter-based contrast
arteriography. Imaging should include the carotid siphon, Circle of Willis, proximal
middle cerebral arteries, and basilar artery. If the SPS3 qualifying stroke involves the
posterior circulation, imaging of vertebral arteries is also required. Note that intracranial
imaging is in addition to cervical carotid imaging to exclude surgically amenable
At most centers, intracranial imaging will be carried-out as part of routine clinical
evaluation of acute ischemic stroke. In some instances when imaging is not justified for
routine clinical care, SPS3 will provide $500 for a noncontrast MRA of the intracranial
arteries (and cervical vertebral arteries if the qualifying stroke involves the posterior
circulation). As with SPS3-funded MRIs, MRAs that are paid for by SPS3 must be pre-
approved by having your SPS3 Neurologist contact the Coordinating Center to discuss
the case. In order to negotiate an MRA for the reduced rate of $500, make it clear that
local interpretation by a radiologist is not required.
Reprints or image files on a CD of all intracranial imaging studies must to be sent to the
Coordinating Center. Receipt is part of the criteria for site reimbursement (see section
ManOp section VII- 06/01/2003
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
VIII. MEASURING BLOOD PRESSURE
The technique is adapted from JNC-VII recommendations.
SPS3 BP MEASUREMENT
1. Measure BP in the morning with morning BP medications held 2. No caffeine or tobacco within prior 60 minutes 3. Sitting position: "Selected" arm relaxed and supported at
level of the heart after 15
minutes of sitting quietly
4. Record the reading using the Colin electronic device 5. The length of bladder of the cuff > 80% of upper arm circumference; the width 40%
of the circumference (recording cuff size)
6. Minimize physical contact during measurement 7. Three readings separated by > 2 minutes 8. Orthostatic (standing) measurements obtained
after sitting BPs are measured 9. If the measured BP is unexpectedly high or low, re-check with a recently calibrated
(preferably mercury) sphygmomanometer
• The temporal effects of anti-HTN meds on BP in relationship to dose vary greatly
between agents (for example, little intra-dose variability for thiazides, a large dose
effect for direct acting vasodilators).
As a general rule, SPS3 BP measurements
should be taken at a time reflecting trough activity relative to the dosing of long-
acting antiHTN medication. It is also optimal to measure BP at about the same
time (within 2 hrs) of day for individual participants, to minimize fluctuations in BP.
To achieve this end, we recommend that SPS3 clinics be held in the morning, and
that patients hold all morning doses of antiHTN medications, whether it is an OD,
BID, or TID dosing. You may need to contact patients the day before clinic to
remind them to hold and bring the morning dose. (If morning visits are not possible
for a patient, measure BP at approximately the same time of day and avoid
measurement within 3 hrs of taking BP lowering meds. A patient seen in the
afternoon needs to take morning doses of antiHTN meds, but hold any noon-
afternoon doses.)
ManOp section VIII - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
In morning clinics, after the BP measurement, ask the patient to take all held OD
medication doses. Also, advise the patient to take the held morning dose of a
BID/TID medication if the next dose is a bedtime dose, the BP is quite elevated, or
the appointment was early morning. Alternatively, have the patient skip the held
morning dose of a BID/TID medication if the appointment is in late morning and the
next dose taken in the afternoon, or if the patient's BP is not too high. (We want to
avoid taking two doses of the BID/TID drug close together and precipitating
hypotension.) Be sure patients bring their antiHTN medications with them to clinic
so that you can supervise taking/skipping the held medications.
• A set of three SPS3 BP measurements for each arm is done at the initial SPS3
measurement and recorded on the Baseline form. All subsequent BP
measurements are done in the "Selected" arm. The right arm is the "Selected" arm
unless the left arm mean systolic BP measures > 10 mmHg than the right arm at
the initial measurement.
• Cuffs that are too short or too narrow may give falsely high readings, so be sure to
use the correct size cuff. Four cuff sizes are available for the Colin BP
measurement device: regular, large, short-wide, and thigh. It is important to
use
the same cuff size when repeating BP measurements over time in an individual
Colin cuff size Bladder
Fits arm circumference
regular blue-black
short-wide blue-red
Each site will receive both the regular and large cuffs with the Colin automated BP
machine. If you find that neither of these cuffs is the correct size for a patient and
ManOp section VIII - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
that you need to use either a short-wide cuff or a thigh cuff, measure the BP using
an available non-SPS3 manual or electronic instrument for which a proper sized
cuff is available for the short term. Contact the Coordinating Center, and the proper
sized Colin cuff will be forwarded to you for subsequent BP measurements.
• To avoid elevating BP in some individuals, minimize physical contact when
measuring the official SPS3 sitting BPs. Rest the patient's arm comfortably on a
table. For standing BPs done later, supporting the patient's arm at heart level often
requires contact - use a supporting adjustable table if possible.
• The Colin automated BP machine is used in SPS3. Review of data from the
literature concerning the Colin devise as well as our preliminary observations in the
SPS3 pilot study support the use of automated electronic BP measurement.
Electronic BP measurement is widely used in clinical practice, and several large
randomized clinical trials (such as HOT) have used high-quality, validated
electronic BP measurements. The inherent day-to-day variability in BP as well as
the intrinsic variability in manual measurement of BP is likely to exceed small,
largely random, differences between carefully measured manual vs. electronic
Electronically measured BPs have the advantage of avoiding any bias in BP
measurement by investigators, seeking to achieve targets. Additionally, it is likely
that inter-observer variability in manual BP measurement in a multicenter trial
involving 100 or more investigators would be comparable to or more than that
achieved with high-quality electronic measurements. Finally, use of electronic BP
measurements is in-line with trends in clinical practice at many large hospitals and
In SPS3, a single type of electronic BP device is used at all sites, to minimize
variability and simplify quality control. For "routine" SPS3 BP determinations, three
ManOp section VIII - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
recordings taken at least 2 minutes apart are made using the electronic device, and
then averaged to determine the mean BP for that visit.
The Colin automated BP machines will be centrally calibrated annually during
•
BP Checks During Home Visits: Because the initial months of SPS3
participation require multiple clinic visits (averaging 4-6 during the first few months),
BP checks by SPS3 personnel at the participant's home may be selectively used to
decrease the burden on the participant (and increase protocol adherence). Post-
randomization BP checks may be occasionally done by the research coordinator or
physician using the standardized protocol. BPs from home visits are identified in
the computerized database for comparison with those obtained from clinic
measurements. If the home visit is carried-out by the research nurse, an SPS3
physician must be contacted via telephone to discuss any elicited side effects and
for verbal orders regarding adjustment of anti-HTN meds.
•
24-hour ambulatory blood pressure monitoring: A Spacelabs 24 hour blood
pressure monitoring device is provided to selected sites to record ambulatory BPs
and to categorize as dippers/nondippers:
1. In each participant, at the selected site, as soon as possible after becoming
stable on antihypertensive medications and achieving target BP
2. In participants who are having difficulty staying within the assigned target, i.e.
when you declare Failure-to-Achieve Assigned Target (FAAT)
3. In normotensive patients
See Instructions for the Ambulatory BP -13 form and Section XXVIII for more detail.
ManOp section VIII - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
IX. CHARACTERIZATION OF HYPERTENSION AT STUDY ENTRY
All SPS3 participants will have their blood pressure (BP) carefully measured on
at least two occasions as outpatients before randomization, with the first SPS3
BP measurement done at least one week after the qualifying stroke. (see below)
Qualifying
Consecutive BP Measurements
Participants will not be randomized (and consequently will not have medications
adjusted to reach the assigned BP target) sooner than two weeks after their qualifying
Characterization of hypertension at study entry is based on two criteria:
1. A definite history of hypertension prior to the qualifying stroke and
currently receiving
medications given to lower blood pressure.
2. The
SPS3 BP at entry: the average reading in the "selected" arm from at least two
consecutive outpatient visits, the first at least one week after the qualifying stroke and
at least one week prior to the second.
A
definite history of hypertension prior to the qualifying stroke
is based on:
• consistent recording of "hx of HTN' in medical records spanning > 1 yr prior to
qualifying stroke
• medical records or clear patient report describing treatment with anti-HTN meds
prior to qualifying stroke
ManOp section IX - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
• medical records prior to qualifying stroke showing elevation of outpatient BPs
sustained spanning > 3 months
• use of one or more meds specifically and unequivocally for BP lowering by medical
records or patient report prior to qualifying stroke
• history of HTN coupled with target organ damage attributed to HTN (e.g., left
ventricular hypertrophy)
The SPS3 BP at entry is based on the average measured BP of six BP measurements
taken during two (2) consecutive outpatient visits, the first at least one week after stroke
and the second at least one week later (see technique above) and done prior to
randomization. An average systolic > 140 mmHg or average diastolic >90 mmHg is
required to meet this criterion for hypertension, regardless of concurrent anti-HTN
SPS3 participants will be considered to have "high-normal" BP if there is no history of
hypertension, and their
SPS3 BP average systolic is 130-139 mmHg or the average
diastolic is 85-89 while on no hypertensive medications.
All patients regardless of hypertension and SPS3 BP at entry will be randomized to
blood pressure treatment target ranges. If a normotensive patient subsequently
becomes hypertensive during follow-up, blood pressure will be managed according to
the assigned blood pressure target. No systematic blood pressure management will be
provided to those participants who are normotensive at baseline and remain
normotensive throughout follow-up.
ManOp section IX - 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
X. STUDY ENTRY/RANDOMIZATION
After a patient is determined to be eligible to participate in SPS3 and written informed
consent has been obtained, there are six steps to complete study entry:
1. Be sure that results of all required tests/procedures are available.
2. Have physician investigator complete necessary forms required prior to
3. Complete other forms necessary at study entry.
4. Randomize the patient into SPS3.
5. Dispense study medications and review excluded meds.
6. Schedule next study visit.
Remember: Once a patient has been randomized, the patient must be
followed, on or off therapy, wherever he/she lives, until the end of the study.
1. Tests/Procedures Required Before Randomization
Results of the following tests/procedures must be available and reviewed prior to
randomization, primarily for the purpose of being certain that the patient meets
eligibility criteria for the study:
¾ Neuroimaging – MRI
¾ ECG – most recent since qualifying stroke
¾ Transthoracic or transesophageal echocardiogram – since qualifying stroke
¾ Cervical carotid imaging – since qualifying stroke: sonography, MRA, spiral CT,
and/or angiography
¾ Lab tests – since qualifying stroke: hemoglobin, hematocrit, platelet count, BUN,
creatinine, INR, sodium, potassium, lipids, glucose, HbA1C (diabetics only, may
be within 6 mo preceding the qualifying stroke)
If any of these tests/procedures have not been done or the results are not available
to you, arrange with the patient's personal physician to obtain them, as appropriate,
ManOp section X - 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
so that the patient is inconvenienced as little as possible, but before randomization.
All are considered part of routine clinical work-up for the acute stroke. If an MRI was
not done as part of the clinical work-up following the qualifying stroke, contact the
principal investigator at the Coordinating Center for permission to obtain an SPS3-
funded MRI prior to randomization.
2. Forms to be Completed Prior to Randomization
All patients will have their medical history taken and undergo a general physical and
neurological examination by the SPS3 neurologist investigator within the four weeks
prior to study entry. Additionally, the Edinburgh Stroke Outcome questions will be
asked, and the modified Rankin Scale and Barthel Index administered.
SPS3 blood pressures measured on two occasions using the SPS3 technique at
least one week after the qualifying stroke and at least one week apart must be done
(see Section VIII above). Hypertension status must be determined prior to
randomization into SPS3.
The following forms must be completed by a study physician prior to randomization
(may be completed within four weeks prior to randomization):
¾ Baseline History – Medical History section
¾ Qualifying Stroke
Additionally, the following forms must be carefully reviewed by a study physician
prior to randomization:
¾ Eligibility/Randomization
The Patient Contact form must also be completed. Since all randomized patients
are followed until the end of the study, it is important to collect contact information
ManOp section X - 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
about family members and friends. You may assure the patient that this confidential
information will only be kept in a file at your site and will be destroyed after the study
3. Other forms to Complete at Study Entry
The following forms must be completed on or prior to date of randomization. They
may be completed at a patient visit within the four weeks prior to the randomization
visit, or sometime during the randomization visit.
¾ Baseline History – non-physician portion of the form
¾ Post-Stroke Disability
¾ Hypertension QOL
¾ Cognitive Scales - to be completed by neuropsychological test administrators
¾ Intracranial Vascular Imaging - complete if IC imaging done, otherwise complete
4. Randomizing the Patient
The decision to randomize a patient is not made lightly. All patients that are
randomized will be followed until the end of SPS3. Randomizing an ineligible patient
or one that will be lost-to-follow-up or withdraw consent is a big mistake, so before
you push the button, be sure that the patient is eligible, has signed the consent form,
and is likely to adhere to the SPS3 protocol until the end of SPS3
. Patients who
have reached 4.5 years of follow-up (as stated on the SPS3 consent form template
and most clinical site consent forms) will be invited to re-consent and be followed
until the common study end date. To avoid a potential selection bias, it is critical that
as many patients as possible agree to be re-consented and continued to be followed
as per protocol.
ManOp section X - 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
Participants are randomized into SPS3 using a password protected Web based
system accessible from a "preloaded" PC with web access (see section XXVI for
details). To randomize a patient, have the completed Patient Contact and
Eligibility/Randomization forms with you at the PC. Be sure that the antiplatelet drug
kits are proximate to the PC, as you will need to select the assigned kit for the
participant. Randomization of the participant into the antiplatelet portion of the trial
and the HTN trial are done concurrently.
About 10-15% of SPS3 patients are anticipated to be normotensive at study entry.
These patients will be randomized into the HTN trial, and if they become
hypertensive during follow-up, their target BP will be that which was assigned at
Access the SPS3 web site by entering your username and password from a
previously "loaded" PC (see section XXVI for details), and then click on the
Randomization box. You will then enter the responses for the series of questions on
the Eligibility/Randomization form leading up to the question of whether you are
ready to randomize the patient, and the patient's acrostic (see next paragraph).
After you have confirmed that you wish to randomize the patient, the patient is
officially considered an SPS3 patient, and a unique id number (corresponding to the
AP therapy kit number) and target BP group assignment for that patient will be
assigned. Print off the Randomization form using the Print Forms option under the
Reports/Labels/Forms option in the data entry system and keep in the patient's
ID numbers have the format ss-ppp-c AAAA, where ss is your site number, ppp is
the patient number at your site, c is a check digit, and AAAA is the patient's acrostic.
Randomization is stratified by hypertension status. This means that patients with
hypertension by SPS3 criteria (see Eligibility/Randomization form) are assigned
patient numbers between 000-099, while normotensive patients are assigned patient
ManOp section X - 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
numbers starting at 100.
The patient acrostic is composed of the first letter of the
patient's first name followed by the first three letters of the patient's last name, e.g.
John Smith has the acrostic JSMI. The patient id number is used on all forms to
uniquely identify a patient and also appears on all antiplatelet study drug kits
dispensed to that patient. For example, patient id 01-011-1 LCHA belongs to a
patient 011 randomized into the hypertension stratum at site 01. The patient's first
initial is L and the first 3 letters of his last name are CHA.
A summary of the randomization process, the study id number assigned, the target
BP assigned, and a schedule of quarterly follow-ups visits are generated and printed
at this time. For additional information, see Sections XII (follow-up) and XXVI
(training materials).
5. Dispensing Study Medications
The antiplatelet study kit that you dispense to the patient has the same study id
number on it (without acrostic) as that assigned to the patient at the time of
randomization. Two bottles are dispensed to the patient: one bottle contains aspirin
and one bottle contains clopidogrel or placebo. Before you dispense each bottle of
study drug, remove the part of the perforated label that contains the patient ID and
place this in the patient file. If the bottle number is not noted on the label, please
write it on the label along with the date dispensed and the patient's acrostic. You can
decide where you wish to place this but please be consistent in its placement so that
it can be easily located. Add the patient's name and your contact information to
each bottle, and instruct the patient to take one tablet daily from each bottle at
approximately the same time of day. Also, review with the patient the list of
excluded medications.
It is very important that the patient take only the antiplatelet
therapy assigned and dispensed to him/her by you, and not others. A list of
excluded medications is included in the participant brochure, which should be
reviewed with the patient. Encourage the patient to contact you about any questions
ManOp section X - 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
Excluded medications:
Non-study aspirin containing medications (regularly)
Non-study clopidogrel
Dipyridamole (Aggrenox)
Warfarin (Coumadin) / Ximelagratran/Other oral anticoagulants
You will also be dispensing antihypertensive medications to the patient as
determined by your hypertension specialist. Again, as common sense dictates,
review the instructions for each medication with the patient, and be sure the patient
understands and knows how to contact you if he/she has any questions.
Make certain that the patients are aware that the SPS3 physicians will be monitoring
and supervising their antihypertensive therapy (and not their primary care physician).
Let patients know that you will regularly contact their primary care physician
regarding their blood pressure status.
Give the participant the SPS3 brochure and wallet card.
Send or fax a letter to their primary care physician (sample provided in Section XVII)
informing the physician of the patient's participation in SPS3 and that SPS3 will
manage blood pressure.
6. Scheduling a Follow-Up Visit
There are two types of follow-up visits to schedule:
1) BP Checks: Schedule these as needed between quarterly follow-up visits
to check the patient's blood pressure and make any necessary
antihypertensive medication changes. All patients (hypertensive and
ManOp section X - 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
normotensive) are required to have a BP check during each of month one
and month two post-randomization.
2) Quarterly follow-ups: Schedule these every 3 months based on date of
randomization into SPS3 (within a 2-week window on either side)
To help you with scheduling follow-up for a patient, print off the follow-up schedule
available from the report menu options on the data entry system (see section XXVI).
All patients need to be scheduled to return in about 1 month for a BP Check.
Patients whose blood pressure is not in target range on date of randomization may
need their antihypertensive medications changed, and then need to return to clinic in
2 weeks (4 weeks if thiazides) for a BP Check.
You will also need to schedule intracranial vascular imaging within 1 mo post-
randomization if it was not already done as part of clinical care. If imaging cannot be
justified as part of routine clinical care, your neuro-PI will need to contact the
Coordinating Center for pre-approval for an SPS3-funded intracranial MRA.
7. Entering Data Forms
Enter and lock the completed data forms into the DE system within one week of
randomization - sooner is better.
8. Mailing to Coordinating Center
Obtain the image files on CD or a reprint of the MRI film and mail it to the
Coordinating Center. If the intracranial vascular imaging has been done, include a
reprint or image files on CD of this as well. You also need to send a copy of the
completed Cognitive Scales form (all 18 pages please including the CLOX drawing
and the handwriting sample) along with the audiotape to the Coordinating Center,
which may go in the same package.
ManOp section X - 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
XI. MANAGEMENT OF BLOOD PRESSURES
Overview
Blood pressure management in SPS3 will be based on national guidelines for treatment
of hypertension, specifically the Joint National Committee Report (currently VIIth report).
SPS3 does
not test specific agents (like the recently reported PROGRESS and ALLHAT
trials), but rather seeks to lower blood pressure according to best available practice for
individual patients, as currently applied in the US.
Hypertension management for SPS3 participants will be supervised by the designated
SPS3-affiliated hypertension specialist at each clinical site, who has special experience
and expertise in management of blood pressure. The local specialist at your site will
make the specific management decisions for individual patients beneath the broad
umbrella of management guidelines based on co-morbidity and side-effects. The use of
specific agents for each SPS3 participant will be recorded during the trial. Blood
pressure management at each clinical site will be reviewed on an ongoing basis by the
Coordinating Center Hypertension Expert and SPS3 Steering Committee regarding
achieving targets, choice of agents (in-line with national guidelines by correlating with
baseline demographic features) and side-effects/complications. The local Hypertension
Specialist will interact regularly with the Coordinating Center Hypertension Expert about
problems with individual participants.
There is ongoing controversy about whether specific types of antihypertensive
medications offer special protection against stroke and/or other vascular events.
Individual SPS3 physicians at clinical sites may well view this controversy differently
and may favor use of specific agents, albeit in accordance with practice guidelines. The
distribution of antihypertensive drug use across clinical sites will be monitored closely
(and individual quirks should average-out!). The SPS3 protocol will be modified if
convincing data become available supporting the use of specific agents (such data
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
would likely influence national guideline recommendations which form the basis of
SPS3 blood pressure management).
It is important to communicate with the participant's primary care physician regarding
SPS3 management of blood pressure. Prior to study entry, the primary care physician
must agree to allow the local SPS3 Hypertension Specialist to supervise the patient's
BP management. The primary care physician and the participant may need to be
reminded periodically that antihypertensive medications will be adjusted only by the
SPS3 team (and not other physicians, except in emergency situations) while he/she is
active in the study. Good control of hypertension by experts is one of the benefits of
SPS3 Study participation, and this should be emphasized to the participant to
encourage commitment to the multiple clinic visits and medication adjustments that are
often required to achieve control.
All SPS3 participants will be instructed and encouraged to pursue lifestyle modifications
for hypertension control (Table below).
Lifestyle Modifications for Hypertension Management
Approx Systolic BP
Reduction, Range
Weight reduction
Maintain normal body weight (BMI, 18.5-24.9)
5-20 mm Hg/10 kg weight loss
Adopt DASH eating plan
Diet rich in fruits, vegetables, and low fat dairy products with a
reduced content of saturated and total fat
Dietary sodium reduction
Reduce dietary sodium intake to <100mEq/L (2.4 g sodium or
6 g sodium chloride)
Physical activity
Engage in regular aerobic physical activity such as brisk
walking (> 30 min/day, most days of week)
Moderation of alcohol
Limit to < 2 drinks per day in most men (i.e.1 oz or 30mL
ethanol; e.g. 24 oz beer, 10 oz wine, or 3 oz 80-proof whiskey)
and < 1 drink per day in women and lighter-weight persons
Stop smoking for overall cardiovascular risk reduction.
From JNC-VII (JAMA 2003;289:2560-75)
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
Antihypertensive Medications
SPS3 antihypertensive drugs are purchased and supplied to each clinical site through
collaboration with the VA Cooperative Studies Program Central Research Pharmacy in
Albuquerque (the SPS3 Drug Distribution Center). At least one drug from each of the
major classes of antihypertensive medications is available, specifically: an ACE
inhibitor (lisinopril), a beta-blocker (atenolol), two long-acting calcium channel
antagonists (felodipine, verapamil), four diuretics (HCTZ, chlorthalidone, furosemide,
spironolactone), two vasodilators (minoxidil, hydralazine), an angiotensin receptor
blocker (candesartan), a central alpha agonist (clonidine), a potassium supplement, and
additional medications on a case-by-case basis. These meds were chosen based on
the JNC-VII recommendations, cost, and strongly favoring ease of dosing for
Treatment of hypertension is good clinical practice, and SPS3 is not obliged to supply
all antihypertensive medications to participants if it is feasible to obtain them through the
participant's health plan. For example, if a patient is treated with thiazide, Altace and
Norvasc prescribed by the primary care physician prior to SPS3 participation and these
medications are deemed appropriate by the local SPS3-affililated hypertension
specialist, they should continue to be obtained through the local health plan, both to
spare the SPS3 medication budget and to allow the participant to continue on the same
medications (it is unlikely that these specific agents will be on the SPS3 formulary).
In other situations, good compliance with antihypertensive drug therapy requires that
these medications be supplied by SPS3 (e.g. medically indigent participants who would
have to pay for drugs out-of-pocket). This decision will be made on a case-by-case
basis by the local SPS3 investigators, with the goals of getting BP controlled,
encouraging participant compliance with the frequent clinic visits and medication
adjustments, and sparing the SPS3 medication budget, when appropriate. To maximize
compliance, blood pressure medications will be available to all participants during the
titration phase and to selected participants as deemed appropriate by the local
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
hypertension expert.
Initial Adjustment of Antihypertensive Drugs
The most recent of the pre-randomization SPS3 BPs will be used to assess whether the
participant is within the assigned target ("usual care" of 130-149 mmHg vs. "intensive" of
<130 mmHg) at time of randomization. (If this SPS3 BP is not at least two weeks post-
qualifying event, have the patient return to the clinic for a BP check at that time.) If the
participant's blood pressure is not in assigned target range, antihypertensive
medications may need to be adjusted by the local hypertension specialist, and the
participant should return in two weeks to measure BP (after four weeks if thiazides are
added, due to the longer interval to see the full BP lowering effects). In some instances
(e.g. the patient had not been taking the medications, concurrent illness), it may be
sensible to observe the patient on the same antihypertensive drugs rather than make
adjustments.
SPS3 participants should return for BP checks and adjustment of
antihypertensive drugs every 2-4 weeks until in-target on two consecutive
measurements. At that point, the participant will be seen quarterly unless additional
medication adjustment is required.
Selection of initial antihypertensive medication, adjustment of dosage, addition of other
medications, etc will be made following the
current JNC guidelines (JNC-VII 2003, see
table below). Titration of doses as well as
No response or side effects
Inadequate response & no side effects
addition of agents follows a stepwise
approach, monitoring carefully for specific
Substitute BP drug
Add second BP drug
side effects or agents or due to lowering of
blood pressure (see Figure). Uncomplicated
Figure: General algorithm for treatment of hypertension
hypertension warrants starting with a low
(adapt to individual patients per JNC guidelines)
dose of a long acting once daily drug
Continue Adding Agents from Other Classes
(diuretic or beta-blocker), and titrating the
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
dose. Low dose combination may also be appropriate. The initial drug choice for
participants with compelling reasons (e.g. diabetes) or specific indications (e.g. African-
American) may be different.
Antihypertensive drugs given to participants for reasons other than blood pressure
control (e.g. beta-blockers after myocardial infarction, ACE inhibitors for diabetic
nephropathy) will not be discontinued in order to achieve the SPS3-assigned BP target.
Drugs to increase BP will not be administered to achieve SPS3-assigned targets.
Participants will be told of their assigned target level at their request, but they should be
encouraged not to undertake extensive home monitoring in most cases and in the event
that they provide you with their home readings, these are not to be entered into the DE
system. Both target ranges are within generally good control, based on control of
hypertension clinics in the U.S.
As you dispense antihypertensive agents to individual SPS3 participants, the type
and dose are recorded on the individual Receiving, Assignment and Destruction records
and in your patient records. Please attach the perforated label from each bottle of
medication to the patient record. On a weekly basis (at least), please enter dispensed
medications into the Drug Distribution web-based inventory and your drug supply will
automatically be replenished by the DDC. If you have an emergency, contact the Study
Coordinator. Needless to say, SPS3 antihypertensive medications should never be
given to nonSPS3 participants. (See section XVIII for additional information about Study
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
JNC-VII ALGORITHM FOR DRUG TREATMENT OF HIGH BLOOD PRESSURE
HTN with Compelling Indications for Use of Specific Agent
4. Angina/coronary risk factors
■ Angiotensin receptor blocker
■ Calcium channel blocker
■ Calcium channel blocker
2. Heart failure
5. Chronic kidney disease
■ ARB ■ Aldosterone antagonist
6. Secondary stroke prevention
JAMA 2003; 289:2560-75
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
SPS3 Blood Pressure Follow-up
SPS3 includes intensive BP management until the target levels are achieved, often
requiring returning to clinic 4-6 times during the first few months for a "BP Check."
(Patients that are not returning to clinic at least monthly for BP checks during the first 6
months ought to be contacted by phone to encourage compliance.) It should be
stressed to participants that good control of BP (which is currently achieved in only
about one-third of hypertensives in the U.S.) is an investment in good health and that it
may take awhile to find the best medicines with the least toxicity to control BP. Close
monitoring and good control of BP by experts is a clear benefit of joining SPS3.
Noncompliance is a major reason for failure to control BP, and this should be explored
by interview and supplemented by pill counts. In selected patients, use of the 24hour
ambulatory BP device may help clarify hypertension management.
After a patient's BP is "in-target" on two consecutive measurements, then the participant
reverts to the quarterly follow-up schedule.
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
SPS3 hypertension treatment algorithm:
SBP ≤
15 mmHg above goal
SBP ≥
15 mmHg above goal
Start 1 drug
Start 2 drugs
Thiazide Diuretic
1 OR Angiotensin converting enzyme
Thiazide Diuretic
1 AND Angiotensin converting
inhibitor
2 (ACEI)
enzyme inhibitor
2 (ACEI)
SBP not at goal 3
SBP not at goal 3
Increase dose
Increase doses
Diuretic and monitor potassium (high potassium diet
Diuretic and monitor potassium (high potassium
diet recommended)
ACEI (lisinopril) up to 40 mg/day
Increase dose ACEI (lisinopril) up to maximal dose (max dose 80 mg/day in single or divided doses)
SBP not at goal 3
SBP not at goal 3
Add a drug (3 drugs)
Add the other drug (2 drugs)
•
If Heart Rate >80 bpm: Beta blocker (BB)
4 or a non-
not in the regimen already
dihydropyridine calcium channel blocker (CCB)
5:
(ACEI or Diuretic, starting dose depends on BP level)
atenolol 25 mg/day
Or verapamil 120 mg/day
•
If Heart Rate <80 bpm: add dihydropyridine CCB
6:
felodipine 5 mg/day
SBP not at goal 3
SBP not at goal 3
Already on 2 drugs at moderate to maximal doses.
Increase dose of 3rd drug
See algorithm for SBP ≥
15 mmHg above goal
• Verapamil up to 360-480 mg/day • Atenolol up to 100 mg/day • Felodipine up to 10 mg/day
SBP not at goal 3
Add 4th drug
• If on atenolol (+ diuretic and ACEI or ARB) add felodipine
• If on verapamil (+ diuretic and ACEI or ARB) add felodipine or hydralazine
7 25-50 mg (BID)
• If on felodipine (+ diuretic and ACEI or ARB) add atenolol
SBP not at goal 3
Titrate 4th drug
• Felodipine up to 10 mg/day • Hydralazine up to 100-150 mg (BID); it can be given every 6-8 h but not recommended due to poor compliance • Atenolol up to 100 mg/day (some prefer to use atenolol 50 BID instead)
SBP not at goal 3
Add 5th drug
• Candesartan 8-32 mg/day (if not already substituted for ACEI in intolerant patients).
• Clonidine8 0.1-0.3 mg BID (
OK if young, avoid if >70 y/o or if HR already <70 bpm, monitor side effects).
• Hydralazine or minoxidil to regimens without them (monitor for edema and tachycardia, MUST be on beta
blockers – or other rate control agents – and diuretics before adding direct vasodilators).
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
Always recommend a low sodium diet. The DASH diet has been proven to
decrease BP in clinical studies
(www.nhlbi.nih.gov/health/public/heart/hbp/dash/). Smoking cessation is
imperative. Limit alcohol consumption to 1 drink per day in women and 2 in
men. Weight loss and exercise programs also need to be started and
maintained for successful BP control.
Algorithm is based mostly on SPS3 formulary.
1. Thiazide diuretic: In patients with normal kidney function start chlorthalidone
6.25 to 12.5 mg per day or hydrochlorothiazide (HCTZ) 12.5 mg/day. Increase
either one to 25 mg per day as needed (HCTZ is approximately 60% as potent as
chlorthalidone). In some cases higher doses can be used (rarely >50mg per day)
but must monitor serum potassium frequently. In patients with kidney failure and
decreased GFR loop diuretics may be used instead.
Always use furosemide at
least twice a day. A useful loop diuretic dosed only daily is torsemide (Demadex,
start at 10-20 mg/day).
2. Angiotensin converting enzyme inhibitors (ACEI): Lisinopril is the drug in the
SPS3 formulary. Many other agents are available (in the USA enalapril, captopril and benazepril are also generic and very affordable). Start lisinopril at 10-20 mg per day and increase to the maximum of 80 mg per day. Some experts prefer to use lisinopril twice a day when in high doses (remember compliance decreases sharply if drugs are used more than daily). Can substitute for candesartan (the angiotensin receptor blocker in the SPS3 formulary) if known intolerance to ACEI. Start at 8mg per day, increase up to 32 mg per day in daily doses. Other good ARBs are available but none generic yet (and thus expensive).
3. Blood pressure not at goal: If compliance is good adjust doses of medications
every 4 weeks. In some cases (except for diuretics) changes can be made more
often (every 2 weeks).
It is better to start drugs in moderate doses (1 or 2
agents) and wait than start very low and make frequent adjustments (because it
usually takes a minimum of 2 wks to see the full effect of most drugs).
4. Beta Blocker: The beta blocker in the SPS3 formulary is atenolol. Depending on
the desired effect start at no more than 25 mg daily (in some cases,like in patients
with kidney failure, 12.5 mg daily could be adequate). Other generic beta
blockers are also adequate. Bisoprolol and short acting metoprolol tartrate
(Lopressor) are generic drugs also. Always use metoprolol tartrate twice a day.
Metoprolol succinate (Toprol XL) is used once a day.
Monitor HR and do not
titrate dose up if resting HR is <60 bpm.
5. Non-DHP Calcium channel blockers (CCB): Verapamil SR (long acting
formulation) is in the SPS3 formulary (the other agent in this class is diltiazem).
These agents are vasodilators but also have negative chronotropic and inotropic
effects.
Always use long acting preparations. Do not use in patients with
known congestive heart failure. Peripheral edema is a common side effect.
Monitor HR and do not titrate dose up if resting HR is <60 bpm. Do not use
in combination with BB as life threatening bradycardia can occur.
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
6. DHP Calcium channel blockers (CCB): Felodipine is the drug in the SPS3
formulary. Start in doses of 5 mg per day and increase as tolerated to 10 mg per
day. Higher doses do not usually decrease BP more than 10 mg/day would. Can
use felodipine as
second line agent after diuretics or ACEI/ARB but do not use
alone as it (like amlodipine and nifedipine) can increase the incidence of
congestive heart failure. Other popular drugs in this class are amlodipine
(Norvasc) and nifedipine (Adalat). These are effective vasodilators. Better
combined with long acting diuretics and ACEI to diminish the peripheral edema
common to these agents. Some peripheral edema may persist despite addition of
diuretics and ACEI, while generally benign, if patient comfort is an issue may
need to discontinue use.
NEVER use short acting nifedipine.
7. Hydralazine: This is an effective vasodilator that can be used very successfully
twice a day (never less). Can be added to regimens that MUST include a diuretic and a rate controlling agent like a BB or non-DHP CCB. I have used it in addition to CCB with success. As with minoxidil must monitor for CHF and volume overload carefully.
8. Clonidine: This is a popular centrally acting alpha blocker.
It is short acting! BP
tends to oscillate rapidly and the rebound effect can be serious if stopped
abruptly. It is better used as a patch every 7 days (it is expensive if not
covered by a drug plan). If a centrally acting drug is needed consider
reserpine at low dose (0.1 to 0.2 mg per day). It takes at least 4 wks to see the effect of this
drug but it can be very effective combined with diuretics.
In difficult to control hypertensive patients BB can be substituted successfully for
labetalol. It is a selective alpha1-adrenergic and nonselective beta-adrenergic
receptor blocker. Labetalol is generic; start at 100 mg twice daily and increase the
dose up to 1200 mg twice a day. Carvedilol another agent in the same class is
typically reserved for patients with CHF (it is expensive).
In elderly patients maximal doses of most agents are typically lower than those
recommended for younger patients.
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
Frequently Asked Questions
Q: If an SPS3 participant who is randomized to the "usual care" group (i.e. 130-149
mmHg) has a measured systolic BP of 126 mmHg on an ACE inhibitor and diuretic,
should antihypertensive therapy be reduced?
Ans: Yes. Either the dose should be reduced or one agent discontinued to bring the
patient into the assigned range (it might be sensible to bring the patient back in two
weeks for a repeat measurement before changing medications). Is this ethical? Yes. If
we were certain that the lower blood pressure range was better for the patient, there
would be no need for the trial. A small percentage of patients will be hypertensive with
an SPS3-measured systolic BP < 130 on a concurrent antihypertensive medication,
which cannot be discontinued due to a co-existing medical condition (e.g. diabetics with
microalbuminuria on ACE inhibitors). ACE inhibitors initiated by primary care physicians
in diabetic patients will not be discontinued nor dose decreased for the purpose of
achieving target blood pressure. The same is true for patients with coronary artery
disease on beta-blockers.
Q. What about diabetics? If my diabetic hypertensive patient is assigned "usual care",
and their systolic BP is 144 mmHg, should it be lowered further?
Ans: It is important to emphasize that no clinical trials have established optimal levels
of BP control for diabetic patients with established cerebral small artery disease. If it
were certain that lower BPs were better for these patients, there would be no
justification for including them in SPS3. As always, there is legitimate debate about how
far to extrapolate observations from other clinical settings, and many physicians might
feel uneasy. It should be emphasized to primary care physicians that guidelines for BP
management reflect the best practice based on available data, but they should not
restrict additional research if they are not based on hard evidence. This is all to say that
there has been much debate about this issue, weighing the strength of evidence and its
applicability to S3 participants. Of note, the SPS3 PSMB will be asked to monitor the
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
hypertension trial separately in the subgroup of diabetic participants and to recommend
that the trial be stopped or modified if concerns emerge.
In the situation described above, the patient should continue on the current
antihypertensive regimen, per SPS3 protocol. At the option of the local hypertension
specialist, BP in diabetic patients assigned "usual care" can be lowered to <140 mmHg
(still within the usual care range of 130-149 mmHg). Actual achieved BP among
diabetic participants will be monitored closely during the early course of SPS3, and the
protocol modified if need be.
Q: We are having trouble achieving the assigned target. When should the participant
be declared "failure to achieve assigned target (FAAT)?"
Ans: This is a matter of judgment that is influenced mainly by the participant's
willingness to continue vigorous attempts to control blood pressure. Most SPS3
participants will be able to achieve the assigned target BP levels if they are willing to
return for regular monitoring and adjustment of antihypertensive medications by a
skilled, experienced clinician. The need for frequent clinic visits and medication
changes/dosage adjustments should be made clear to potential participants before
study entry. At some point after entry, a participant may decide that he/she is unwilling
to continue to return to clinic and/or try new antihypertensive medications to control
blood pressure (possibly because of minor side-effects, etc). Declaring "failure to
achieve assigned target" does not mean that SPS3 management of BP stops, only that
it is pursued less aggressively, in accordance with the participant's tolerance. Recall
that all participants, whether FAAT or inactive, will be followed every 3 months and their
outcomes analyzed according to the original treatment assignment ("intention-to-treat").
Effort to control BP must continue in all active SPS3 participants.
Before declaring a participant "failure to achieve assigned target", the local hypertension
specialist should discuss the situation with the Coordinating Center Hypertension
Expert. The number of participants who are "failure to achieve assigned target" will be
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
monitored carefully across the study and at each clinical site, seeking common
problems that can be avoided.
Q: An SPS3 participant is taking ramapril prior to joining the study. The ACE inhibitor
available on the SPS3 formulary is lisinopril. Can the patient continue ramapril?
Ans: Yes, if the patient is able and willing to obtain the ramapril. We encourage
continuing antihypertensive agents initiated pre-SPS3 that can be funded by the
participant's HMO/medical insurance (justified as for routine clinical care) as
appropriate. If special antihypertensive medications that are not part of the SPS3
formulary are required, as prescribed by the SPS3-affiliated local hypertension
specialist for a good reason, efforts will be made to obtain the medication, as the budget
allows (usually including solicitation of donations from pharmaceutical companies). E-
mail the specific circumstances to the Coordinating Center Hypertension Expert, who
will consider the necessity and work to obtain the medication, if deemed appropriate.
Sample Cases
For each of the following 10 cases, consider the following options, and select the correct
answer based on the SPS3 protocol.
Options: Adjust medications now and recheck in 2-4 weeks. Recheck at next quarterly follow-up No meds adjustment, recheck in 1-2 weeks No meds adjustment, failure to achieve assigned target (FAAT) Other, explain
1. Hispanic, female patient randomized to BP target 130-149. Current medications:
HCTZ 12.5 mg qd, Fosinopril 40 mg qd, Metoprolol 50 mg qd. Patient is seen at the clinic today for her 6 month follow-up visit, and she reports she has not been taking her BP medications for last 2 weeks because "she does not feel like it". Her SPS3 BP is 156/79. Her most recent SPS3 BP prior to today was done at the 3 month follow-up and was 145/75. What is your plan?
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
2. 75 year old, white, male with history of HTN was randomized to BP target 130-149
and is in the clinic for his 9 month follow-up. Patient was in target at last quarterly follow-up. Current medications: Terazosin 5 mg every other night, Prinzide 20/25 mg qd. SPS3 BP today is 130/77. What is your plan?
3. Patient was randomized to BP target 130-149 with an SPS3 BP on date of
randomization of 170/95. Patient's SPS3 BP has not been in target since randomization. Most recent BP SPS3 BP was 160/88 four weeks ago on atenolol 25 mg qd. Today at his 3 month follow-up, his SPS3 BP is 154/85. Current medications: Atenolol 50 mg qd, HCTZ 12.5 mg qd.
What is your plan?
4. 75 year old female patient with history of DM type II was randomized to BP target
130-149. Current medications: Captopril 50 mg qd, HCTZ 12.5 mg qd. Patient comes to the clinic today for a BP check complaining of feeling very tired and getting dizzy every time she stands up. Her SPS3 BP is 125/53.
What is your plan?
5. Patient was randomized to target BP < 130. His SPS3 BP today is 125/69, and at
his last BP Check it was 128/71. Current medications: HCTZ 12.5 mg qd and Fosinopril 20 mg bid.
What is your plan?
6. Diabetic patient is randomized today to BP < 130. His SPS3 BP today is 135/63.
Current meds: Verapamil 180 mg qhs.
What is your plan?
7. Patient randomized to target BP 130-149 reports today at his regular follow-up that
he has not taken his medications for the last week because he lost them vacationing and decided to wait until his appointment today to contact us. His SPS3 BP today is 156/74. Prior to this, patient BP was stable on current meds. Current meds: Propanolol 40 mg bid, HCTZ 25 mg qd.
What is your plan?
8. Diabetic patient randomized to BP target 130-149 returns to clinic for a regular
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
follow-up. His SPS3 BP today is 137/76, and his only complaint is dry cough once and a while. Current meds: Fosinopril 40 mg bid.
What is your plan?
9. Non-diabetic patient randomized to target BP 130-149 comes to the clinic for her first
post-randomization BP check. Her BP is 128/75 today with no complaints. SPS3 BP on date of randomization was 132/82 on the same meds. Current medications: atenolol 25 mg qd.
What is your plan?
10. Hispanic female with history of DM type II and HTN for 23 years treated with a large
number of anti-hypertensives was randomized to target BP <130. Patient comes to the clinic today for her 1 year follow-up; her SPS3 BP is 136/69. Patient states that she is tired of trying a lot of medicines and that her BP is good enough, and she feels well. Current meds: Amlodipine 5 mg qd, Fosinopril 40 mg bid, Furosemide 40 mg bid, Propanolol 40 mg bid, Hydralazine 100 mg bid.
What is your plan?
ANSWERS AND JUSTIFICATIONS 1. Other.
Patient needs to re-start the same therapy she was receiving and return to the clinic for BP check in 2-4 weeks.
2. Recheck at next quarterly follow-up.
Stable patients (i.e. on target twice and then seen only at quarterly follow-ups until out-of-range) with an on target BP at a quarterly follow-up return for their next BP at their next regular follow-up in 3 months.
3. Adjust medications now and recheck BP in 2-4 weeks.
Increase HCTZ to 25 mg qd and recheck in 4 weeks (2 wks if medication change did not involve thiazide).
4. Adjust medications now and recheck BP in 2-4 weeks.
Patient's BP is below assigned target and patient complains of not feeling well, so a decrease in antihypertensive medication is needed. The patient was started on her ACE inhibitor for her diabetes, so it ought not to be discontinued. In this case it is
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
appropriate to reduce or stop the HCTZ, and recheck in 2-4 weeks.
5. Recheck at next quarterly follow-up.
When the patient completes two consecutive BP checks on target, next appointment will be the next quarterly follow-up.
6. Adjust medications now and recheck in 2-4 weeks. Starting an ACE inhibitor as part of the anti-hypertensive therapy for this patient
would be appropriate. Recheck BP in two weeks.
Instruct the patient to resume the same medications at the same doses and recheck the BP in two weeks.
8. Recheck at next quarterly follow-up.
This patient can be seen again in three months in a regular follow-up. Even though he reports a side effect from the medications, no change of anti-hypertensive therapy is required. Instruct patient to call the study coordinator if the dry cough becomes a problem.
9. No meds adjustment, recheck in 1-2 weeks.
Patient needs two consecutive BP checks in target on the same medications to be able to revert to the quarterly follow-up schedule. Patient was either not taking her medications at her post-randomization SPS3 BP that was in target, or the medication was not yet stable. Recheck and if again below target, then either change medication or reduce to q48h.
10. No medications adjustment, Failure to Achieve Assigned Target (FAAT). In this particular case, the patient completed a year in the study suggesting that
multiple attempts were done to keep the BP on target assigned. The patient is already showing discomfort with the frequent changes in the therapy and is pleased with her current BP. The physician must declare Failure to Achieve Assigned Target and keep the BP under control, so the patient's compliance will be not jeopardized. The next appointment will be at the one year + 3 month follow-up.
ManOp section XI – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
XII. STUDY FOLLOW-UP
Regular in-clinic follow-up of SPS3 patients is extremely important. Patients seen in
clinic on a regular basis are more likely to stay on assigned antiplatelet therapy, have
their blood pressures in the target range, take their medications as directed, and have
potential events detected and documented in a timely fashion. When you schedule a
quarterly follow-up visit, remember that part of the visit is BP measurement, so if the
patient is taking any antihypertensive medications, he/she will need special scheduling
considerations and prompting about taking medications (see Section VIII).
The SPS3 study physician should physically evaluate the patient at all in-clinic visits,
even if it is a seemingly routine visit with no suspicious symptoms elicited by the study
coordinator. The participant's expectation of being seen by a physician regularly (and
for free!) is often a factor that encourages patients to join the study (although it is
generally rapport with the study coordinator that keeps them in!).
Goals of follow-up are seven-fold:
1. To keep participants on assigned antiplatelet therapy
Analysis of the SPS3 endpoint data is on an intention-to-treat basis. This means
that all events that occur after the moment of randomization until the end-of study
date are counted against the assigned therapies. Events occurring in patients,
whom are non-compliant, discontinue study medication for whatever reason, or take
excluded medications are counted the same as those events occurring in the
perfectly compliant patients. If excessive numbers of participants are not taking
assigned therapies per protocol, the results of SPS3 will be worse than worthless!
To minimize such circumstances, we must select patients carefully and encourage
compliance during follow-up, avoiding non-clinical withdrawals.
Keeping patients active on assigned therapy involves continuous re-education about
the rationale and importance of the study. SPS3 physicians should emphasize the
ManOp section XII – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
importance of the project and of the crucial participation of each patient at follow-up
visits. Whenever possible, avoid long waiting times for follow-up appointments, and
be sure to communicate with the patient's primary care doctor to keep his/her critical
Patients who are intolerant of aspirin are ineligible for the study, however, very
occasionally a patient will be randomized who is unaware of being aspirin intolerant.
During follow-up, if a patient experiences difficulty tolerating the 325 mg aspirin, the
clinical decision may be to try a dose of 81mg. If the patient takes the 81 mg aspirin
and continues to take the other ‘antiplatelet' medication (clopidogrel/placebo), this
patient is still considered active in the antiplatelet arm of the trial. Please contact
the Coordinating Center and make them aware of this change in aspirin dose. This
change to 81mg of aspirin also needs to be recorded on the next quarterly follow-up
form (See specific instructions for ‘Antiplatelet Trial' questions of Follow-Up form in
2. To monitor blood pressures and adjust antihypertensive medications to keep
BPs in target
Management of hypertension is discussed in detail in Section XI. It is imperative
that all efforts within the patient's tolerance and limits of safety be made to achieve
and maintain blood pressures in the assigned target range. When a blood pressure
is out of range, it must be compulsively rechecked in two to four weeks as per
protocol. We need to show that the achieved blood pressures are importantly
different between the two BP target groups, and this means consistent follow-up and
action on out-of-range BPs. If you are having difficulty with a patient, ask for help! If
excessive numbers of participants have BPs out of range, the results of SPS3 will be
uninterpretable! To minimize such circumstances, we must select patients carefully
and work to achieve in target blood pressures.
ManOp section XII – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
3. For patient safety
Remind patients that they need to bring all their medications, both study and non-
study, with them to each study visit. This allows you to record which medications the
patient takes, not just the ones they can recall. When a patient forgets to bring with
them any of either the study or non-study medications, have the patient call you
when he/she returns home, or better yet call them. Pill counts for antiplatelet
therapy compliance can be done over the phone, but be sure to tell the patient to
destroy any pills leftover.
Patients should be told to contact you if any medication is changed, if any
medication is lost, if they have any symptoms of stroke, TIA, or if they are
hospitalized for any reason. Although SPS3 patients have all experienced a stroke,
they will still need to be re-educated periodically about the warning signs of stroke.
4. To complete follow-ups as scheduled
Quarterly follow-ups need to be compulsively completed within the follow-up window.
Missed or late follow-ups are usually a sign of poor patient compliance or
disorganization of study personnel - both of great concern. This will be monitored
carefully at each center, and a report will be sent to your center and the Coordinating
Each patient is required to have regularly scheduled follow-up visits every 3 months.
The follow-up schedule is generated and printed at the time of randomization.
Target dates for these visits are based on date of randomization and have a two-
week window on either side. A patient randomized on April 4th, 2003 has the
following schedule of quarterly follow-ups.
ManOp section XII – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
It is not necessary to schedule these Scheduled follow-up Target Date of Follow-up
Follow-up Window
quarterly follow-ups on the exact target 3 month
06/20/03 – 07/18/03
09/20/03 – 10/18/03
date. A follow-up is considered "on 9 month
12/21/03 – 01/18/04
time" if it is completed anytime during 1 year
03/21/04 – 04/18/04
06/20/04 – 07/18/04
the follow-up window. In fact, it is 1 yr + 6 mo
09/20/04 – 10/18/04
suggested that follow-ups be 1 yr + 9 mo
12/21/04 – 01/18/05
03/21/05 – 04/18/05
scheduled early in the window; if a 2 yr + 3 mo
06/20/05 – 07/18/05
patient needs to change their 2 yr + 6 mo
09/20/05 – 10/18/05
12/21/05 – 01/18/06
appointment at the last minute, then 3 year
03/21/06 – 04/18/06
there is adequate time to schedule 3 yr + 3 mo
06/20/06 – 07/18/06
09/20/06 – 10/18/06
another within the follow-up window. If 3 yr + 9 mo
12/21/06 – 01/18/07
a patient needs to have a BP check, 4 year
03/21/07 – 04/18/07
06/20/07 – 07/18/07
and the appointment date is within the 4 yr + 6 mo
09/20/07 – 10/18/07
follow-up window, complete only the
Follow-up form as blood pressure data are collected as part of the follow-up.
In-clinic visits are the expected; however, occasionally it may be necessary to do a
follow-up by telephone or with a non-study physician. For example, if a patient
moves far away, it may be possible to arrange for a physician in their new location to
see the patient, or to alternate follow-ups by phone with in-clinic visits. For some
patients, particularly those who come long distances, compensation for travel
expenses of taxi fare may be considered, but this is the prerogative of the local
clinical center using credit dollars. It is the SPS3 policy that patients will not be paid
for participating in the study; non-monetary benefits include potential benefit of study
drug and antihypertensive medications, regular follow-up of blood pressure and
symptoms, free regular contact with terrific doctors and nurses experienced in stroke
prevention. Home visits may be done at the discretion of the local investigators.
Follow-ups not occurring in the designated window are considered missed. If a
patient misses a follow-up, contact them immediately. A patient may miss a follow-
ManOp section XII – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
up for a number of reasons, e.g. forgot the appointment, had an event, was in the
hospital, losing interest in the study, doesn't want to participate in the study, etc. If a
patient is hospitalized, you may complete the follow-up in-hospital. This gives you
an opportunity to make sure that no unnecessary changes are made in the patient's
study drug use, and that the patient is not placed on any excluded or contraindicated
medications unless absolutely necessary.
If a patient is absolutely unable to come
to the clinic during the entire follow-up window, and a home visit is not possible,
conduct a phone follow-up, send their antiplatelet medications to them ahead of
time, and arrange for an in-clinic BP check as soon as possible. While adherence to
this window may seem unduly rigid, it is impossible to analyze the over 40,000
expected follow-up forms on a catch-as-catch-can basis!
Patients who discontinue all assigned SPS3 intervention(s), i.e. both their
antiplatelet therapy and SPS3 management of their blood pressure, still need to
continue to be followed every 3 months in clinic for the purpose of measuring blood
pressure and event detection. If this is not possible, at a minimum, have the patient
come for annual visits with phone follow-ups for the other quarterly follow-ups.
5. To detect and document events
This involves alerting the SPS3 physician, enlisting the blinded neurologist event
assessor (BEA) for ischemic stroke/TIA and CNS hemorrhage events, and timely
completion of appropriate forms. Events and their procedures are defined in detail in
Section XIII and in the instructions for the forms (section XX). As events occur
infrequently, call the Study Coordinator at the Coordinating Center for help if you
have any questions.
6. To identify other cerebrovascular risk factors (e.g. diabetes,hyperlipidemia)
and assist their primary care physician with their management
Management of other cerebrovascular risk factors will be under the supervision of
the patient's primary care physician; however, the SPS3 study team will assist their
ManOp section XII – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
efforts. Algorithms for management of risk factors other than hypertension are in
7. Establish a good rapport with the patient
The rapport that the study coordinator establishes with the patient is the major factor
keeping the patient active in the study. Get to know your patients, their families,
their interests, etc. As a general rule, patients are considerate and appreciative of
your interest and help.
ManOp section XII – 07/01/07
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
XIII. EVENT DOCUMENTATION AND PROCESSING
Event Documentation
There are nine categories of events during SPS3 follow-up that require scrupulous,
standardized documentation and validation using separate data forms listed below:
1. Ischemic stroke 2. CNS Hemorrhage 3. Major Cognitive Decline (not due to clinical stroke) 4. TIA 5. Major non-CNS Hemorrhage 6. Serious Complication of Hypotension (e.g. orthostatic fall causing a broken bone, etc.)
7. Other SPS3-related Serious Adverse Event (e.g. T.T.P., severe allergic reaction to an
SPS3 prescribed med)
8. Acute MI or other non-CNS Thromboembolism (including venous thrombosis and
pulmonary emboli)
9. Death (categorized as vascular, nonvascular, and uncertain)
Specific definitions for each study event appear in the line-by-line for the Event
Notification form and as part of the protocol. All events, including those that occur in
patients who have discontinued all SPS3 antiplatelet therapy and BP management, and
in those with previous events of the same type, will be assessed and recorded for
central adjudication. All possible events should undergo assessment and central
adjudication in order to minimize detection bias. (Note: if all submitted events are
verified, then it is likely that minor events are under-detected.)
For safety monitoring purposes, all events need to be reported, via the Event
Notification Form 20 and the SPS3 Data Entry System (DES), within 24hr of their
identification. When you learn of a possible event, carefully review the SPS3 event-
specific definition for that event category in the line-by-line of the Event Notification form
or event-specific form and discuss with your local physician investigator. Check that the
event meets SPS3 criteria, then complete and enter the Event Notification form. If
ManOp section XIII – 08/15/09
Secondary
Prevention of
Manual of Operations
Small
Subcortical
Strokes
possible, the local physician investigator should complete the narrative summary on the
Event Notification form, but occasionally, the SPS3 research coordinator may need to
do so in order to submit it within 24 hr. Contact the SPS3 Coordinator if you have any
Reporting of four categories of events will be forwarded by the Statistical Center (SC) to
the independent Medical Safety Monitor immediately upon their receipt. The Medical
Safety Monitor (MSM) uses the web-based monitoring system to process events
assessments. First, the clinical coordinator enters an Event Form 20 record into their
local DES. Next, the overnight process sends an email to the MSM for major events
(event codes: 3 – CNS hemorrhage, 4 – major non-CNS hemorrhage, 5 – serious
complication of hypotension, and 6 – other serious adverse event) and to data
managers at the SC for all events, both major and minor. Within the SC, the e-mail is
forwarded to staff members at four levels of notification, with the level of notification
increasing for each day that there is no response from the MSM. Initially, only the data
management staff, study statisticians, and programmer receive the notification. Level 2
then serves to resend the e-mail to the initial group of contacts. Level 3 forwards the
email to the principal investigator at the SC along with the initial group of contacts.
Finally, level 4 adds other key members, including the manager of information systems
and other statisticians.
The MSM then goes to the specific website (See Fig. 1 and 2), at
http://138.26.144.17/evtnot/ack.asp, in order to acknowledge receipt of the event
notification. Next, the site coordinator enters into the DES the appropriate auxiliary
event form for the event (form 22 for a CNS hemorrhage, form 23 for a major non-CNS
hemorrhage, form 24 for a serious complication of hypotension, or form 25 for another
type of event). For major events, the overnight process sends an email to the MSM that
new information is available regarding the event. The Monitor may then choose either
to close the event case, having received enough information, or request more
information from the coordinator, with both options being available via the website. If
ManOp section XIII – 08/15/09
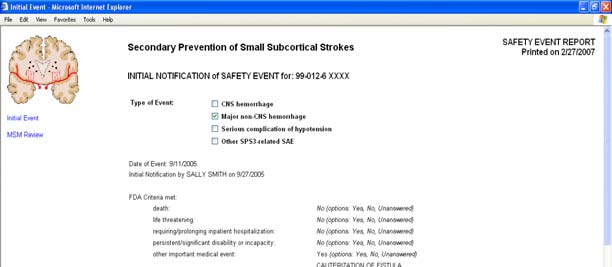
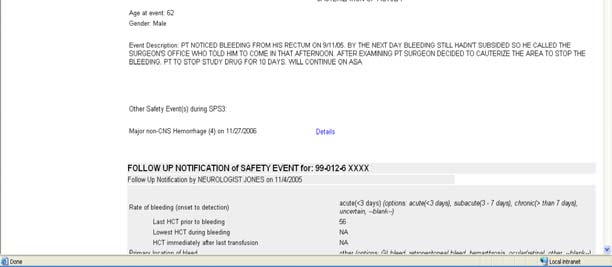
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
the MSM requests more information from the coordinator, the overnight process sends
an email to both the coordinator and to the SC data management with the text of
request. If no further information is needed, the MSM will finally close the event case by
selecting the appropriate box listed on the website.
Figure 1 – The Medical Safety Monitor Website – Site 99 Sample Event
ManOp section XIII – 08/15/09
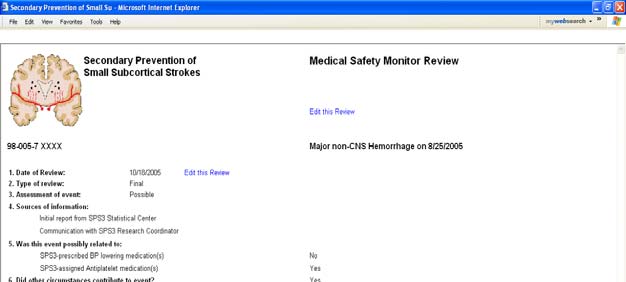
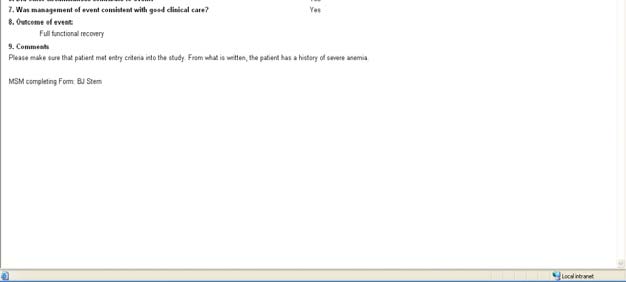
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Figure 2 – Medical Safety Monitor's review webpage on MSM website
BEA Assessment
The event-specific form for an event will be completed by an SPS3 physician who may
be unblinded to BP target assignment. Separately the Blinded Event Assessor (BEA)
will complete the BEA event-specific form, if applicable. Additional forms to be
completed and by whom are event-specific and indicated on the relevant event-specific
form. The event-specific form, the BEA event-specific form if applicable, and any
accompanying forms need to be completed and entered within 30 days of identification
of the event. Call the Coordinating Center if you are having trouble doing so!
Three event categories require the BEA to evaluate the patient: 1) Ischemic stroke, 2)
TIA, and 3) CNS hemorrhage. Since the HTN trial target BP group is not blinded, the
BEA will offer unbiased determination of whether a stroke (ischemic or hemorrhagic) or
ManOp section XIII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
TIA event meets study criteria and, in the case of strokes, whether there is persisting
neurological disability. The BEA is an SPS3-affiliated neurologist who is unaware of the
assigned BP target and the status of antithrombotic therapy (i.e. active or inactive).
During assessment of an event, the BEA should avoid learning about BP meds,
compliance, antithrombotic therapy, etc.
The specific event-related materials to be collected for an event vary with event type
and are listed at the end of each event-specific form. Start collecting event materials in
the participant's event folder as soon as you submit the Event Notification form.
Adequate documentation is extremely important, so err on the side of collecting too
much information rather than too little. Obtaining event materials when participants are
hospitalized at non-affiliated hospitals requires extra efforts, but still must be done.
Copy the event materials, including the event narrative, and send to the Events
Coordinator within 30 days.
Clinical neuroimaging following an event will be determined by the participant's personal
physician. In the event of a stroke, if a CT was done as part of routine care and
demonstrates the lesion, please submit these images. If the CT does not demonstrate
the lesion or if neuroimaging was not done as part of routine stroke care, then contact
the Coordinating Center principal investigator for approval for an SPS3-funded MRI. In
the event of a Major Cognitive Decline event, we want to be sure to obtain an MRI,
preferably with vascular imaging. If an MRI + vascular imaging was done as part of
routine care, then obtain a reprint/image files on CD. If not, then contact the
Coordinating Center for approval to have an SPS3-funded study done. As the
diagnosis of TIA is clinical, we are not asking for a CT/MRI in the event of a TIA. If
neuro-imaging is obtained as part of the TIA clinical work-up, please submit the images
to the Coordinating Center. Note that the permission of the participant and the personal
physician should be obtained prior to scheduling this post-event study, which although
covered by the initial informed consent, remains voluntary. Send the reprint/image files
on CD to the Coordinating Center within 30 days.
ManOp section XIII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
7 steps to happy and complete event documentation:
Reporting of all possible events must be done on a timely basis with supporting
documentation submitted to the Events Coordinator for any necessary blinding and
subsequent submission to the Events Adjudication Committee.
1) Complete and enter the Event Notification form for each and every possible
event that is identified, within 24 hrs of identification. Report all events
considered "possible" by the SPS3 physician. If in doubt, submit!
2) Have the SPS3 physician evaluate the patient and complete the event-specific
form and any additional forms indicated on the event specific form within 30 days
of event identification.
3) Have the BEA evaluate the patient as soon as possible (within 30 days) for all
possible ischemic strokes, TIAs, and CNS hemorrhages. All possible events
detected by investigators, all suspicious symptoms elicited by a standardized
questionnaire, and all patients at their end-study visit are evaluated by a
neurologist blinded to treatment assignments.
4) Collect copies of the specific event-related documents and place in a file folder to
remain permanently with the participant's SPS3 records.
5) Copy the narrative summary and the event-related documents and send to the
Events Coordinator within 30 days of the event.
6) Send a reprint/imaging files on CD of the relevant neuroimaging study if the
event was an ischemic stroke, TIA, CNS hemorrhage, or major cognitive decline
to the Coordinating Center.
7) Events occur infrequently. Contact the Study Coordinator or Events Coordinator
at the Coordinating Center if you have any questions about what to do.
ManOp section XIII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Event Adjudication
After the clinical site has submitted all relevant forms electronically and sent copies of
the event documentation to the Events Coordinator, the event will be sent for verification
by the Events Adjudication Committee. A report summarizing relevant diagnostic
information from the database and the blinded event documentation will accompany an
Event Adjudication form for the adjudicator to complete. Ischemic Stroke, TIA, and CNS
Hemorrhage events will be reviewed by two members; other event types will be
reviewed by one.
Clinical Centers • Event Notification form to Statistical Center within 24 hr • Event-specific form (and auxiliary forms, if applicable) to Statistical Center within 30 days • Event documents to Events Coordinator within 30 days • Blinded event assessment for primary events
Events Adjudication Committee
• Events Coordinator: purge medical records of treatment information • Event reviewed by physician adjudicators within 30 days of receipt • Event tracked by Events Coordinator
Event Processing: Safety Events
The processing of Safety Events has two stages: the initial stage occurring within 24 hr
of identification of the event, and the subsequent follow-up stage within 30 days of the
event when more information becomes available. The adjudication of Safety Events is
Clinical Centers
• Event Notification form to Statistical Center within 24 hr • Event-specific form (and auxiliary forms, if applicable) to Statistical Center within 30 days
ManOp section XIII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Medical Safety Monitor
• Notified of Safety events within 24 hr • Interacts with clinical sites re: management p.r.n. • Reports to DSMB every 3 months or p.r.n. • Safety Events include all: CNS hemorrhage
Major non-CNS hemorrhage
Major Complication of Hypotension
Other SPS3-related SAEs
ManOp section XIII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
ManOp section XIII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XIV. PREMATURE STUDY TERMINATION
All randomized patients should be followed until the end-study date in 2012, regardless
of whether or not they are actively receiving assigned SPS3 intervention(s). Patients
who end all contact with SPS3 before end-study either by withdrawing consent or losing
contact with SPS3 can have a serious impact on the outcome and validity of the study
and this should be avoided. It is not unreasonable to expect 100% follow-up at the end
of the trial!
If a participant indicates that he/she no longer wants to participate in SPS3, ask if you
may contact him/her by phone rather than requiring in-clinic follow-up. This
compromise allows detection of major study events and avoids "premature study
termination". Some participants who may initially request no further contact due to an
adverse event, etc. may agree to telephone contact with you or an SPS3 investigator. If
the patient is willing to maintain contact but refuses one or both assigned SPS3
intervention(s), complete the Status of Therapy form. If the patient does withdraw
consent despite your very best efforts, complete the Study Termination form.
If a participant is moving away from your site, they may be followed by another SPS3
site. Contact the Coordinating Center if this is a possibility. If this is not possible, then
arrange episodic telephone follow-up with the patient. For the end-study visit in 2012,
arrangements may be made with a local physician to complete most of the forms due at
end-study. If you are able to maintain contact with the patient, but the patient will not
actively receive the assigned SPS3 intervention(s), complete a Status of Therapy form.
In the very rare situation that a patient is moving and episodic telephone contact will not
be possible, complete the Study Termination form along with the other forms required at
No patient is lost to follow-up, but some are harder to find than others. The best
protection against "lost" patients is a good relationship with the patients and their
ManOp section XIV – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
families. Prompt attention to missed appointments is also important. The better you
know your patients, the more resources you will have for tracking them down if they
seem to disappear. Furthermore, ensuring that you record a minimum of two contact
persons and their numbers at study entry with quarterly review will ensure that you have
current information to locate a participant if necessary. A patient should not be declared
lost until all resources (including a house-to-house search) have been exhausted. If you
think you have a patient lost-to-follow-up, contact the SPS3 Study Coordinator at the
Coordinating Center. A search in the National Death Index will be done at study
completion for any patient "lost" during the study. It is critical to have the patient's SSN
to do so; be sure to record it on the Patient Contact Sheet at study entry.
Completion of the Study Termination form for those patients who terminate early
requires contacting the Coordinating Center, either the Study Coordinator, Oscar
Benavente, or Bob Hart. Both premature study termination and discontinuing SPS3
assigned interventions are measures of study execution and will be closely monitored
by the Steering Committee. If you are having trouble with a patient, don't wait until it is
too late - Contact the Study Coordinator at the Coordinating Center for help.
Related to the extended follow-up, participants who reach the originally planned 4.5
years of follow-up will be approached to re-consent to further follow-up. It is critical to
the validity of the study that re-consenting is not differential by treatment group or in
relation to the outcomes under study. The rate of re-consenting and also remaining
active in the assigned treatment groups and follow-up is also important for precision of
the results. If a patient refuses to re-consent for extended follow-up or re-consents and
then at a later time withdraws consent, complete the Study Termination form.
ManOp section XIV – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XV. COMMON ANTIHYPERTENSIVE MEDICATIONS
This section contains three reference tables. The first table (Table 1 below) lists
categories of BP lowering meds and generic names of commonly found meds in each
category. The next table is lengthy and lists BP lowering medications by Trade Name
(first letter capitalized) and generic name (no capitalization). You may find it useful to
help you complete the Medications and BP Check forms. If you need to know how to
categorize a medication that is not listed in Table 2, contact the Study Coordinator for
help. Table 3 lists the more common side-effects of BP lowering medications by
medication category.
Table 1. BP Lowering Meds by Category.
Category Generic
Thiazide Diuretic
chlorothiazide; hydrochlorothiazide (HCTZ); chlorthalidone; indapamide; metolazone
furosemide; bumetanide; ethacrynic acid; torsemide
Potassium Sparing
amiloride; spironolactone; triamterene;
Beta-blocker acebutolol;
betaxolol; bisoprolol; carteolol;
carvedilol; labetalol; metoprolol; nadolol; penbutolol; pindolol; propranolol; timolol
amlodipine, diltiazem, felodipine, isradipine,
nicardipine,nifedipine, nisoldipine, verapamil
benazepril; captopril; enalapril; fosinopril; lisinopril; moexipril; perindopril; quinapril; ramipril; trandolapril
Angiotensin Receptor candesartan; eprosartan; irbesartan; losartan; olmesartan; Blocker
telmisartan; valsartan
Central Alpha Agonist clonidine; guanabenz; guanfacine; methyldopa
doxazosin; prazosin; terazosin
Vasodilator hydralazine;
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Table 2. Classification of BP Lowering Meds by Name
CLASS, category on form
acetazolamide acetazolamide
Accupril quinapril
quinapril + HCTZ
ACE inhibitor, other + Diuretic, thiazide
Beta blocker, other
Aceon perindopril ACE
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Alatone spironolactone Diuretic,
spironolactone + HCTZ
Diuretic, potassium sparing + Diuretic, thiazide
Aldactone spironolactone Diuretic,
methyldopa + chlorothiazide
Misc, central alpha agonist + Diuretic, thiazide
Miscellaneous, central alpha agonist
methyldopate HCl
Miscellaneous, central alpha agonist
methyldopa + HCTZ
Misc, central alpha agonist + Diuretic, thiazide
ACE inhibitor, ramipril
amiloride amiloride
Calcium channel blocker, amlodipine
Apo-Hydro hydrochlorothiazide Diuretic,
Diuretic, thiazide
hydralazine + HCTZ
Misc, vasodilator + Diuretic, thiazide
Miscellaneous, vasodilator - hydralizine
Angiotensin receptor blocker, candesartan
candesartan + HCTZ
ARB, candesartan + Diuretic, thiazide
Beta blocker, atenolol
irbesartan + HCTZ
ARB, other + Diuretic, thiazide
Angiotensin receptor blocker, other
amlodipine + olmesartan
Calcium channel blocker, amlodipine + Angiotensin receptor blocker, other
benazepril benazepril
bendroflumethazide bendroflumethiazide
Angiotensin receptor blocker, other
olmesartan + HCTZ
ARB, other + Diuretic, thiazide
Beta blocker, metoprolol
Beta blocker, other
hydralazine + isosorbide dinitrate
Miscellaneous vasodilator – hydralazine + Miscellaneous long-acting nitrate
Beta blocker, other
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
CLASS, category on form
Beta blocker, other
Diuretic, furosemide/loop diuretic
Diuretic, furosemide/loop diuretic
Diuretic, furosemide/loop diuretic
amlodipine + atorvastatin
Calcium channel blocker, amlodipine + Lipid lowering agent, statin
Calcium channel blocker, verapamil
Angiotensin receptor blocker, candesartan
Capoten captopril
captopril + HCTZ
ACE inhibitor, other + Diuretic, thiazide
captopril captopril
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Cardura doxazosin
Beta blocker, other
Calcium channel blocker, other
Beta blocker, other
carvedilol carvedilol
Miscellaneous, central alpha agonist
Miscellaneous, central alpha agonist
chlorothiazide chlorothiazide
chlorthalidone chlorthalidone
Diuretic,thiazide
Calcium channel blocker, verapamil
Miscellaneous, central alpha agonist
clonidine + chlorthalidone
Misc, central alpha agonist + Diuretic, thiazide
Coreg carvedilol
blocker, carvedilol
Beta blocker, other
Beta blocker, other
nadolol + bendroflumethiazide
B-blocker, other + Diuretic, thiazide
Calcium channel blocker, verapamil
Coversyl perindopril
Angiotensin receptor blocker, other
Diuretic, furosemide/loop diuretic
Miscellaneous, other antihypertensive
Diamox acetazolamide Diuretic,
Calcium channel blocker, other
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
CLASS, category on form
Calcium channel blocker, other
Calcium channel blocker, other
Angiotensin receptor blocker, other
valsartan + HCTZ
ARB, other + Diuretic, thiazide
Diuril chlorothiazide Diuretic,
reserpine + methyclothiazide
Misc, other antihypertensive + Diuretic, thiazide
doxazosin doxazosin
triamterene + HCTZ
Diuretic, potassium sparing + Diuretic, thiazide
Calcium channel blocker, other
Calcium channel blocker, other
Dyrenium triamterene
Diuretic, furosemide/loop diuretic
enalapril enalapril
deserpidine + methyclothiazide
Misc, other antihypertensive + Diuretic, thiazide
eplerenone eplerenone
Angiotensin receptor blocker, other
Espirolona spironolactone Diuretic,
Diuretic, furosemide/loop diuretic
Calcium channel blocker, felodipine
ACE inhibitor, fosinopril
Diuretic, furosemide/loop diuretic
Diuretic, furosemide/loop diuretic
Miscellaneous, central alpha agonist
guanadrel sulfate
guanadrel sulfate
Miscellaneous, central alpha agonist
Miscellaneous, central alpha agonist
hydrochlorothiazide
Diuretic, thiazide
Hidroclorotiazida hydrochlorothiazide
Hidrosaluretil hydrochlorothiazide Diuretic,
hydrochlorothiazide + hydralazine
Diuretic, thiazide + Miscellaneous vasodilator - hydralazine
Miscellaneous, vasodilator - hydralizine
hydrochlorothiazide + hydralazine
Diuretic, thiazide + Miscellaneous vasodilator - hydralazine
HydroDIURIL HCTZ
Hydrozide (PLEASE
hydrochlorothiazide +/- amiloride
Diuretic, thiazide +/- Diuretic, potassium sparing
Hygroton chlorthalidone Diuretic,thiazide
guanadrel sulfate
Miscellaneous, central alpha agonist
Hytrin terazosin
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
CLASS, category on form
ARB, other + Diuretic, thiazide
isosorbide mononitrate
Miscellaneous long-acting nitrate
indapamide indapamide
Diuretic,thiazide
Beta blocker, propanolol
Beta blocker, propanolol
propranolol + HCTZ
B-blocker, propranolol + Diuretic, thiazide
propranolol + HCTZ
B-blocker, propranolol + Diuretic, thiazide
Inhibace cilazapril
Beta blocker, propanolol
Inspra eplerenone Diuretic,
Angiotensin receptor blocker, other
Calcium channel blocker, verapamil
Calcium channel blocker, other
Beta blocker, other
Beta blocker, other
Diuretic, furosemide/loop diuretic
Calcium channel blocker, other
Beta blocker, other
enalapril + felodipine
ACE inhibitor, enalapril + Ca-Channel blkr, felodipine
ACE inhibitor, lisinopril
Miscellaneous, vasodilator - minoxidil
benazepril + HCTZ
ACE inhibitor, other + Diuretic, thiazide
Beta blocker, metoprolol
metoprolol + HCTZ
B-blocker, metoprolol + Diuretic, thiazide
Angiotensin receptor blocker, other
Lotensin benazepril
amlodipine + benazepril
Ca-Channel blkr, amlodipine + ACE inhibitor, other
Lozide indapamide Diuretic,thiazide
Lozol indapamide Diuretic,thiazide
Mavik trandolapril ACE
triamterene + HCTZ
Diuretic, potassium sparing + Diuretic, thiazide
triamterene + HCTZ
Diuretic, potassium sparing + Diuretic, thiazide
methazolamide methazolamide
methylclothiazide methylclothiazide
Miscellaneous, central alpha agonist
methyldopate HCl
methyldopate HCl
Miscellaneous, central alpha agonist
metolazone metolazone
Beta blocker, metoprolol
Angiotensin receptor blocker, other
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
CLASS, category on form
telmisartan +HCTZ
ARB, other + Diuretic, thiazide
Midamor amiloride
Minipress prazosin
prazosin + polythiazide
Misc, alpha blocker + Diuretic, thiazide
Miscel aneous, vasodilator - minoxidil
amiloride + HCTZ
Diuretic, potassium sparing + Diuretic, thiazide
moexipril moexipril
Beta blocker, other
Beta blocker, other
ACE inhibitor, fosinopril
fosinopril + HCTZ
ACE inhibitor, fosinopril + Diuretic, thiazide
Mykrox metolazone Diuretic,
Beta blocker, other
Beta blocker, other
Neptazane methazolamide Diuretic,
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Calcium channel blocker, other
Beta blocker, other
Calcium channel blocker, other
Novo-Hydrazide hydrochlorothiazide
Miscellaneous vasodilator - hydralazine
Diuretic, furosemude/loop diuretic
hydrochlorothiazide + triamterene
Diuretic, thiazide + Diuretic, potassium sparing
Calcium channel blocker, amlodipine
Angiotensin receptor blocker, other
Beta blocker, other
perindopril perindopril
Beta blocker, other
Beta blocker, other
Calcium channel blocker, felodipine
polythiazide polythiazide
prazosin prazosin
ACE inhibitor, lisinopril
lisinopril + HCTZ
ACE inhibitor, lisinopril + Diuretic, thiazide
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
CLASS, category on form
Calcium channel blocker, other
Calcium channel blocker, other
Beta blocker, propanolol
quinapril quinapril
ACE inhibitor, ramipril
Calcium channel blocker, felodipine
reserpine + polythiazide
Misc, other antihypertensive + Diuretic, thiazide
reserpine reserpine
Miscellaneous, other antihypertensive
Beta blocker, other
Beta blocker, other
Miscellaneous, other antihypertensive
hydralazine + HCTZ + reserpine
Misc, vasodilator + Diuretic, thiazide + Misc, other
antihypertensive
spironolactone spironolactone
Calcium channel blocker, other
trandolapril + verapamil
ACE inhibitor, other + Ca-Channel blkr, verapamil
Calcium channel blocker, other
Miscellaneous direct rennin inhibitor - aliskiren
Angiotensin receptor blocker, other
Miscellaneous, central alpha agonist
atenolol + chlorthalidone
B-blocker, atenolol + Diuretic, thiazide
Beta blocker, atenolol
terazosin terazosin
Angiotensin receptor blocker, other
eprosartan + HCTZ
ARB, other + Diuretic, thiazide
Thalitone chlorthalidone Diuretic,thiazide
Calcium channel blocker, other
B-blocker, other + Diuretic, thiazide
Beta blocker, other
Beta blocker, metoprolol
Diuretic, furosemude/loop diuretic
Diuretic, furosemide/loop diuretic
Beta blocker, other
trandolapril trandolapril
triamterene triamterene
moexipril + HCTZ
ACE inhibitor, other + Diuretic, thiazide
Univasc moexipril
Angiotensin receptor blocker, other
enalapril + HCTZ
ACE inhibitor, enalapril + Diuretic, thiazide
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
CLASS, category on form
Vasotec enalapril
Calcium channel blocker, verapamil
Calcium channel blocker, verapamil
Calcium channel blocker, verapamil
Miscellaneous, central alpha agonist
Zaroxolyn metolazone
Beta blocker, other
lisinopril + HCTZ
ACE inhibitor, lisinopril + Diuretic, thiazide
ACE inhibitor, lisinopril
bisoprolol + HCTZ
B-blocker, other + Diuretic, thiazide
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Table 3. More Common Side Effects of Antihypertensive Medications:
Thiazide diuretics: GI complaints, leg cramps, rash, volume depletion and hypokalemia
(about 30%), caution in patients on digoxin, arrhythmias, loss of blood
sugar control in diabetes
Potassium sparing diuretics: gynecomastia (high dose of spironolactone), hyperkalemia
Loop diuretics: volume depletion, hypokalemia, GI intolerance, elevated creatinine and
Beta-blockers: fatigue, somnolence, nightmares, impotence and cold extremities,
bradycardia, bronchospasm (if COPD), inability to sense hypoglycemia
and loss of blood sugar control (if diabetic)
ACE inhibitors: cough, rash, increased potassium concentration, possible increased
creatinine (if hypovolemic or pre-existing renal dysfunction), hypotension
is usually dose-limiting adverse effect
Ca-channel blockers: constipation (particularly verapamil), hypotension, peripheral
edema, bradycardia (verapamil and diltiazem), potential for heart failure (if
poor LV function), heart block
Angiotension II receptor blocker: dizziness (?hypotension), diarrhea (same frequency as
placebo), cough (losartin) same frequency as placebo, hyperkalemia
Alpha blocker: postural hypotension (most common with prazosin; give first dose at
bedtime), conscious sedation, tachyphylaxis (loss of effectiveness of a
given dose with chronic therapy), GI complaints
Central alpha antagonist: fatigue, somnolence, depression, hypotension, impotence,
Vasodilator: sodium and water retention (edema, heart failure and loss of BP control),
lupus syndrome (hydralazine), hypertrichosis (excess hair growth,
minoxidil), hepatitis, hypotension
ManOp section XV - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XVI. MANAGEMENT GUIDELINES FOR OTHER CEREBROVASCULAR
RISK FACTORS
• Pharmacologic
for type 2 DM in adults
• Exercise algorithm for type 2 DM prevention and therapy • General lipid treatment algorithm • Lipid Treatment algorithm for type 1 and 2 DM in adults
ManOp section XVI - 04/15/03
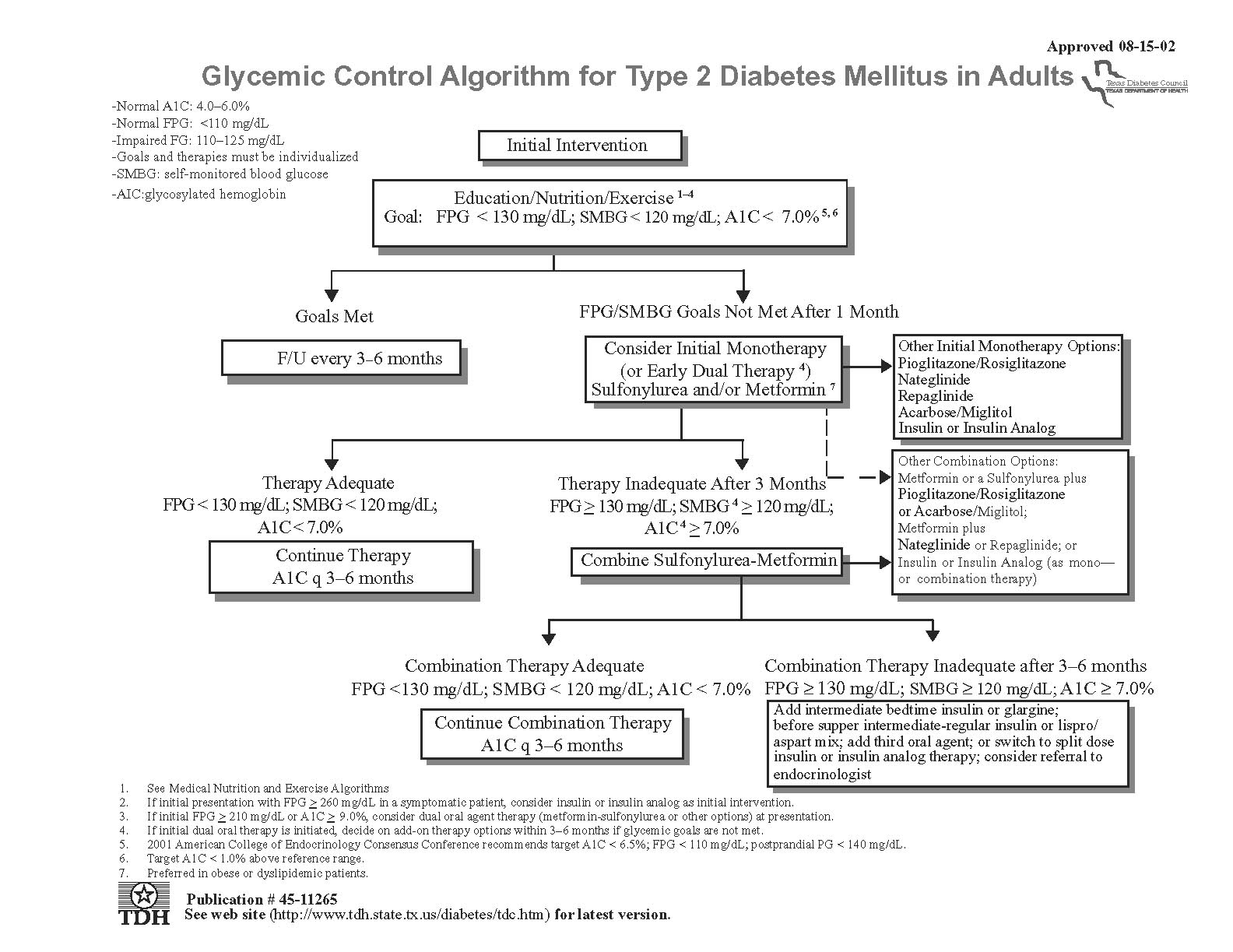
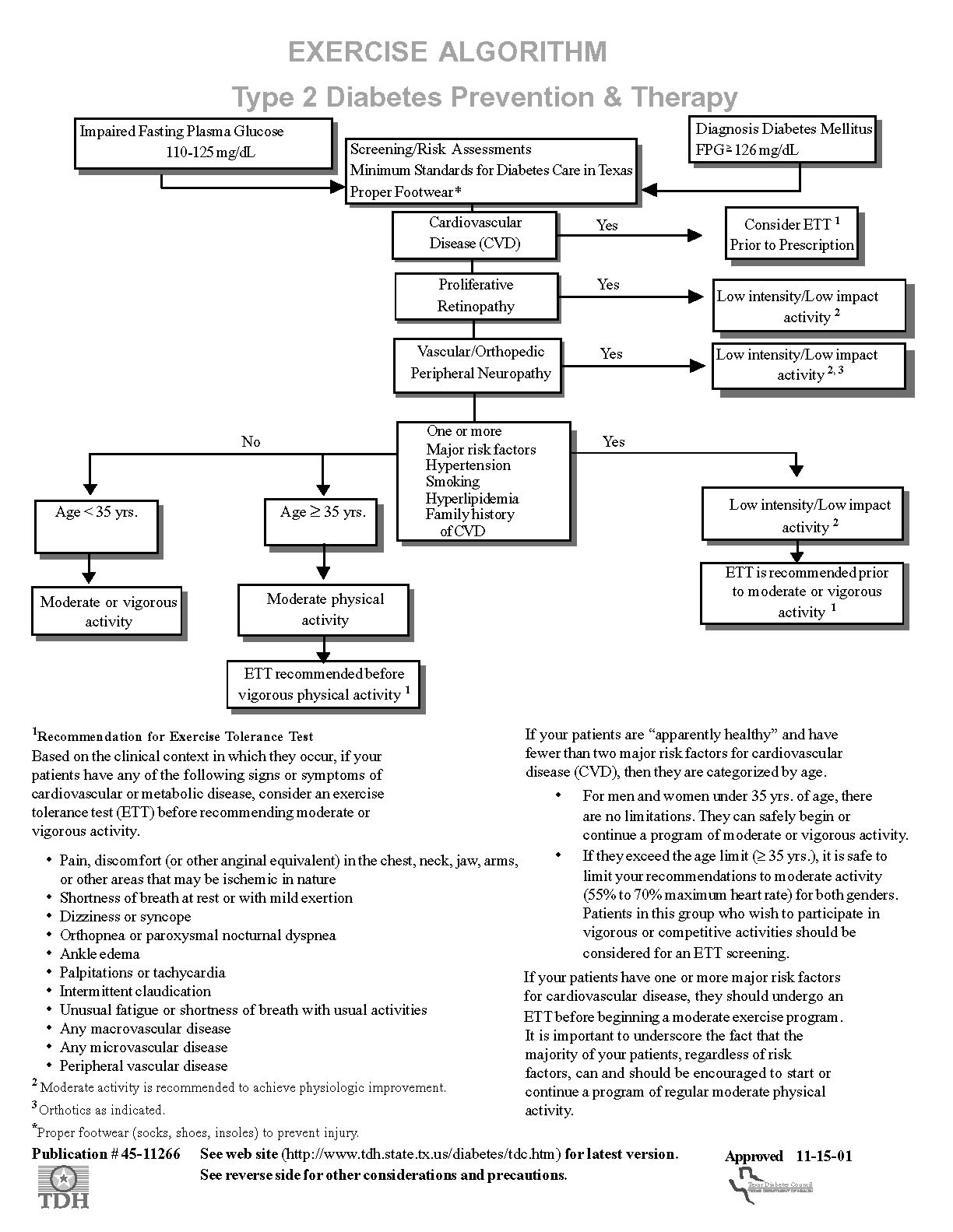
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
ManOp section XVI - 04/15/03

Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
ManOp section XVI - 04/15/03
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
General Lipid Treatment Algorithm
Recommendations for evaluation and treatment of high cholesterol in adults are based on the
Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection,
Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or
ATP III). JAMA 2001; 285:2486-2497.
New Features of ATP III:
1. Recommends a complete lipoprotein profile (total, LDL, and HDL cholesterol and
triglycerides) as the preferred initial test.
2. Encourages use of plant stanols/stenoids and viscous (soluble) fiber as therapeutic
dietary options to enhance lowering of LDL cholesterol.
3. Recommends treatment beyond LDL lowering for persons with triglycerides ≥200 mg/dl.
Table 1. ATP III classification of LDL, total
Table 2. Major risk factors (exclusive of LDL
and HDL cholesterol (mg/dl)
cholesterol) that modify LDL goals
LDL cholesterol
> 140/90 or on antiHTN meds
Near or above normal
• Low HDL cholesterol (< 40 mg/dl)
• Family history of premature CHD*
(CHD in male first degree relative < 55 yr;
CHD in female first degree relative < 65 yr
> 45 yr; female > 55)
Total cholesterol
*Diabetes is regarded as CAD risk equivalent.
HDL cholesterol
Table 3. LDL Cholesterol Goals and Cutpoints for Therapeutic Lifestyle Changes
(TLC) and Drug Therapy by Risk Category
InitiateTLC if LDL…
Consider Drug Therapy if
Category
CHD or risk equivalents
(10 years risk >20%)
(100-129:drug optional)
(10 year risk ≤20%)
10 year risk <10%: ≥160
0 – 1 Risk factor
160-189: LDL-lowering drug opt'l
ManOp section XVI - 04/15/03
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Figure 1. Steps in Therapeutic Lifestyle Changes
• Reduction of saturated fat and
• Moderate physical activity. • Consider referral to a dietitian.
• Reinforce reduction in
saturated fat and cholesterol.
Consider adding plant
stanois/sterois.
achieved, intensify If
• Increase fiber intake.
on atenolol (+ diuretic
• Consider referral to a dietitian
and ACEI or ARB)
• Initiate therapy for metabolic
Intensify weight management
and physical activity
• Consider referral to a dietitian
achieved, consider
adding drug therapy
Every 4-6 Months
Subsequent Visit
Monitor adherence to
ManOp section XVI - 04/15/03
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Figure 2. Progression of Drug Therapy in Primary Prevention
Initiate LDL-Lowering
Start statin or bile acid sequestrant or nicotinic acid
Consider higher dose of statin, or adding bile acid sequestrant or
achieved, intensify
LDL-lowering drug
If LDL goal achieved, treat other
lipid risk factors
achieved, intensify
LDL-lowering drug
therapy or refer to a
Every 4-6 Months
Subsequent Visit
Monitor adherence to
ManOp section XVI - 04/15/03
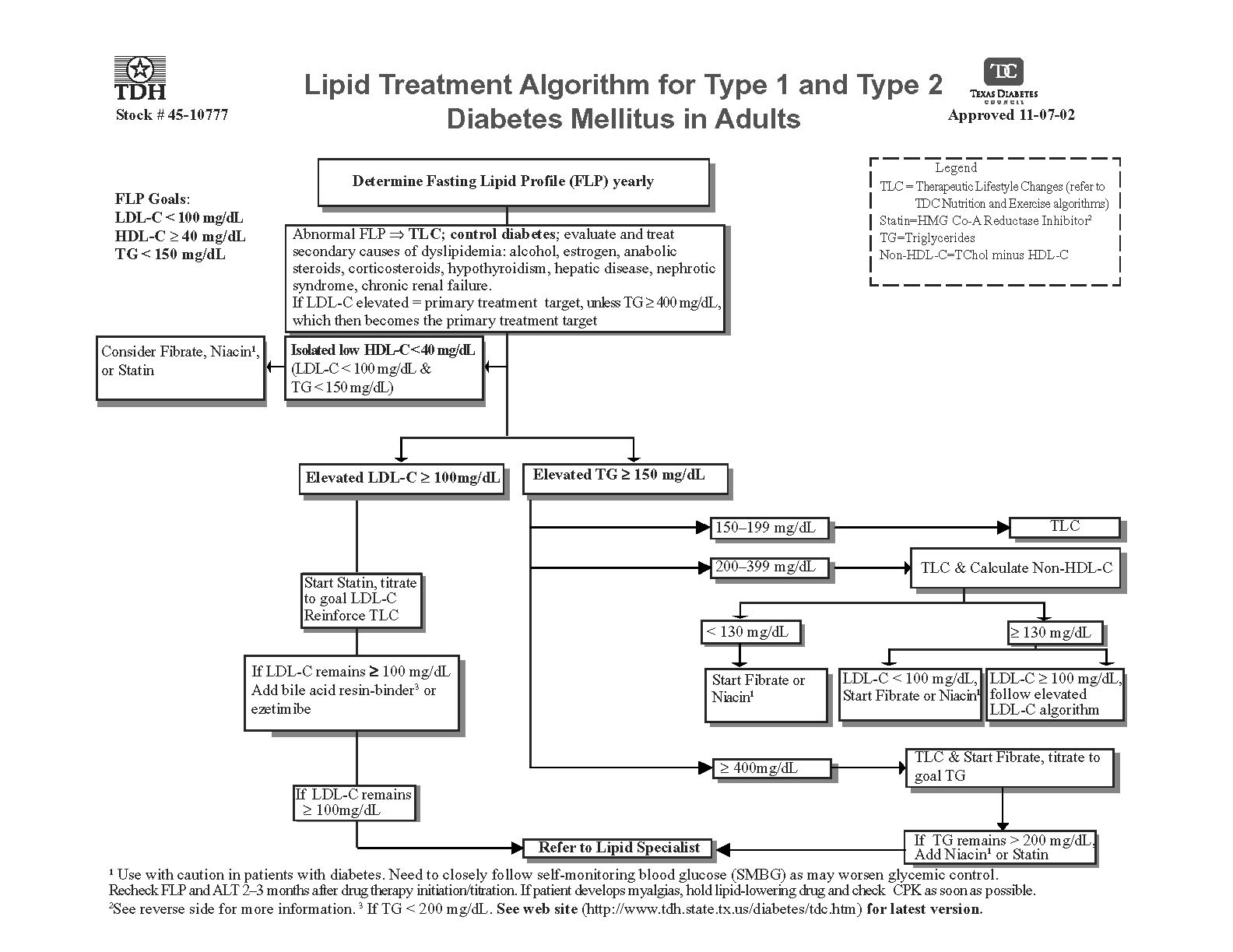
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XVII. SAMPLE CONSENT FORM AND PARTICIPANT MATERIALS
Following is a template of the consent form in English. Spanish and French versions
are also available from the Coordinating Center. Each IRB has its own slightly different
requirements, and you will need to work with your local IRB to meet its specifications.
Contact the Study Coordinator at the Coordinating Center if you have any questions or
Copies of the SPS3 information brochure, participant booklets, and participant wallet
cards will be provided to you by the Coordinating Center. There is space on them to
personalize them for your site.
Sample letters to a patient's primary care physician are also included in this section.
Two additional patient information handouts, "How I Can Reduce High Blood Pressure"
and "What is High Blood Pressure Medicine", which are produced by the American
Heart Association, are also included here for distribution to your patients if you wish.
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
SUBJECT CONSENT TO TAKE PART IN A STUDY OF
SECONDARY PREVENTION OF SMALL, SUBCORTICAL STROKES (SPS3)
The University of Texas Health Science Center at San Antonio
University Hospital and University Physicians Group (UPG)
PRINCIPAL INVESTIGATOR:
CO-INVESTIGATOR(S):
SPONSOR:
National Institute of Neurological Disorders and Stroke (NINDS).
University of British Columbia, Vancouver, Canada
INTRODUCTION
We are inviting you to take part in a research study about stroke prevention. Stroke is
damage to the brain caused by problems in the blood vessels. Strokes often cause
paralysis, loss of sensation, loss of speech and other problems. One type of stroke
affects the inner part of the brain causing small "pea-sized" areas of damage due to
blockage of small blood vessels within the brain. Doctors often call this type of stroke
"lacunar stroke" or "small subcortical stroke." About 150,000 strokes of this type
happen each year in the United States and close to 20,000 in Canada. We are asking
you to take part in this study because you have recently had a transient ischemic attack
or stroke of this type. We are aiming to enroll up to _ participants in this center, and up
to 3000 subjects across North America, Latin America, and Spain. Your participation in
this study could continue until April 2012.
PURPOSE
The purpose of this research study is to gather information about a new treatment that
might help prevent another stroke from happening in people who have suffered a
"lacunar stroke" or transient ischemic attack. Aspirin is the medicine most widely used
and is the standard therapy for this type of stroke. Clopidogrel is another medicine
doctors use to prevent stroke. Both of these medicines have an effect on blood clotting
and are now widely prescribed by doctors for stroke prevention. However, using them
together for the type of stroke that you have had has not been tested. We want to learn
if using them together is more effective than using aspirin alone. We also want to learn if
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
lowering a patient's blood pressure below the usual limits will also help prevent stroke and maintain thinking ability. If you do not have hypertension (high blood pressure), your blood pressure will not be lowered.
PROCEDURES
If you decide to take part in this research study, you will be assigned by chance (like
flipping a coin, called randomization) to one of two types of treatment to reduce clotting.
You will have an equal chance of being randomized to receive either aspirin alone or
the combination of aspirin and clopidogrel. Neither you, your doctor, nor the research
staff (investigators and nurses) will know until the end of the study whether you are
receiving the combination of aspirin plus clopidogrel or aspirin alone. However, if we
need to know for an emergency situation, we can quickly find out which medicine you
are taking.
We will give you two bottles of pills. One will contain aspirin (325 mg) and will have a label marked "aspirin." The other bottle will be labeled "study drug" and will contain either clopidogrel or placebo (a pill that contains no medicine). You will take one pill from the "aspirin" bottle and one from the "study drug" bottle (a total of two pills a day) every day until the end of the study.
You will also be assigned by chance to one of two groups of blood pressure control. The
difference between the two groups is the target level of systolic blood pressure: either
130-149 or below 130. While it is generally recommended that systolic blood pressure
be controlled below 140 for most people with high blood pressure, we do not know what
target levels are best for people who have already had a stroke. No one knows whether
lowering blood pressure after a stroke might help prevent another stroke or cause
further damage; this is the goal of the blood pressure aspect of the study. You will not
be told to which target blood pressure group you are assigned, but we will give you your
blood pressure readings if you wish.
With the permission of your doctor, the study doctors will treat your blood pressure
using standard medicines according to accepted guidelines in order to lower your blood
pressure to the goal assigned to you.
No experimental blood pressure medicines will be used, just those used everyday in
clinic that you may be receiving now. You will know the names and doses of the blood
pressure medications, and these medications will be provided at no cost to you. We will
ask you and your doctor to allow us to adjust your blood pressure medicine during the
study.
STUDY VISITS
At your first two visits, we will review your medical history, give you a physical
examination, and carefully measure your blood pressure. We will also ask you
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
questions to test your memory and thinking which will be audio taped. You will then be randomized (assigned by chance) to receive one of two types of treatment to reduce clotting and to one of two blood pressure groups (explained above). These visits will each take about two hours.
You will return to clinic to have your blood pressure measured at least once each month for the first two months. If you do not have high blood pressure, you will come to clinic at three months and every three months thereafter. If you have high blood pressure it may take 4 to 5 visits (at least monthly) until your blood pressure meets your assigned goal. At these visits, your blood pressure medications will be adjusted if necessary. Each visit will take about half an hour.
After your blood pressure has reached the goal, you will come to the clinic every 3 months. These visits will take about 45 minutes. We will ask you questions about your health, possible side effects of the medicines, examine you, take your blood pressure, and adjust your blood pressure medicines if needed. You will need to bring your bottles of study medicines with you to each clinic visit. Once every year, we will ask you questions to test your memory and thinking and these tests will be audio taped. As part of routine clinical care, we will also obtain a yearly blood sample to look at your cholesterol levels, kidney function, and your sugar control if you are diabetic.
We would also like to invite you to participate in an optional sub study. We will collect blood samples (about two tablespoons) at study entry and at your first yearly visit to evaluate for levels of blood proteins that may predict risk of another stroke and the effect of the protocol medicines. There may be minor discomfort associated with the drawing of blood. Apart from this, no blood will be drawn or any special tests done unless you are having symptoms or problems.
As part of this research study, we want to learn about changes in a person's brain after a stroke. If you did not have an MRI (magnetic resonance image) and MRA (magnetic resonance angiogram) scan of your brain following your stroke, you will need to have one done. Another scan will also be done if you suffer a stroke or major change in thinking ability during the study. An MRI scan of the brain provides a clear picture of the brain by using magnetic signals (not x-rays) and an MRA provides a clear picture of the intracranial vessels. For this test, you will be asked to lie quietly on a table for about 30 minutes with your head in a tube that extends down over your shoulders. If you have a tendency to get nervous in small spaces, a mild sedative can be given before the test (but you will not be able to drive for 8 hours if a sedative is used). People with artificial heart valves, pacemakers and certain other types of metal in their bodies cannot undergo MRI/MRA safely. If you cannot undergo an MRI/MRA safely, please tell us now. The results of any MRI/MRA done will be provided to you and your doctor, but only at the end of the study. By signing this form, you are giving permission to have these MRI/MRA scans if needed, for the investigators to look at the results, and for a print-out of the scan to be sent to the SPS3 Coordinating Center, with any identifying information
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
kept confidential. Information from this research about individual patients will not be available and will not be used for any future treatment.
RISKS AND DISCOMFORTS
If you have high blood pressure and receive medicines to control your blood pressure
as part of this study, there may be side effects of these blood pressure medicines. The
chances for side effects generally increase with the higher doses sometimes needed to
achieve lower blood pressures. Common side effects include impotence and
gastrointestinal side effects. The specific side effects depend on which medications are
used. The choice of blood pressure medicine to be used for you will depend on whether
you have other health problems (such as diabetes or heart failure), what medicines you
are taking now, and how you may have reacted to medicines in the past. When we
determine which medications are best for you (based on standard guidelines and help
from our high blood pressure experts), you will be given an information sheet about the
possible side-effects that might occur with each of the medicines you are taking. These
side effects, if they do occur, are seldom life-threatening, and they usually go away
when the medicine is stopped. We will review possible side effects at each clinic visit,
and you should call us if you have any questions about possible side effects or your
symptoms.
Combination of Aspirin and Clopidogrel
It is possible that treatment with the combination of aspirin and clopidogrel will diminish
the risk of having another stroke. There is also the possibility that the risk of stomach
upset as well as abnormal bleeding might increase. In a study of heart attack patients
and another study of persons who had suffered a stroke, when aspirin and clopidogrel
were taken together, very serious bleeding occurred in about 3 of 100 patients per year.
Clopidogrel
In previous studies of taking clopidogrel alone (without aspirin), side effects have
occurred in a small number of persons. These include petechiae (small, purplish spots
on the skin); bruises (purplish patch on the skin); or bleeding (such as bleeding in the
stomach or nosebleed) (1 in 10) and life-threatening bleeding (about 1 of 100 patients
per year). A serious side effect called thrombotic thrombocytopenic purpura (TTP) which
affects clotting function (marked reduction of the platelets which would cause
hemorrhages), and bone marrow, may affect on rare occasions people taking
clopidogrel (1 in 400,000). Diarrhea (1 in 200), and rashes were also noted. These side
effects went away after stopping the medicine.
Aspirin
Some of the side effects of aspirin include tinnitus (ringing in the ears) (1 in 100), and
stomach upset, nausea and vomiting were present in about 1 in 10 of the people taking
aspirin. The increased risk of bleeding in the stomach while taking aspirin every day is
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
about 1%, or 1 chance in 100 per year and life-threatening bleeding is about 1 to 1.5 out of 100 patients per year.
If you are going to have general surgery or dental surgery, it is important that you tell
your doctors (surgeon and anesthesiologist) or dentist that you are taking part in a
research study. Both aspirin and clopidogrel are medicines that can increase the risk of
abnormal bleeding during an operation. We will give you an identification card to carry in
case of an emergency or if additional information is needed about the study.
PREGNANCY
If you are pregnant, you cannot take part in this study. We will perform a pregnancy test
for you before you begin taking the medicines to make sure that you are not pregnant.
You should use birth control while you are taking part in the study because we do not
know what effect the combination of aspirin and clopidogrel may have on an unborn
child. If you think you might be pregnant at any time during the study, you should tell us.
BENEFITS
You may benefit from taking part in this study because the medicines may decrease
your chances of having another stroke. You will have your blood pressure checked
more often than usual and you will receive close observation while you are taking part in
the study. We do not guarantee, however, that you will directly benefit from taking part
in the study.
PARTICIPATION COSTS
You will not be charged for the clinic visits, examinations, MRI scans, the anti-clotting
drugs (clopidogrel and aspirin), or the high blood pressure medications that are
provided as part of the study. If you are taking another medication for your blood
pressure that is not provided by the study and your blood pressure is stable, you have
the choice to continue your medications at your own expense. If you were hospitalized
for a stroke before you joined this study, you are responsible for all costs of
hospitalization. If you have another stroke while taking part in this study, you will be
responsible for all costs relating to treatment. If you are injured as a result of the
research procedures, medical care will be provided. You will be responsible for all
charges. We are not able to give you money if you are injured.
CONFIDENTIALITY AND PROTECTED HEALTH INFORMATION
Every effort will be made to protect your privacy and, in so doing, the study will use your
initials and your study number rather than your name on any copies of your study
records, and on any blood/tissue samples and results of investigations that are sent
outside of the local research institution for review or testing. If the results of this study
are reported in medical journals or at meetings, your personal identity will remain
confidential.
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
You will be asked to provide your social security number and other identification to the study nurse. This information will be used only to contact you during the study and at the end of the study, if we are unable to locate you. We are also requesting permission to review and obtain copies of your medical records relevant to your recent stroke and any physician visits/hospitalizations during the study. These records are needed in order to have accurate information about your stroke and any medical event that happens to you during the study. This information and all study information will be confidential. Under federal privacy regulations, you have the right to determine who has access to your personal health information (called "protected health information" or PHI). If you choose to take part in this study, you will be giving your permission to the investigators and the research study staff (individuals involved in carrying out the study) to collect and use your personal health information for this research study. In carrying out this research, the PHI we will collect and use about you will include: •
information obtained from procedures used to determine your eligibility to participate in the study, including medical history (past and current), vital signs, physical examination, electrocardiogram, echocardiogram, brain MRI/MRA and blood tests;
information that is created or collected from you during your participation in the study, including physical exam, current medications, vital signs, results of tests (done for the study or primary care doctor visits), any records from hospitalizations while participating in the study and any information from out patient clinic records;
information contained in your underlying medical records related to your medical history and treatment prior to the study;
demographic information which includes things like your age, marital status, type of work you do, years of education completed, gender, date of birth, race.
We will obtain this information in different ways (mentioned above). We will ask you personally for information and we will get information about you from your physicians and from facilities where you have received health care. Because of the research goals of this study, however, your PHI in the study records cannot be kept absolutely confidential. Your PHI may be shared with other persons and organizations involved in the conduct or oversight of this research including: •
the sponsor of this study, National Institute of Health (NIH) and representatives of the sponsor or other agents designated by the NIH, to monitor or inspect study
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
data as well as regulatory authorities such as the Food and Drug Administration and Health Canada, Therapeutic Products Directorate and its agents,
the study's Data and Safety Monitoring Board (DSMB);
representatives of (your site specific) Institutional Review Board (IRB), the Compliance Office, the research subject advocate and representatives of other groups that have the responsibility of monitoring and overseeing research studies at your institution.
parts of your PHI that pertain to your participation in this study may be photocopied and sent to a central location for review or electronically transmitted.
You need to be aware that these organizations receiving your PHI may not have the same obligations to protect your PHI and may further disclose your PHI to groups not named here. Information released to these parties is no longer under the control of the study doctor and can no longer be protected by Federal Privacy Rules. The purposes of disclosing your PHI to these entities are to collect the data necessary to complete the research, to properly monitor how this study is carried out and to answer research questions related to this research study. The Institutional Review Board may approve additional use and disclosure of your PHI for other research by qualified researchers under circumstances permitted by the regulations.
If you decide to participate in the study, by signing this form you will be authorizing
these uses and disclosures of your PHI. If you choose not to authorize these uses and
disclosures of your PHI, you will not be able to participate in the research study.
Finally, the federal regulations allow you to obtain access to your PHI collected or used in this study. While the research study is in progress, your access to the PHI in your study records will be temporarily suspended. You will be able to access your information when the research study is completed. At that time, you will have the right to see and copy the medical information collected from you in the course of the study, for as long as that information is maintained by the study staff and other entities, subject to federal privacy regulation.
The research information from this study will be stored for a period of _ years.
VOLUNTARY PARTICIPATION AND STUDY DISCONTINUATION
Your participation in this study is voluntary. If you decide not to take part in the study,
you will continue with the standard treatment. The treatment recommended to prevent
another stroke is to take either aspirin or clopidogrel. Good control of high blood
pressure is standard care after a stroke, although doctors disagree about what blood
pressure level is best for persons who have suffered a stroke and to control high blood
pressure.
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
You are free to choose not to participate in this study or to withdraw from it at any time. You are also free at any time to cancel the authorization of the use and disclosure of your PHI. If you choose to not be in the study or to not authorize the use of your PHI, there will be no penalties. In other words, you will not be denied any treatments you may need and it will not affect your eligibility for any health plan or any health plan benefits or payments for which you may be eligible.
You may cancel or "revoke" your authorization for the researchers to collect, use and disclose your PHI at any time. However the request to revoke the authorization must be sent in writing to Dr. or _ at . If you revoke your authorization, your participation in the study will end and the study staff will stop collecting medical information from you and about you. However, the study staff will continue to use the PHI collected about you before you cancelled your authorization to the extent that, in order to preserve the scientific integrity of the study, it is needed to complete the research.
Your participation in the study may be terminated if, among other reasons:
You ask to stop taking part in the study; Your doctor decides to take you off the study should he/she feel that it is in your
best interest, for your well being and health;
The sponsor has decided to terminate the study; Any other exclusion criteria stated in the study protocol.
This authorization expires in 2012.
CONTACT PERSONS
If you have any questions now, feel free to ask us. If you have additional questions later
or you wish to report a medical problem that may be related to this study, your doctors
can be reached at the following numbers below:
Coordinator Neuro
To reach someone in an emergency, please page _
The University committee that reviews research on human subjects
(Institutional Review Board) will answer any questions about your rights as a research
subject (tel: ).
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
INFORMED CONSENT FORM
We will give you a signed copy of this form to keep.
Sign this form only if all of the following are true:
I have read (or someone has read to me) the above information I have decided to take part in this research study I understand the information given about my protected health information and
authorize the collection, uses and disclosures of my protected health information as described in this form
My questions have been answered to my satisfaction and I believe I understand
all of the information given to me about this study
Signature of Subject
Signature of Witness
Signature of Person Obtaining Consent Date/Time
Printed Name and Title of Person
In addition to the main study for which I have signed above, I also agree to participate in the additional part of the study: □
taking a blood sample at study entry and one year later to see if there are blood proteins which predict the risk of another stroke and also indicate the body's response to the anti-clotting drugs (as explained on page 3)
Signature of Subject
Signature of Witness
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
S econdary Prevention of
Smal Subcortical Strokes
<physician address>
RE: <patient name>
This letter is to inform you that your patient has elected to take part in a research study entitled,
"Secondary Prevention of Small Subcortical Strokes (SPS3)." The study is sponsored by the National
Institutes of Health and is being conducted at 70 sites in the US, Canada, Mexico, South America, and
Spain including <name of your site>.
This is a phase III study designed to assess the effect of two interventions on prevention of recurrent
stroke and maintenance of cognitive function. Patients will be randomized to receive 1) either aspirin
alone or aspirin plus clopidogrel (Plavix), and 2) to a systolic blood pressure control target, either 130-
149 or below 130. While it is generally recommended that blood pressure be controlled below 140/85
for most people with high blood pressure, we do not know what target levels are best for people who
have already had a stroke. No one knows whether lowering blood pressure after a stroke might help
prevent another stroke or cause further damage; this is the goal of the blood pressure aspect of the
study. (If your patient does not have high blood pressure, no blood pressure management will be
done.)
We will treat your patient's blood pressure using standard medicines according to accepted JNC
guidelines, lowering your patient's blood pressure to the assigned goal. No experimental blood
pressure medicines will be used. Your patient will have information about the names and doses of the
blood pressure medications prescribed. There is no cost to the patient for anti-hypertensive
medications or antiplatelet therapy.
If you have questions, please do not hesitate to contact our Study Coordinator, _
at ( ) _ or one of us.
Sincerely yours,
<Neuro principal investigator>
<Hypertension specialist>
<contact information>
<contact information>
ManOp section XVII – 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
S econdary Prevention of
Smal Subcortical Strokes
<physician address>
RE: <patient name>
was seen today in the Stroke Prevention Clinic.
The mean electronic blood pressure was _ mmHg. We recommended that the patient continue on the following anti-HTN therapy:
Your patient is scheduled to return to the clinic on _.
Please contact Dr. <neuro PI> at or Dr. <htn specialist> at _
if you have any questions about this therapy or the study.
Thank you,
<study coordinator >
<contact information>
ManOp section XVII – 08/15/09
Secondary Prevention of
Small Subcortical Strokes
ManOp section XVII – 08/15/09
Secondary Prevention of
Small Subcortical Strokes
ManOp section XVII – 08/15/09
Secondary Prevention of
Small Subcortical Strokes
ManOp section XVII – 08/15/09
Secondary Prevention of
Small Subcortical Strokes
ManOp section XVII – 08/15/09
Manual of Operations
Small Subcortical Strokes
E. The DASH diet (Adapted from the Mayo Clinic Health Letter)
For years doctors have recommended reducing sodium to help lower your blood
pressure. Now they've discovered that a diet rich in fruits, vegetables, and low-fat dairy
products also can lower your blood pressure. An eating guide called the Dietary
Approaches to Stop Hypertension (DASH) diet may help you lower high blood pressure.
And the diet may also provide other health benefits.
Diet comparison
The DASH diet evolved from the DASH-Sodium study. For 8 weeks, participants
followed one of three diets:
• A diet that matched the average American diet • A diet rich in fruits and vegetables • A combination diet that was reduced in saturated fat and emphasized fruits,
vegetables, and low-fat dairy products
Sodium consumption in all three diets was about 3,000 mg per day.
The result: The fruit and vegetable and combination diets both lowered blood pressure,
but the combination diet was most effective. In that group the decrease was greatest
for those with high blood pressure - with an average drop of 11 points in the systolic (top
number) and 5 points in the diastolic (bottom number) blood pressure.
Researchers aren't sure why the combination diet fared better. However they believe it
is due to the mixture of nutrients provided rather than any single ingredient.
What about sodium?
The benefits of the DASH diet are improved even further by the reduction of salt. A
1997 study of 412 people was conducted at five US hospitals and sponsored by the
ManOp section XVII – 08/15/09
Manual of Operations
Small Subcortical Strokes
National Heart, Lung and Blood Institute (NHLBI). Those in the study were divided into
two groups and put on controlled-salt diets for 14 weeks.
One group ate an average American diet composed of foods such as roast beef, ham,
turkey, high fat sweets, and some fruit and vegetables. The other group followed the
DASH diet. Members of both groups consumed three levels of sodium during different
stages of the trial - a higher intake of 3300 mg daily, an intermediate intake of 2400 mg
and a lower intake of 1500 mg.
Current dietary guidelines say that you should limit your daily intake of sodium to no
more than 2400 mg. Government researchers say that many Americans typically
consume more than 4000 mg of sodium daily.
The group on the DASH diet experienced a dramatic average blood pressure drop of 9
mmHg in the systolic (top number) blood pressure reading and a 4 mmHg drop in the
diastolic (bottom number) when ingesting 1500 mg of sodium. This represented the
biggest reduction in the study and indicates that sodium is a very important factor in
blood pressure control.
Widespread benefits
The DASH diet may improve your health in other ways. Fruits and vegetables may
reduce your risk of some cancers. The calcium in dairy products can lower your risk of
osteoporosis. And a diet low in saturated fat and cholesterol can reduce your
cardiovascular disease risk. The diet also lowers the amount of homocysteine in your
blood, which when high is another risk factor for cardiovascular disease. An additional
plus: The diet is made up of foods readily available at your local grocery store.
The DASH diet can't do it alone, though. It's important that you take other steps to
control hypertension, such as: exercising, losing excess weight, not smoking, and
limiting alcohol.
ManOp section XVII – 08/15/09
Manual of Operations
Small Subcortical Strokes
The Specifics of the DASH Diet
Here are the number of servings you should consume daily from each food group.
Serving amounts are based on a diet of 2,000 calories per daily.
Food: Daily Servings
1 serving equals
Grains and grain products: 1 slice bread
Whole wheat breads,
Servings: 7-8 daily
1/2 cup dry cereal
English muffins, pita bread, bagels, cereals,
1/2 cup cooked rice
1/2 cup cooked pasta 1/2 cup cooked cereal
Fruits and Vegetables:
6 oz. Fruit or vegetable juice
Apricots, bananas,
Servings: 4-5 fruit daily and
grapes, oranges,
4-5 vegetable daily
grapefruit, melons,
1/2 cup frozen or canned fruit strawberries, tomatoes, 1 cup raw, leafy vegetables
peas, carrots, potatoes, broccoli, squash, leafy
1/2 cup cooked vegetables
Dairy: (low-fat or non-fat)
Skim or 1% milk, non-fat
Servings: 2-3 daily
or low-fat yogurt, non-fat or part-skim cheese
1 1/2 oz. Cheese
Meats, poultry & fish:
3 oz. cooked meat
Lean meats only; trim
Servings: 2 or fewer daily
3 oz. cooked poultry
visible fat, remove skin from poultry; broil, roast
3 oz. cooked fish
Nuts, seeds and legumes:
Almonds, peanuts, mixed
Servings: 3-4 per week
2 tablespoons seeds
nuts, sunflower seeds, kidney beans, lentils
1/2 cup cooked legumes
ManOp section XVII – 08/15/09
Manual of Operations
Small Subcortical Strokes
XVIII. STUDY MEDICATIONS
The principal (PI) investigator at each clinical site is responsible for a complete and
accurate accounting of all SPS3 study medications received from the Drug Distribution
(DDC). The DDC will provide forms, instructions, and assistance as necessary to
assure proper use and accountability of all drugs in this regard. Each site must observe
local policies and applicable state and federal regulations concerning custody,
dispensing, labeling, and disposition of medications. (See http://www.nabp.net for links
to individual state pharmacy boards)
A. Antiplatelet Medications
Antiplatelet medications are packaged in kits with each kit designed to last one specific
patient six months. There are 4 bottles in a kit, 2 bottles each of aspirin 325 mg and 75
mg clopidogrel/placebo, with each bottle containing a three month supply of medication
(100 capsules/tablets). One bottle type contains aspirin and has the manufacturer's
label on it. The other bottle type contains either clopidogrel or placebo and is labeled
"clopidogrel/placebo" and both these bottles are labeled with the same patient ID. Two
bottles (one bottle of each type) are dispensed to a patient at randomization and at each
quarterly follow-up with bottles being dispensed consecutively according to the bottle
Each kit has a patient id on it. The first two numbers are your site number. The next
three numbers tell you whether it is a HTN stratum kit or a normotensive stratum kit. If
this number is below 100, it is a HTN stratum kit, and if it is 100 or higher, it is a
normotensive stratum kit. For example, the kit with the number 01-000-6 is a HTN
stratum kit for site 01, whereas the kit with the number 24-100-8 is a normotensive
stratum kit for site 24.
B. Antihypertensive Medications
These medications are packaged so that they can be dispensed directly to the patient
without transfer to another container. Each bottle contains 100 tablets (the exception is
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
candesartan which contains 90 tablets and depending on the manufacturer, Felodipine
may also be packaged in bottles of 90 tablets) and has the manufacturer's label which
contains the lot number and expiration date. In addition, a label affixed by the DDC
contains study name, bottle number, applicable federal warnings, and a contact number
at the DDC. Bottles are shipped in boxes containing 4 bottles.
C. Custody and Storage Requirements
SPS3 study meds should be stored at room temperature between 15° and 30°C (59°
and 86°F) in a secure area. Drugs may be stored either in the study clinic or pharmacy
depending on local policy. If the study drug is not stored in a pharmacy, it must be kept
in a locked room or locked cabinet. Each clinical site must be in compliance with state
regulations and Board of Pharmacy requirements for storage of study medication.
D. Shipment of Study Medications
After receiving notification from the SPS3
Coordinating Center that all required regulatory
documents (i.e. IRB approvals) are received
and that all necessary training has been
completed, the DDC will send the clinical site
an initial shipment of study meds. A Shipping
Notice listing items and quantities of all
supplies shipped is included with each
shipment. In addition, a Receiving,
Assignment, and Destruction Record is
enclosed for each box of medications.
Upon receipt of the shipment, check that you
have received each medication as listed on the
Shipping Notice, and that the Receiving,
Assignment, and Destruction Record is correct for each medication. If there appears to
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
be a discrepancy, or you have a question regarding the shipment contents, contact the
DDC (Dave Hunt or Robert Ringer (505) 248-3203). We are providing you with a binder
in which to file Receiving, Assignment, and Destruction Records for each medication.
E. Dispensing Study Meds
When a patient is randomized, the randomization program on the computer assigns a
patient id number with the format ss-ppp-c XXXX, where ss is the site, ppp is patient
number, c is the checkdigit, and XXXX is the patient acrostic (e.g. 01-011-1 JSMI). The
corresponding kit with aspirin and clopidogrel/placebo bottles will have the
corresponding label except without the patient acrostic. The clopidogrel/placebo bottles
within the kit will have the patient id number on them, but the aspirin bottles will not.
You will need to put patient id labels on the aspirin bottles. Dispense the bottles within
a kit in order by bottle number. In most cases you will start with bottle 1, bottle 2, etc.
Related to expiration of the study drug, there may be occasions where bottle 1 will not
be the first bottle dispensed or where you may skip a bottle number. Complete the
information for each bottle dispensed on the Receiving, Assignment, and Destruction
Record for that kit. Alternatively, you can use the web
site to maintain your records and indicate the date the
bottle was dispensed and to which patient using the u
pop-up menu and print or save the file on your computer.
Regardless of whether you use the Receiving,
Assignment, and Destruction Record forms or the
website, you must have a record of what medication was
dispensed to which patient on which day.
The bottles of antihypertensive medications do not
contain a patient id number, and when you dispense a
bottle to a patient, you will need to put a patient id label
on that bottle. Complete the information for each bottle
dispensed on the Receiving, Assignment, and
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
Destruction Record for that medication or alternatively using the web-based inventory,
indicate the patient ID and date of dispensing for each bottle which is dispensed. This
information can be printed and filed or stored as a file on your computer. When
dispensing study medications, consider whether the medication will expire before the
bottle is completely used.
Whenever you dispense a study medication, you also need to affix a prescription label
to each bottle that is dispensed. Labels need to contain the following information:
Prescription number (if dispensed by pharmacy) Patient's name Drug name, strength, and quantity Directions for use Prescriber's
Name of facility Emergency phone number (local) Do not cover labels applied by the DDC or the manufacturer's lot number and expiration
date. Formatted prescription labels are supplied by the DDC for those sites not utilizing
a pharmacy. The individual dispensing the drug simply fills in the necessary information
on the formatted label using a typewriter or with pen and ink.
Prescription of study-related medications must be documented as part of the medical
record or on a standard prescription blank. Documentation needs to conclude the
following information:
Patient's name and current address Study name [SPS3 Trial] and patient id number Drug Name, Strength and Quantity to be dispensed Directions for use Signature of investigator or other authorized physician Date prescription or order is written No refills (applicable only to medications on the SPS3 formulary)
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
Each bottle of medication (blinded study drug, ASA, and antihypertensive medication)
has a perforated label on the bottle. Please detach the perforated portion and attach to
the patient file in a consistent place. This label provides a space to enter the date and
the patient's acrostic (an additional check that the correct bottle is being dispensed to
the correct patient).
F. Web-Based Drug Inventory
Sites will access their inventory via an Internet web page hosted in Albuquerque. There
are no computer system requirements to use this website. Authorized personnel will use
the web pages to indicate dispensing of medications from their current inventory. This
information will be retrieved from the website by DDC personnel on a weekly basis and
used to generate new drug orders.
The website address is: https://www.csp.gov/ctsc/login.cfm
Site personnel will register on the website
and be authorized to manage inventory by
website administrators. This process
provides a level of security as well as an
opportunity to ensure that users are
assigned to the correct site. New users
will click on the "Would you like to
register?" link and fill out the form which
appears. Existing users will enter their
User ID and Password and click the Log
In button. This will take the user to their
site-specific inventory control page.
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
Users will see the current inventory at their site, according to DDC records. The default
page is the antihypertensive inventory. By moving the radial button to the
Dispensed/Destroyed,
you are able to view all
antihypertensive
medications that you
have dispensed or
destroyed. By selecting
Antiplatelets and clicking
on Inventory, you will
view your current
antiplatelet inventory and
you can also view what
you have dispensed or destroyed by selecting that option.
Drugs listed can be sorted and filtered in many ways, making it easier to locate specific
bottle numbers. The inventory
can be filtered in a variety of
ways including medication
name, bottle number, and
ship date. Filtering by
medication name is most
likely how ongoing
management will be handled.
The screen here shows a filter
of "ATEN" applied to this site's
Whether or not a filter is applied, records can be sorted in order to facilitate inventory
management. To the right of the screen, you will see the options for ordering (sorting)
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
the medications. You have several options for ordering including generic name, bottle
number, kit number, and kit by patient. Ordering the antiplatelets by ‘Kit by Patient' sorts
your antiplatelet kits in ascending order by patient ID and places the ASA and study
drug that make up that kit together in the inventory. This makes it much easier for you
for indicating the dispensed study drug and ASA. This report is a little slow and will not
function correctly using some filter options.
F.1 Indicating Dispensed/Destroyed Medications
To indicate medications as dispensed, please mark the Dispensed box for each item
and click the Submit button at the top of the page. If you wish to enter the patient ID and
the dispense date if different from current date, please select the update link to the right
of the dispense check box. The current date is displayed by default (in the pop-up
window) but can be changed. Once you have clicked the "update" button on the small
popup window you do not have to press submit unless you have checked off bottles
without using the u link (see below). If you do however submit the form after dispensing
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
by using the u update popup this will NOT over-write the date you entered in the popup.
After selecting update on the popup the page will not display submitted data until you
refresh the page. In this case it will remove records from list under inventory and they
will show up in the dispensed/ destroyed page. Until the DDC uploads the information
on dispensing/ destroying to
their database, you will see that
the check mark (in the
dispensed/ destroyed window)
is still active. This means that
if you made an error (for
example, indicated the incorrect
bottle as dispensed), you could
click on this and it would go
back into your inventory. If the
DDC has already picked up the
orders the button is no longer
active and if you need to make
a change, you will need to
contact the DDC directly.
F.2 Special Ordering Medication
Clicking on Request Medication brings up the following page where you can order ASA
81mg (with approval from the CC) and extra boxes of medications. After entering the
number of boxes, users will click Submit to add the order. The order will be processed
by the DDC at the same time as other orders. If your par has been set to 0 for a
medication, ordering one box of this medication will re-set your par to one box (4
bottles). If you already have a par level for a certain medication, ordering an additional
box will not reset your par. If you determine that you need to keep more of a specific
medication in stock, you can contact the DDC (from this page) and request that your par
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
be increased. You can view your par
and order level on each medication
by selecting that option under the
F.3 Re-supplying Study
Medications
The DDC will monitor inventory levels at each site through the web-based inventory.
For each medication (based on your usage), you are assigned a par level (order level)
and an order point (the number of bottles on hand that will trigger a shipment – this is
usually 50% of the order level). You can view this information for your site by selecting
Par Levels. This will show you your previous six months usage. As your usage of a
medication increases, the inventory level will automatically increase. As well, you can
make adjustments to your par levels by contacting the CC or the DDC.
Once you dispense or destroy a medication, please indicate this on the website. Once
you reach your order level, this will trigger a new shipment of medication. The DDC will
upload the information on what you have dispensed and destroyed on a weekly basis
(Tuesday morning). Orders will be process for shipment by the DDC the following
week. As a shipment leaves the DDC, it is scanned as it is picked up by Fed Ex (for
overnight delivery). Once it is scanned, this information will appear on your inventory
under Ship Date and you can expect to receive it the following day (or shortly after that
if it needs to clear customs).
The DDC is only able to re-supply your inventory as you indicate medications as
dispensed or destroyed. If you do not complete this step, the DDC assumes this
medication is available on your shelf for dispensing and it will not be replenished. Once
the two bottles of study drug (from the anti-platelet kit) have been indicated as
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
dispensed, this will trigger a new kit to be sent to your site within 8 working days of the
order being picked up by the DDC.
Please indicate all dispensed medications on at least a weekly basis (before the weekly
upload to the DDC). This will ensure that you have medications available for all patient
follow-up visits.
F.4 Replenishing Expiring Medications
For the antihypertensive medications and the ASA, you will see the expiration date
indicated in the Expiration Date column. When a medication is within 3 months of the
expiration date, this will be highlighted in red. Please do not dispense medication that
will expire before you have a chance to replace it. Please destroy any medication that
is expiring and indicate this as destroyed in your web-based inventory. It will only be
replaced as you indicate that it has been dispensed or destroyed. It is important that
you do this in a timely manner so that you receive replacements for expiring
When ASA that is part of the anti-platelet kit is expiring, please do not destroy the entire
kit (the blinded study drug). Destroy the expiring ASA and indicate this as destroyed on
the website. To replenish the EC-ASA 325mg, please request the number of boxes
needed (4 bottles per box) under Request Medication menu.
The expiration date is not provided for the blinded study drug. This is to maintain the
blinding. When either the Clopidogrel or the placebo is expiring, the DDC will notify you
and will send you replacement kits for the expiring lots of study drug.
Unless otherwise directed by the DDC, expired drugs are destroyed by the local clinical
sites, in accordance with local policies for drug destruction.
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
G. Unblinding
Antiplatelet therapy (clopidogrel vs. placebo; all patients are assigned aspirin) is given in
a double-blind manner. The treatment code will only be broken if the knowledge of
whether the patient is receiving clopidogrel vs. placebo is essential to the medical
management of the patient. Unblindings are discouraged; however, the patient's safety
must always be paramount. Study personnel will remain blinded if at all possible.
If the clopidogrel/placebo blind needs to be broken, the site or physician directly
involved in the patient's care will contact Benavente/Hart at the CC for permission).
Assuming that Benavente/Hart agrees, the physician directly involved in the patient care
will then contact the DDC (Rob/Dave (505) 248-3203; pager 1-877-561-1862).
H. Completion of Study
At the end of the study, each site will inventory all remaining study medications. Once
inventory is complete, all study drugs will be destroyed on site.
ManOp section XVIII –08/15/09
Manual of Operations
Small Subcortical Strokes
XIX. COMPLETING SPS3 DATA FORMS
Data will be collected and recorded on paper forms at the clinical sites. You will be
provided with a CD-ROM from which to print the forms, and you may also print them
from the SPS3 Web site. Each form must be completed neatly and carefully to ensure
data quality, and then stored securely. Please check your forms for completeness
before you enter them.
Form Completion Guidelines:
• Affix a patient id label to the top of each page of each form. Do not write the
patient's name or other patient identifying information on the form.
• Staple all pages for each form together
• Use a pen, not a pencil to complete forms.
• Mark boxes clearly with an X or 9.
• Write numbers clearly in the number of spaces provided. A value of 123 will fit
into an area of " " but will not fit into an answer area of " ". Similarly,
a value of 0.3 will fit into an area of " • ", but is smaller than expected for a
space of " ". If you have a value that appears too large or too small for the
space indicated, check the units of your measurement against that indicated on
the form. (Do not write in a decimal point.) If this does not resolve the problem,
contact the Study Coordinator. If you have a value that is more precise than
requested, round the value (go up on 5). For example, a hemoglobin value of
14.5 would be recorded as 1 5 and a value of 14.2 would be recorded as 1
• Print letters so that they can be clearly read for data entry. It may be necessary
to use abbreviations. If specified, do not exceed the number of characters
allowed for an answer.
ManOp section XIX – 02/01/10
Manual of Operations
Small Subcortical Strokes
• If you make a mistake on a form, label the mistake as an "error", indicate the
correct response, date and initial nearby.
• If you are asked to indicate one answer (i.e. 9one), then mark only one box. If
you feel that it is necessary to mark more than one, contact the Study
Coordinator for help.
• More than one answer may be marked only if indicated (i.e. 9 all that apply).
• Record dates in the format given, usually mm/dd/yy or mm/yy where mm
indicates the month, dd is the day of the month, and yy is the year. Occasionally
you will be asked to record a date with a four-digit year.
• Check the alternate responses carefully before you mark the box "Other". It is
highly likely that one of the options is appropriate. The forms have been
designed to minimize the number of "other" responses.
• If you need to write additional information on the form, write in the white space
away from the printed questions and answers on the form.
• Each form must be signed at the end by the indicated SPS3 personnel. "MD
completing form" means that the signature of the physician is required. If you are
a research-nurse, do not allow your study physician to coerce you into
completing forms that are designated for physicians to complete. During our site
inspections, this will become clear (i.e. we'll find out). Call Oscar Benavente or
Bob Hart at the Coordinating Center to initiate an attitude adjustment for your
study physician.
• The paper forms may in some cases be the source documents (i.e. the quality of
life forms) so it is essential that the paper form matches the DE system for all
• If you don't understand a question, please don't hesitate to contact the Study
Coordinator at the Coordinating Center. We are here to help!
ManOp section XIX – 02/01/10
Manual of Operations
Small Subcortical Strokes
SPS3 Patient Forms:
Form Name and Number
Follow-up Contact
Quarterly Annual Add'l
Baseline History
Patient Contact (site use only)
Review regularly; update as needed
Qualifying Stroke
Post-Stroke Disability
Cognitive Scales*
HTN Quality of Life
Current Meds (site use only)
Update as needed after each patient visit
24 hr Ambulatory BP
Stroke Symptoms QOL
Intracranial Vascular Imaging
Failure to Achieve Assigned Target
Stroke Symptoms Questionnaire
Event Notification
Ischemic Stroke/TIA Event
Major non-CNS Hemorrhage
Serious Complication of Hypotension
Other SPS3-related SAE
Acute MI & Other Thromboembolism
Major Cognitive Decline
Death Notification
BEA Ischemic Stroke/TIA
BEA CNS Hemorrhage
Status of Therapy
Premature Study Termination
Cognitive Screening
± See HTN trial protocol/form for instructions; * Cog Screening Form 90 completed after year 5 annual If indicated. See specific form for instructions.
ManOp section XIX – 02/01/10
Manual of Operations
Small Subcortical Strokes
XX. SPS3 DATA FORMS AND LINE-BY-LINES
Form version numbers and dates are found in the bottom right hand corner of the form and line-by-line instructions
SPS3 Form/Line-by-Line Name
Line-by-Line Version
07/01/07 01.Screening.03
Baseline History
07/01/07 02.Baseline.06
Patient Contact (not entered on PC) 03.02
Medications 04.04
Qualifying Stroke
07/01/07 06.QualStrk.03
Post-Stroke Disability
06/15/04 07.PostStrk.02
06/15/04 08.NeuroExam.01
Cognitive Scales (English, Spanish, French) 09.04
Cognitive Screening Form
08/15/09 90.CogScreen.01
HTN QoL (English, Spanish, French) 10.03
Current Meds (not entered on PC) 11.01
11.CurrentMeds. 01
Eligibility/Randomization 12.03
24 hr Ambulatory BP
06/15/04 13.AmbulBP.02
Stroke Specific QOL (English, Spanish, French) 14.02
14.StrokeSxQOL.01
Intracranial Vascular Imaging
06/01/03 15.ICImage.01
07/01/07 17.BPCheck.04
Stroke Sx Questionnaire
06/06/05 19.StrokeSxQx.02
Event Notification
06/15/04 20.Event.01
Ischemic Stroke/TIA Event
06/15/04 21.StrokeTIA.01
07/01/07 22.CNS-Hemorrhage.01
Major non-CNS Hemorrhage
07/01/07 23.Major-Hemorrhage.01
Serious Comp of Hypotension
06/15/04 24.Hypotension-Comp.01
Other SPS3-related SAE
06/15/04 25.OtherSPS3-SAE.01
Acute MI & Other Thromboembolism
06/15/04 26.MIOther-Thromb.01
Major Cognitive Decline
06/15/04 27.MajorCog-Decline.01
Death Notification
06/15/04 31.BEAStroke-TIA.01
BEA CNS Hemorrhage
07/01/07 32.BEACNSHemm.01
Status of Therapy
06/06/05 36.StatusTherapy.02
Premature Study Termination
04/04/03 37.StudyTerm.01
06/15/04 38.RepeatMRI.01
Cognitive Screening
08/15/09 90.CogScreen.01
03/09/09 99. StrokeSx99.01
ManOp section XX – 02/01/10
Manual of Operations
Small Subcortical Strokes
XXI. DATA TRANSFER AND MONITORING
Data will be entered at the clinical sites and transferred electronically to the Statistical
Center on a real-time basis by use of a secure web-based system. Each clinical site
will receive a CD-ROM with the complete SPS3 randomization/data entry system
installation package and receive training on using the system. After the initial
installation of the software, whenever a clinic starts up the SPS3 system, the system will
automatically communicate with the SPS3 server at the Statistical Center. Data will be
continuously transferred to the main SPS3 database, and the local system will be
updated with the latest version of the program. No patient-identifying information will be
stored in the database. This SPS3 randomization/data entry system is used at a clinical
site to randomize a patient, print labels, enter forms, view and edit previously entered
data, and print data reports. See Section XXVI for instructions on how to install and use
the SPS3 system.
Data reports summarize complete forms, locked forms, overdue forms, and forms with
questionable data that have been entered at your site. A complete form has all relevant
questions answered and no unresolved validation checks. Complete forms should be
locked when you are satisfied that the data are accurate, correct and complete. You
are encouraged to enter and lock forms as soon as possible so that the
procedures/visits are credited as done. Locked forms are required for site payment
(see section XXIII). Overdue forms are forms expected to have been done and locked
more than 1 week ago, but which have not yet been entered at all, have questionable
data, or are complete but not yet locked. Forms with questionable data have at least
one of the following: non-confirmed response(s) outside the anticipated range,
unexpectedly blank response(s), conflicting responses within or across forms, etc. If
you do not understand an item on a report, contact the Study Coordinator at the
Coordinating Center or the Data Manager at the Statistical Center.
All clinic personnel using the system will be assigned a login name and password. Both
ManOp section XXI – 07/01/07
Manual of Operations
Small Subcortical Strokes
are required to logon to the system. Use only your assigned login name and password,
and do not share them with others.
Each user will be trained and certified by the Statistical Center to use the
randomization/data entry system. Following training, a mock set of completed forms will
be given to you to enter using the system. To obtain certification, you will need to
successfully enter these forms with an error rate below 0.5%. Those with higher error
rates will need to re-enter these forms until the error rate falls below this threshold.
Only those certified will have access to the randomization/data entry system.
Data Monitoring
Site visits are undertaken by the Coordinating Center (CC). Frequency of the visit to a
particular site varies according to site performance. During the site visit, approximately
50% of the patients' binders are selected for random review for completeness of source
documentation, informed consent and verification/accuracy of key fields (e.g. recorded
risk factor data at study entry) including inclusion/exclusion criteria. Any site violating
inclusion or exclusion criteria will need to contact the CC prior to enrolling each
subsequent patient for review of their entry criteria. With initial and ongoing training of
physician investigators, few protocol violations are expected.
Reports summarizing the completeness and consistency of forms completed at study
entry, during follow-up and for events for individual patients are available to the sites
weekly via the Data Completeness Report website.
The data entry error rate for each user will be closely monitored during the study.
Specifically, during the study on a regular basis, the Statistical Center will send you a
list of patient forms entered at your site to photocopy and send back to them within 2
weeks. Responses recorded on the paper forms will be compared with those entered in
the database. Any user with an error rate exceeding 0.5% will be re-trained and
required to double-enter forms.
ManOp section XXI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XXII. SITE VISITS
Site visits are made primarily by personnel from the SPS3 Coordinating Center and
occasionally by those from the Statistical Center (e.g. if data entry problems are
paramount) or by other members of the SPS3 Executive Committee. All SPS3 sites
will be visited within the first year after entering patients, at least one time thereafter
during the course of the trial, and more often as needed depending on problems with
recruitment, execution, or data-quality concerns.
Sites will be notified well in advance of the visits. The study coordinator will need to
arrange access to a selected group of participant's medical records. During these visits,
the Coordinating Center visitor would also like to meet with key study personnel both
together and individually to discuss study progress and to solicit your views about the
status at your site.
On-site activities will require an average of one-half to one full day to complete. Sites at
which there are special problems may require longer visits.
Purposes
• To document compliance with human subjects protections • To verify a sample of participant data by comparing medical records with the SPS3
forms, particularly focusing on eligibility criteria, key patient features, and event-
related materials
• To review local SPS3 study activities and procedures to ensure adequate physician
investigator involvement and documentation of day-to-day activities, to ensure
adequate commitment of time and resources for SPS3, and to identify areas of
• To encourage interest and enthusiasm among SPS3 collaborators and others for
study recruitment and execution (via meetings with investigators, presentation of
ManOp section XXII – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Policies and Procedures
• Compliance with Human Subjects Protections
The research chart of each SPS3 participant will be reviewed to verify the presence
of a signed study consent form that has been approved by the local committee
governing research in human subjects. The site visitor will ask the study coordinator
to describe the informed consent process including the involvement of study-
affiliated physicians.
• Verification of Participant Data
Baseline data forms will be reviewed and verified against source documentation for
6-12 randomly selected patients enrolled within the previous year or since last site
visit. Particular attention will be paid to on-site verification of eligibility criteria
(excluding MRI, which is done centrally) including the Qualifying Stroke form and the
Medical History and test results sections of the Baseline form. Sites at which
ineligible patients were entered will need to contact the Coordinating Center prior to
enrolling subsequent SPS3 participants for review of entry criteria.
• SPS3 Physician Involvement in Follow-up
SPS3 research charts will be reviewed for 6-12 randomly selected patients seeking
evidence of on-going physician investigator participation in follow-up visits and BP
• Review SPS3 Study Activities and Procedures
The site visitor will meet with the SPS3 study coordinator to review day-to-day
activities involved in recruitment and execution, including the time allocated to these
activities. Emphasis will be on assessing for adequate physician investigator
involvement and supervision in recruitment, blood pressure management, and
endpoint detection and processing. At centers where recruitment is lagging or
ManOp section XXII – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
follow-up is sub-optimal, options for improvement will be explored with the study
coordinator and discussed with the local principal investigator. Competing studies at
the site and/or studies or other commitments of the study team will be noted.
The site visitor will meet with the local Neurology PI and the local hypertension
specialist to discuss problems or issues at the site, make recommendations, and
answer questions. Whenever possible, the site visitor will meet again with the local
neurology PI at the end of the visit to debrief.
• Encourage Interest and Enthusiasm
During most site visits, the site visitor will meet with the entire local SPS3
investigators team over breakfast/lunch to present an update on the trial and to
discuss local performance, and to initiate discussion of ways to improve recruitment
and execution. As appropriate, the site visitor may present lectures to local
physicians who might refer participants to encourage their interest in SPS3.
• Site Visit Reports
Written feedback from the site visitor to the local SPS3 investigators, usually via e-
mail, will be provided within a few days of the site visit. This can be addressed to
the neurology PI or additionally to the study coordinator and hypertension specialist
at the discretion of the site visitor.
A site visit report will be prepared within two weeks (see below) and reviewed by the
SPS3 Executive Committee at its proximate meeting.
Protocol violations involving individual SPS3 participants identified during the site
visit will be recorded on the SPS3 Protocol Deviations Form (#40) by the site visitor
and entered into the SPS3 database by Coordinating Center staff. A copy of the
form will be sent to the site at the reviewer's discretion.
ManOp section XXII – 07/01/07
Secondary Prevention of
SPS3 Site Visit Form
Small Subcortical Strokes
SITE VISITED:
Site Number:
Site Neurology PI
Study Coordinator
1. Date(s) of visit
2. Site visitor(s)
3. Compliance with Human Subjects Protections
Signed informed consent form on file for each participant checked
(Append list of patients checked. Indicate status for each.)
Adequate consent process with appropriate physician involvement
If either not checked, explain:
4. Verification of Participant Data:
Complete site visit form below for each patient
a) Eligibility criteria (excluding MRI) verified for selected participants
b) Key baseline data verified for selected participants
c) BP Check/follow-up visits with physician input verified in SPS3 chart
If any not checked, explain:
ManOp section XXII – 07/01/07
Secondary Prevention of
SPS3 Site Visit Form
Small Subcortical Strokes
5. Review SPS3 Study Activities and Procedures
Site visitor met with:
Neuropsych test administrator(s) and/or supervisor
Blinded event assessor
Pharmacist (if meds stored centrally)
6. Summary
a) What are this site's strengths?
b) What are this site's weaknesses? What problems did you identify during the visit?
c) When do you recommend this site be revisited?
Please append a copy of your feedback sent to the site and any protocol violation forms resulting from this visit.
ManOp section XXII – 07/01/07
Secondary Prevention of
SPS3 Site Visit Form
Small Subcortical Strokes
1. Date of review:
Questions 3-7: Obtain the patient's SPS3 chart and medical records. Compare the completed Qualifying Stroke and Eligibility/Randomization forms with the medical records. Discuss any discrepancies with the local investigators and record them below.
3. Records reviewed:
hospital chart from qualifying stroke outpatient records
4. Patient's date of birth:
5. Do the available medical records support that the qualifying stroke occurred?
Yes, date of stroke
Uncertain/inadeq info
6. Does the qualifying stroke meet SPS3 criteria for S3?
Uncertain/inadequate info
7. SPS3 Eligibility Criteria:
Uncertain/inadeq info
Disabled by stroke (modified Rankin ≥4)
Uncertain/inadeq info
Prior intracranial hemorrhage or hemorrhagic stroke
Uncertain/inadeq info
Prior cortical stroke, clinically or by neuroimaging
Uncertain/inadeq info
Prior cortical or retinal TIA
Uncertain/inadeq info
Ipsilateral carotid stenosis ≥50% or CEA
Uncertain/inadeq info
Major risk for source of cardiogenic emboli
Uncertain/inadeq info
Other specific cause of stroke
Uncertain/inadeq info
Requirement for long-term anticoagulation/other antiplatelet
Uncertain/inadeq info
Intolerance to aspirin or clopidogrel
Uncertain/inadeq info
High risk of bleeding
Uncertain/inadeq info
Pregnancy or female of child-bearing potential
Uncertain/inadeq info
Life expectancy < 2 years
Uncertain/inadeq info
Impaired renal function: estimated GFR < 40 cc/min
Uncertain/inadeq info
Chronic alcohol use
Uncertain/inadeq info
Age < 40 years
ManOp section XXII – 02/23/2007
Secondary Prevention of
SPS3 Site Visit Form
Small Subcortical Strokes
Question 8: Compare the responses of the Medical History question on the Baseline form in the SPS3 chart with the medical records. Discuss any discrepancies with the local investigators and record them below.
8. Key participant features Prior to Qualifying Stroke:
Agree Disagree
Uncertain/inadeq info
Agree Disagree
Uncertain/inadeq info
Hypertension
Agree Disagree
Uncertain/inadeq info
Evidence of coronary artery disease
Agree Disagree
Uncertain/inadeq info
Intermittent claudication/peripheral vascular disease
Agree Disagree
Uncertain/inadeq info
History of major hemorrhage
Question 9: Review the BP Check and Fol ow-up forms in the SPS3 chart for documented physician investigator involvement at these patient visits. Discuss any concerns with the local investigators and record them below.
9. Documented appropriate involvement by physician investigator in BP checks:
Uncertain/inadequate info
10. Documented involvement by physician investigator in follow-up visits:
Uncertain/inadequate info
ManOp section XXII – 02/23/2007
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XXIII. SITE PAYMENTS
Payments to the clinical sites will be made on a monthly basis for each of the following:
randomization of an eligible patient, follow-up, BEA reports, and in participating sites',
sub-study payments (LIMITS, PAS3S, etc). Additional payments will be made for MRIs
and MRAs but only if pre-authorized by the Coordinating Center. Payment is initiated in
the month following, once the data entry criteria have been met. Typically, from visit
date to receipt of payment, the process takes approximately 60 days or longer.
Randomization of an eligible patient:
o Forms due at entry have been completed, entered, and closed, specifically
the Elig/Rand, Screening, Baseline, Medications, Ethnicity, Qualifying Stroke,
Post-Stroke Disability, Neuro Exam, Cognitive Scales, HTN QOL, and
Intracranial Vascular Imaging forms
o Reprints/image files on CD of the MRI scan documenting eligibility and
intracranial vascular imaging study have been received by the Coordinating
Quarterly Follow-up:
o Quarterly Follow-up form with the relevant auxiliary* form(s) have been
completed, entered, and closed
*Auxiliary forms by follow-up:
3 mo: Medications, Post-Stroke Disability, Stroke Symptoms QOL, Stroke
Symptoms Questionnaire if applicable
Annual: Medications, Cognitive Scales, HTN QOL, Stroke Symptoms QOL,
Stroke Symptoms Questionnaire if applicable
Other: Medications, Stroke Symptoms Questionnaire if applicable
While you are not paid specifically for an event during SPS3, events are part of
follow-up. Payment for the next scheduled follow-up visit is predicated on
ManOp section XXIII – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
submission and receipt of event forms and materials. The following are required
o Event documentation#, including the narrative summary, received by the
Events Coordinator
#See individual event-specific forms for listing of event-specific documentation, including neuorimaging study for ischemic stroke, TIA and
CNS hemorrhage events
o All relevant* forms completed, entered and closed
*Relevant forms by event type include the Event Notification, Medications,
Status of Therapy and event-specific forms for all event types, plus the Neuro
Exam form for Ischemic Stroke event
o After assessment and completion of relevant* forms for TIA, ischemic stroke,
or hemorrhagic stroke event and 2) after assessment and completion of the
BEA Ischemic Stroke TIA form due solely to a positive Stroke Symptoms
Questionnaire at a routine follow-up
*Relevant forms include:
Ischemic stroke: BEA Ischemic Stroke/TIA, Post-Stroke Disability
TIA: BEA Ischemic Stroke/TIA
CNS hemorrhage: BEA CNS Hemorrhage, Post-Stroke Disability
ManOp section XXIII – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XXIV. TRAINING MATERIALS: How-to-get started
1. Develop a systematic plan for identifying and screening all patients with subcortical
ischemic stroke. This may include informing your potential contacts such as
residents, nurses, and those in the investigation labs about the study and setting up
a reliable system for ensuring that you are aware of all potential SPS3 participants.
Do not rely on others to call you about potential patients – you will need to take the
initiative and look actively for patients. Test your procedures by beginning to screen
patients using the SPS3 Screening Form.
2. Meet with your SPS3 neurologist, SPS3 hypertension specialist and SPS3
neuropsych test administrator to plan regular clinic times for recruitment and follow-
up. Keep in mind that it is optimal to hold SPS3 clinics in the morning. Plan to have
a meeting between the research coordinator and these three SPS3 colleagues at
least monthly (probably every two weeks during the initial months of the study) to
address issues arising (this might be conveniently held immediately before your
regular SPS3 clinic).
3. Identify clinic space (per #2).
4. Work with neuropsych test administrator to complete SPS3 certification process.
5. Along with your SPS3 PI neurologist, meet with radiology to arrange:
∗ the process for transferring MRI and intracranial arterial imaging, most preferably
image files on CD as opposed to a scan reprint, to the Coordinating Center
∗ negotiate a deal to obtain SPS3-sponsored MRIs and MRAs (without
6. Consider a regular meeting with your SPS3 neurologist for a half-hour early each
week to review screening information and address issues.
7. Begin to organize your office with the written materials related to the study, i.e. IRB
communication, copies of consent forms, patient filing system, manuals, blank
8. Review local policies for storage and destruction of SPS3-supplied medications and
identify suitable storage facilities/cabinets.
ManOp section XXIV - 06/01/03
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
9. Work with the Statistical Center to get the Randomization and Data Entry System
installed on the PC you will use and the certification process completed for yourself
and other persons involved in entering data on the PC.
ManOp section XXIV - 06/01/03
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XXV. TRAINING MATERIALS: Neuropsych Examiners
Cognitive Battery Administration and Instructions
Certification: Prior to administering the SPS3 Cognitive Battery to an SPS3 participant,
the examiner must be SPS3-certified by the SPS3 Coordinating Center (CC) to
administer the test in that language (English, Spanish, or French). Examiners are
typically licensed clinical psychologists experienced in standardized neuropsychological
assessment, or neuropsychology technicians or graduate students under the direct
supervision of the licensed psychologist.
Steps for certification:
1) Review these written materials.
2) Complete the Knowledge test - This is a written test about 25 items in length
designed to assess familiarity with key administration and scoring procedures for
each test. Test is available from the local research coordinator or CC
neuropsychology administrative assistant.
3) View a videotaped administration of the battery and score it on the SPS3 Cognitive
Scales form. The DVD/VHS tape and documentation on the videotaped individual
are available from the CC neuropsychology administrative assistant.
4) Administer the battery to a local study coordinator or other normal volunteer, who will
act as a practice patient. This session needs to be audiotaped or recorded on CD
with the Cognitive Scales form completed.
5) Send the completed Knowledge test (step 2), Cognitive Scales form for sample
patient (step 3), and Cognitive Scales form and audiotape/CD from practice
administration (step 4) to the CC in Vancouver, attention: SPS3 Neuropsychology.
Completed certification materials received at the CC are electronically tracked by an
administrative assistant and reviewed by a neuropsychologist fluent in the testing
language. Specifically, the Knowledge test is corrected, the scoring on the sample
administration is checked, and the practice administration is reviewed with the aid of an
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
electronic-based checklist consisting of key administration and scoring items for each of
the eight tests in the SPS3 battery. After reviewing the certification materials, the
neuropsychologist sends the examiner feedback about his/her performance and
certification status. Certification date(s) and language(s) of certification for each
examiner are tracked electronically at the CC.
General Testing Considerations: Testing should be conducted in a quiet office or
exam room with the subject comfortably seated in a chair with a desk or adjustable tray
table with the testing materials directly in front of them. Test forms, a stopwatch, an
audio-recorder, and the NP materials should be in order and within easy reach. Testing
should be completed in a single session on the same day. Brief breaks are allowed, but
breaks should be limited to between tests/subtests. Breaks should be avoided between
the immediate and delayed recall trials of the CVLT in order to prevent extending the
optimal time ( 20 min) between the two trials. Subjects should be reminded that the test
battery is designed to measure their thinking/attention/memory skills and that everyone
has difficulty with some items.
It is important to remember that we are trying to elicit the best performance possible
from these subjects with injured brains so we need to pay particular attention to the
pace of our administration. Please remember to read directions very deliberately and
sometimes even slower than would be considered normal for ordinary conversation.
Many of our patients have trouble comprehending, and many others process
information very slowly. If we are too fast, s/he may not understand or otherwise feel
intimidated and will not give a best effort. Also, please watch the transitions from test to
test. A well-placed word of encouragement during these transitions may be very helpful
in sustaining motivation. When transitioning from CVLT to COWA remember that these
are both word recall tests. Give the subject time to shift attention by asking how s/he is
doing or making light conversation appropriate to your particular situation before
transitioning between two tests within the same cognitive domain.
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Feedback on specific test performance/results during testing should be avoided, but
patients should be thanked and praised for their efforts during testing to maintain
motivation and rapport. If specifically requested, patients can be informed that a brief
summary of the test results can be released to their physician by the SPS3 Coordinating
Center, but only with their written consent and after the test results have been reviewed
by the study neuropsychologist. In these cases, patients must complete a release of
information form (local to the site/facility) specifying that test results be released to their
physician, who should be listed by name and complete mailing address. A copy of the
form and the SPS3 protocol should then be mailed to us in the SPS3 Coordinating
Center. HIPAA regulations preclude routine faxing of psychological test results, so the
interpretation will be sent by mail.
It is not advisable for a family member or any other person to be present in the room
during testing. Their presence may distract the patient and could affect the test results.
This should be politely explained to the family member with a request that s/he wait
outside. If the patient or family member absolutely insists and testing is not possible
otherwise, then the family member should be seated behind the patient and instructed
not to speak during testing.
The battery may be administered in English, Spanish, or French depending on the
patient's stated preference. Some bilingual patients tested in one language may ask to
have items or instructions translated to another language. If the patient prefers to be
tested in English, instructions in Spanish or French may be given if that would help them
understand the task. However, all test responses must be given in the language of the
test administration. Note that scoring/administration instructions are printed in English
on the Spanish and French versions of the protocol; only the verbatim instructions and
stimulus items are translated into Spanish or French; therefore test administrators must
be fluently bilingual. Tests are not to be administered through an interpreter.
To increase standardization and facilitate easy administration, all test record forms with
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
key administration/scoring instructions have been combined into the SPS3 Cognitive
Scales form. Tests are administered in the order presented in the form with the
exception of adding or postponing tests to maintain the optimal 20 min between the end
of the short delay cued recall and the start of the long delay free recall of the CVLT. The
research coordinator will provide you with a blank form to be used for each
administration, rather than the published record forms. Permission has been received
from the test publishers to reproduce these instructions/forms.
Quality Assurance: All cognitive test administrations in SPS3 need to be audio-
recorded for quality assurance purposes. Completed forms are entered into the
database and filed with the subject's SPS3 records by the local research coordinator.
The audiotape or CD, labeled with a patient id label and the date of test, is sent with a
copy of the Cognitive Scales form (all 18 pages including copies of the CLOX,
handwriting writing sample, and pentagram drawing, any of which may be on the
backside of a page) to the neuropsychology administrative assistant at the Coordinating
Center in Vancouver.
Do not write the subject's full name on the form: this is in violation of HIPAA. Your site
coordinator will affix a patient id label in the upper left hand corner of each page and
record the testing date on each page. Please do identify and keep the pages together
for each test subject until this time.
Forms and tapes received at the Coordinating Center are tracked electronically. Site
coordinators are notified by the neuropsychology administrative assistant of incomplete
materials received on arrival, and outstanding materials are requested on a quarterly
basis. Forms and tapes/CD are labeled with patient id and date of testing and stored by
site in locked secure areas.
At least the first two administrations for each new examiner are fully reviewed by a
neuropsychologist and feedback provided to the examiner. As well, at least two
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
administrations each year for any active examiner are randomly selected for full review
to identify any drift away from the SPS3 administration and scoring protocol. Feedback
on test administration and scoring is given to the examiner, and results of these quality
assurance reviews are tracked electronically. In addition, test scores of 0 and missing
test scores are reviewed for accuracy. Any necessary changes to the original paper
form maintained at the site and/or the study database are done through the site
coordinator and tracked electronically for completion.
General Instructions
Before examining begins, complete questions 1-4 on the SPS3 Cognitive Scales form:
1. Date administered
2. Reason for form: To be completed by research coordinator
3. Tests are administered in: Indicate language of testing
4. Tests are administered by: Tests need to be administered by certified personnel.
See above for certification process.
Cognitive Assessment Screening Instrument (CASI): Pages 1-4
The CASI is an expansion of the Modified Mini Mental Status Examination, modified for
cross cultural use. The SPS3 Cognitive Scales form contains key instructions for
administering and scoring the CASI. Examiners should pay close attention to timing,
both in delivering the stimuli and scoring of responses. Several items have a limited
response window such that any responses given after a specified, short time interval
are counted as errors. For example, on the word memory items the examiner's follow-
up prompts/cues must be given after a specified number of seconds with a resulting
loss of points. Examiners, particularly those accustomed to the MMSE or 3MS which do
not have these time limits, need to pay close attention to the instructions on the form for
correct administration/scoring. This is likely a less familiar test for most psychologists
and technicians.
5. Where were you born? Cue for state if not given spontaneously. Confirm with
significant other if possible, if not go by confidence of answer as this is highly over
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
learned information and rarely missed.
6. When were you born? Prompt for any missing date elements and confirm with
record or site coordinator.
7. How old are you? Score according to accuracy of answer as indicated on test
form. Confirm with birth date in record.
8. How many minutes are there in an hour? If incorrect, ask: How many days are
there in a year?
9. In what direction does the sun go down? (If confused, say north, south, east or
west, or have patient point.)
10. I shall say three words for you to remember. Repeat them after I have said all
three words. Say SHIRT BROWN HONESTY (Presentation rate is 1 word/1.5
seconds.) Score according to test form. Acceptable approximations are given in
parentheses; any other responses are counted as errors. Patients may recall items
in any order. If there are errors on the first trial, repeat the items up to 3 times until
they are learned for later questions. Score on last performance. After the subject
has repeated the three words, do not ask the subject to remember the words again.
This is different from the MMSE procedure, and this subtle difference is intentional.
11. I shall say some numbers, and you repeat what I say backwards. For
example, if I say 1 - 2, then you say 2 - 1. Ok? Remember you repeat what I
say backwards. Presentation rate is 1 digit/second. On the first item, if incorrect
give the correct response and have subject repeat it, but score as incorrect. If the
first two items are missed, skip the third and score it as 0.
12. What 3 words did I ask you to remember earlier? Time this closely! If no
answer after 3 seconds or response is incorrect, give semantic cue. After 2 additional seconds, give multiple choice cue. Tell the subject the correct answer if response is incorrect or no answer. Whenever recall is not perfect, it is very important that you say the three words once more before proceeding to the next item, e.g. "So the three words I asked you to remember are shirt, brown, honesty."
13. From 100, take away 3, equals how many? Provide the correct answer for the
first incorrect subtraction. Do not correct any subsequent subtractions that are incorrect. Stop after 2 errors and score the remaining subtractions as zero (do not leave blank). If the subject asks for the previous number, provide it but count that trial as incorrect. Score responses as correct based on whether the subtraction is correct. For example, if the subject responds 97-95-92-89-85, the scoring would be 1-0-1-1-0.
14. What is today's date? Cue for missing items in date and score according to test
form. Always consider the month and date together in scoring. For example, if the date is March 3, 2007, and a subject reports February 28, 2007. The score for year is 4, score for month is 2 as February 28 is within 5 days from March, and score for date is 1 as February 28 is within 3-5 days from March 3.
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
15. What day of the week is today? Must be exact day of the week.
16. What season are we in? May cue for spring, summer, fall, and winter, if
necessary. Within 1 month is correct (see test form). Seasons start on the 21st of December (winter), March (spring), June (summer), and September (fall).
17. What state are we in? What city/town are we in? Prompt for things not
mentioned. Ask for province or region or comparable area if your country of residence is not divided into states.
18. Are we in a hospital (clinic), store, or home? Use "hospital" or "clinic" as
appropriate to your setting (not both).
19. What animals have four legs? Tell me as many as you can. Immediately start
stop watch. If no response in 10 seconds, ask What (other) animals have four
legs? but continue timing. Do not ask "What else?". Do not tell subject about the 30
sec time limit. Allow exactly 30 seconds for response and write responses in
margin of test form. Maximum score is 10, even if more correct responses are
provided. Score one point for each correct animal name. Do not count repetitions
of the same name. Different names for the same animal of different ages or sexes
count as one, e.g. kitten and cat, puppy and dog, deer and doe. Accept marginal
cases such as monkeys, chimpanzees, baboons, and kangaroos.
20. An orange and a banana are both fruit. Pause for 2 seconds, then ask: An arm
and a leg are both…? You may also ask "In what way are and _ alike
(similar)?" to help the subject comprehend what you want.
For the next two items Laughing and crying are both…? and Eating and
sleeping are both…?, you may repeat the question but no further prompting is
permitted. After each comparison, tell the patient the correct answer if the
response was incorrect. Score according to form. Be stringent in awarding 2
points and lenient awarding 1 point. The response for laughing-crying must include
"emotions" or "feeling" to earn 2 points, and the response for eating-sleeping must
convey "necessary" or "essential" to earn 2 points.
21. What actions would you take if you saw your neighbor's house catching fire?
Prompt "What else might you do?" once only if necessary. Score one point for
each category of correct action. Categories include:
Call 911; inform appropriate authority. Try to alert or evacuate the residents. Try to help put out the fire. Safeguard own property/family. Alert other neighbors.
Repeat for What actions would you take if you lost a borrowed umbrella?.
Score one point for each category of correct action. Categories are 1)
inform/apologize and 2) replace/compensate. If the subject says "I'll look for it," ask
"What would you do if you cannot find it?" and score the response.
Repeat for What would you do if you found an envelope that was sealed,
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
addressed, and had a new stamp? If the subject asks "Where did I find the
envelope," say "on the street" and score the answer.
22. Repeat exactly what I say: He would like to go home. Score 2 points for correct
repetition, 1 point if 1-2 words are missing/incorrect. Then say Now repeat: This
yellow circle is heavier than blue square. For each part of this sentence on the
form score 1 point only if the phrase is exactly as given. Note that the omission of
"the" is intentional on the last item. Score as an error if "the" is added to this item.
Say the first sentence in 2 sec and the second one in 3 sec. Do not stop in the
middle of either sentence. If the subject asks for a sentence to be repeated, say
"Just repeat what you think I said."
23. Please do this. Detach stimulus page at the end of test form package and fold it so
that the statement "Raise Your Hand" is showing. If patient does not do within 5
seconds, prompt by pointing to the sentence and saying, "Read and do this". If
the subject merely reads the command aloud, say "Do what it says". Allow up to 5
sec for response. Score according to test form. Raising either or both hands is
acceptable. Reading the statement aloud is not required.
24. Let me have a sample of your handwriting. Please write "He would like to go
home." This item in the CASI assesses the ability to correctly spell and write five
simple and common words. Say the sentence slowly and distinctly. Repeat the
sentence or dictate the sentence word by word if necessary, but allow a max of 1
minute after first reading. Score 0.5 points for each correct word (give no points for
the word "He"). Do not penalize for self corrected errors, printing, all caps, or
capitalization of the first letter of every word. Mixed capitalization usage counts as
one word/error. Each word with a spelling mistake is scored as an error. For
example, HE wood like to Go homE is awarded a score of 1: no points are awarded
for "would" because it is misspelled, "go" because it has the first letter capitalized
when the other words do not, and "home" because it has mixed capitalization.
25. Please copy this. Fold the stimulus page with the intersecting pentagons so that
only the drawing shows. Place it in front of the patient. Only score parts completed in one minute. Do not penalize for non-straight lines, or minor gaps or overshoots at the corners that seem to be caused by poor motor control. If the subject has made more than one attempt, score the best attempt. Five-sided figures with the longest side less than twice as long as the shortest side are awarded 4 points; five-sided figures with the longest side more than twice as long as the shortest side are awarded 3.
26. Take this paper in your left hand, fold it in half, and hand it back to me. Say
instructions in about 6 seconds. Do not repeat instructions or coach. Ask to take in right hand if patient is left-handed, or right hand dominant, but it is dysfunctional. If the subject asks if both hands can be used for folding, answer "yes." If the subject folds the paper more than once, score the second part as 0.
27. What 3 words did I ask you to remember earlier? Time closely! If no
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
answer/incorrect, give semantic cue after 3 seconds and multiple choice cue after 2 additional sec. Do not repeat the 3 words or provide the correct answer(s) at the end even if recall is less than perfect.
28. What do we call this part of the face or body? Time this closely! Clearly and
slowly point to your forehead, chin, shoulder, elbow, and wrist (flex it a couple of times) in turn. Allow only 2 seconds for each response. Do not wait for patient to mentally search for name before proceeding to next item. Please do not have another object in your hand, such as a pencil, when administering these questions as this may be confusing
29. What is this? Show items (spoon, coin, toothbrush, key, and comb) one at a time
and lay on table in front of patient. Any order is okay. "Quarter" is also correct for
"coin", but if the patient says "money", ask What kind of money is it? If patient
calls the toothbrush a "brush", ask What kind of brush is it? If the subject gives
an incorrect name, tell the correct name and ask the subject to repeat it. When
testing a bilingual subject, answers in either English or other language are
acceptable. Please do not have another object in your hand, such as a pencil,
when administering these questions as this may be confusing.
30. Remember these 5 objects. Wait exactly 5 seconds, then cover the items and
ask: What 5 objects did I just show you? No prompting is allowed. Money and
brush are acceptable responses when recalling the objects.
CVLT (learning & immediate recall trials): Pages 5-6
In introducing the test, it is recommended that the examiner avoid referring to it as a
"memory" or "learning" task. While examinees should not be told whether their
responses are right or wrong, encouragement for effort may always be given. The
Spanish version of this test contains a different list of words.
Trial 1. Say, Let's suppose you were going shopping on Monday. I'm going to
read a list of items for you to buy. Listen carefully, and when I'm through, I want
you to say back as many of the items as you can. It doesn't matter what order
you say them in – just tell me as many as you can. Are you ready?" Read List A
at the rate of 1 word per second. After the last item say, Now, tell me as many as
you can.
Record all words verbatim in the order in which they are given. If words are repeated in
the same trial as a way of self-cuing (e.g., "Let me see, I've said ‘drill', ‘plums,'" etc.),
then the response need not be recorded. If the examinee repeats the response in the
same confidence as he or she reported previous responses on that trial, then record the
response and score it as a perseveration (P). Only if it is unclear why a response is
being repeated, then record it and ask whether the examinee thinks the response has
been said before: Have you said that word before this time? If the response is "no"
or "I don't know" then record the word and score it as a perseveration. If the response
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
is "yes", then cross through the word and do not count as a perseveration. Watch
carefully for intrusions, e.g. pants instead of slacks is incorrect.
About 10-20 seconds after the subject stops responding, provide a single prompt such
as: Tell me anything else you can or See if you can think of any more. Mark a "Q"
on the record form to indicate a prompt was given. This prompt is provided ONE TIME
ONLY for each and EVERY trial.
Trial 2. Say: I'm going to repeat Monday's shopping list. Again, I want you to say
back as many items as you can, in any order. Be sure to also say the items on
the list that you told me the first time. Read, record, and prompt as for Trial 1.
Trials 3-5. Say: I'm going to repeat Monday's shopping list. Again I want you to
say back as many items as you can, in any order, including items you may have
already told me. Read, record, and prompt as for Trial 1.
31. Record the number correct in each of Trials 1-5.
Write the words given by the subject in the order given in the columns provided on the
form. Mark correct responses with a "C", confirmed perseverations with a "P" (cross out
with one line any words determined NOT to be a perseveration), and intrusion errors
(confabulations) with an "I". Count the total number of "C"s and record in the space
provided at the bottom of each column.
Immediate Free Recall, List B (Tuesday List): Say: Now, let's suppose that you
planned to go shopping again on Tuesday. I'm going to read a new list of items
for you to buy. When I'm through, I want you to say back as many as you can, in
any order. Read and record the words from List B as before using the same prompting
procedures as previously described.
Short-Delay Free Recall, List A. Say: Now I'd like you to tell me all the shopping
items you can from the Monday list. Record and prompt for responses as before.
Short-Delay Cued Recall, List A. Say: Tell me all the shopping items from the
Monday list that are spices and herbs. Record responses and give one prompt if all
four items are not correctly recalled. Repeat the same instructions for the Tools,
Fruits, and Clothing categories. If a subject asks what a particular category name
means, you may provide a general definition. Do NOT tell the subject which category
an item belongs to and do NOT warn subjects that there will be later trials.
32. Record number correct for List A: Short Delay Free Recall and Cued Recall.
Write the words produced by the subject in the order they are given in the columns provided on the SPS3 form. Mark correct responses with a "C", confirmed perseverations with a "P" (cross out with one line any words determined NOT to be a perseveration), and intrusion errors (confabulations) with an "I". Count the total number of "Cs" and record in the space provided at the bottom of each column.
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
33. TIME: Record clock time (24 hr clock) at end of short delay cued recall.
WAIS-III Block Design Subtest: Pages 7-8
It is important for the examiner to model the designs in the correct direction relative to the examinee (i.e. booklet with unbound edge closest to patient; test form oriented as labeled). For each design attempted, record the time the subject uses to construct the design. The time limit allowed for each design is listed in the column next to the block design on the scoring page. If the design is incorrect, draw the block design constructed, or write "scattered" if the design is not well formed. Circle the appropriate score in the right-hand column for each design. Credit for designs 1-4 is given when higher level designs are successfully completed and designs were not administered. Discontinue the test after 3 consecutive scores of 0.
Start with Design 5. Place 4 blocks in front of the patient. I'm going to ask you to
make some designs. You see these blocks? They are all alike. On some sides
they are all red, on some all white, on some half red and half white. Turn blocks to
show different sides. Construct Design 5. Do not explain design. Leave model intact.
Place 4 new blocks in front of the patient. Now make one just like this. Tell me
when you have finished. Begin timing. If patient is successful within 60 sec, proceed
to Design 6. If design is incorrect, proceed to Design 5, trial 2.
Design 5, trial 2: Watch me again. Demonstrate correct design with patient's blocks,
then scramble blocks. Now you try it again and be sure to make it just like mine.
Tell me when you have finished. Score performance. Regardless of performance,
proceed to Design 4 and administer Designs 1-4 in reverse order until patient obtains
perfect scores (2 pts) on two consecutive designs. When this criterion is met, give full
credit for any preceding items not administered. Proceed to Design 6.
Design 6: Remove the model and scramble the 4 blocks in front of the patient. Turn to
Design 6 in the booklet and place it in front of the patient with the unbound edge closest
to the patient. This time we are going to put the blocks together to make them
look like this picture. Watch me first. Slowly demonstrate the construction of the
design using the patient's blocks. You see, the tops of the blocks look the same as
this picture. Scramble the blocks. Now look at the picture and make one just like it
with these blocks. Tell me when you have finished. Go ahead. Begin timing.
Record score. If patient is successful within 60 sec, proceed to Design 7. If incorrect,
proceed to Design 6, trial 2.
Design 6, Trial 2: Watch me again. Demonstrate correct design with patient's blocks,
then scramble blocks. Now you try it again and be sure to make it just like the one
on this page. Tell me when you have finished. Begin timing and score performance.
Regardless of performance, proceed to Design 4 and administer Designs 1-4 in reverse
order until patient obtains perfect scores (2 pts) on two consecutive designs. When this
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
criterion is met, give full credit for any preceding items not administered, then proceed to Design 7.
Designs 7-9: Scramble the 4 blocks in front of the patient. Turn to next page in booklet.
Now make one just like this. Try to work as quickly as you can. Tell me when
you have finished. Begin timing. When patient has finished or time limit has elapsed,
score performance, scramble the blocks, and present the next design. Be sure to
remind patients that time is relevant here, by reciting the directions precisely.
Designs 10-14: Scramble 9 blocks in front of patient for designs 10-14 and increase
time limit to 120 sec. For designs 13 and 14, do not allow patient to rotate the book. Be
sure to remind patients that time is relevant here by reciting the directions precisely
Now make one just like this. Try to work as quickly as you can. Tell me when
you have finished.
Example: Subject correctly constructs design 5 on 1st trial, then design 6 on 1st trial. Examinee constructs design 7 incorrectly, design 8 correctly in 55 sec, and designs 9, 10, and 11 incorrectly or over time limit. Score for test is 16 points: 8 points for designs 1-4, 2 points for each of designs 5 and 6, 0 points for design 7, 4 points for design 8, and 0 points for each of designs 9, 10, and 11.
Example: Subject correctly constructs design 5 on 1st trial, is unsuccessful with design 6 on 1st trial, and constructs design 6 correctly on 2nd trial. Examiner then proceeds to design 4. If constructed correctly on 1st trial, then examiner will proceed to design 7, and the total score is 11 (2 pts for each of designs 1-3, 4, 5, and 1 pt for design 6) plus whatever points are scored in designs 7 and higher. If the examinee is unsuccessful on trial 1 of design 4, then the examiner will administer trial 2 of design 4, then design 3, then design 2. If and when the examinee is successful at 2 consecutive trials without awarding 3 scores of 0, then the examiner will return to design 7.
34. Total Score: Score and record totals per instructions on form. Make sure to
include scores for designs 1-4 when higher level designs are completed, and designs 1-4 have not been administered.
WAIS-III Symbol Search Subtest: Page 9
Remove the next 5 pages from the back of the form, i.e. the Sample & Practice Symbol
Search page and the 4 pages of symbol searches. Page numbers are in the upper right
hand corner of the four Symbol Search pages.
In the next task, I want you to look at two target shapes. Then see if you can find
either one of them in the group of shapes next to them. Place the Sample &
Practice page in front of the patient. Look over here. Notice there are two shapes
on the left side and a group of shapes on the right side. You are to mark the YES
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
box if one of the shapes on the left side is the same as any of the shapes from the
group on the right side. For example, this shape here is the same as this shape
here, so you would mark the YES box like this. For the second item, this shape
here is the same as this shape here, so I will mark the YES box like this. Mark the
NO box if none of the shapes is the same as any of the shapes from the group on
the right side. In this case, none of the shapes here is in the group over here, so
this time, I will mark the NO box like this. Now you do these here. Go ahead.
Give feedback and correct any mistakes made in the practice items.
Give the patient a pen and place the pile of 4 symbol pages (stacked in order) in front of
the patient. When I tell you to start, you do these the same way. Begin here and
do as many as you can. When you finish the first page, go on to the next page
and so on. Most people don't do all of them. Work as quickly as you can without
changing your answers. Don't skip any items and don't stop until I tell you to do
so. Any questions? Ok. Ready begin. Begin timing. If necessary, remind the
patient to respond to the items in order. Give no further assistance. After 120 sec,
Stop.
35. TOTAL SCORE: Total score is number of correct responses minus number of
incorrect responses. Items that the examinee did not attempt (either skipped or did not reach before time elapsed) are not included in the score calculation. For example, if the first 20 items were completed with 12 correct and 8 wrong, the score is 4, 12 correct - 8 incorrect. If the score computes to below zero (e.g. first 20 items attempted, 12 incorrect and 8 correct, 8 - 12 = -4), record a score of zero.
Grooved Pegboard - Page 9
Place the pegboard apparatus (25 pegs) in front of the patient on the table. This is a
pegboard and these are the pegs. Examiner points out each and then picks up one of
the pegs and continues. All the pegs are the same. They have a groove, that is, a
round side, and a square side and so do the holes in the boards. What you must
do is match the groove of the peg with the groove of the board and put these
pegs into the holes like this. The examiner demonstrates by filling the top row.
Remove the pegs, putting them back into the tray. When I say go, begin here and put
the pegs into the board as fast as you can, using only your (dominant) hand. Fill
the top row completely from this side to this side. Do not skip any; fill each row
the same way you filled the top row. Any questions? Ready, go.
Start the stopwatch. Record the number of seconds taken to place all the pegs as well as the number of pegs dropped. Discontinue when all 25 pegs are placed or 5 minutes have elapsed (note number of pegs correctly placed at 5 minutes). Repeat with non-dominant hand. If necessary, remind the patient not to use the other hand to help. You
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
may omit the trial on the affected side for patients who are densely hemiparetic (i.e. unable to pick up one peg with affected hand). In this case, record "0" pegs placed, 0 drops and a time of 300 seconds.
Direction of peg placement for the right hand is left to right. Direction of peg placement for the left hand is right to left. Be sure to demonstrate the direction and change in direction of peg placement.
36. Time: Score amount of time elapsed in seconds. Record amount of time that
subject attempted task, regardless of whether all pegs were placed correctly. Maximum time is 300 seconds (5 min).
Drops: Record number of pegs dropped. If a peg is dropped multiple times, count only once. A drop is limited to unintentional peg dropping. Pegs dropped intentionally are not counted, e.g. subject has multiple pegs in hand and lets go or subject lays pegs on board or table to better manipulate peg.
Pegs Placed Correctly: Record number of pegs placed correctly. A peg may be placed correctly even if it is dropped first. Subjects who complete the task will have a score of 25 pegs.
CVLT Delayed (delayed recall & recognition): page 10
37. Time Started: Record clock time at start of Long Delay Free Recall (24 hr clock).
Long Delay Free Recall, List A. Say: I read some shopping items to you earlier. I'd
like you to tell me all the items you can from the "Monday" list—that was the first
list, the one I read to you five times. Go ahead. Record items and provide prompts
as before.
Long-Delay Cued Recall, List A. Say: Tell me all the shopping items on the Monday
list that are clothing. Follow the same procedures as on the earlier cued recall trial,
asking in turn for "fruits", "tools", and "spices and herbs" categories and cueing after
each category. Record responses then move immediately to the Long-Delay
Recognition trial.
Long-Delay Recognition, List A. Say: I'm going to read a list of shopping items.
After I read each item, say "Yes" if the item was from the Monday list and say
"No" if it was not. Read aloud the 44 words on the record form and note the response
as Y or N next to the word. If the subject says "I don't know" or does not respond at all,
provide a single prompt such as: Tell me whether you think (repeat item) was on the
Monday list. If the subject still says "I don't know" or does not respond, write a "?" and
proceed to the next item.
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
38. Number Correct:
As with the Immediate and Short Delay, write the words produced by the examinee in
the order they are given in the columns provided on the SPS3 form. Mark correct
responses with a "C", confirmed perseverations with a "P" (cross out with one line any
words determined NOT to be a perseveration), and intrusion errors (confabulations) with
an "I". Count the total number of "Cs" and record in the space provided at the bottom of
each column.
39. Totals:
Mark a white box for each "yes" response. Do not mark any box for a "no" response.
Words that were on the Monday list have a white box in the "Hits" column and words
that were not on the Monday list have a white box in the "FP" for false positive column.
Count the number of hits (first column - white boxes) and false positives (2nd column -
white boxes) and record the total at the bottom of the column. "No" responses are not
totaled up.
Controlled Oral Word Association Test - page 11
Say: I am going to say a letter of the alphabet, and I want you to say as quickly as
you can all the words you can think of which begin with that letter. You may say
any word except for proper names, such as names of people or places. So you
would not say "Texas" or "Robert". Also, do not repeat the same word or use the
same word again with a different ending, such as "eat" and "eating." Do you
understand? Remember no names or people or places, just ordinary words. You
will have one minute for each letter. The first letter is "F". Go ahead. Record
words produced in order as you time exactly 60 seconds. Then repeat with the next two
letters. For the English and French versions, the letters read are F-A-S. For the Spanish
version, the letters read are P-M-R.
40. NUMBER CORRECT: Count the number of correct responses. Do not count
perseverative responses or words beginning with letters other than the target letter as correct. Proper nouns other than the names of people or places are acceptable, e.g. Saturday. Also, words from other languages that are familiar in the testing language are acceptable.
Digit Span Subtest - Page 12
There are two trials for each level. Administer BOTH trials for each level. Stop when
the subject gets both trials in one level incorrect. Read the digits evenly at a rate of 1
per second. Do not chunk the digits, e.g. say 6-1-9-4-7-3 rather than 6-1-9-pause-4-7-
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
3. Drop your voice inflection slightly on the last digit of each series. Record the
subject's responses verbatim on the answer sheet in the column labeled "Responses".
Score 1 point for each correct trial.
Digits Forward. Say: I am going to say some numbers. Listen carefully, and when
I am through, I want you to say them right after me. Just say what I say. Start at
Item 1, Trial 1.
Digits Backward: Now I am going to say some more numbers. But this time when
I stop, I want you to say them backward. For example, if I say 7-1-9 what would
you say? If the patient responds incorrectly, state No, you would say 9-1-7. I said 7-
1-9, so to say it backward, you would say 9-1-7. Now try these numbers.
Remember you are to say them backward: 3-4-8. Regardless of correctness of this
response, proceed to trial 1 of Level 1.
41. Digits Forward and Backward Total Score: Score one point for each correct item,
i.e. 2 points per level if both were correct.
CLOX - Page 12
The CLOX is administered on the back side of the paper that has the circle drawn on it.
Turn the paper over on a light-colored surface so that the circle shows through faintly.
Instruct the patient to: Draw me a clock that says 1:45. Set the hands and numbers
on the face so that a child could read them. Repeat the instructions until they are
clearly understood. Do not draw attention to the pre-drawn paper that is faintly showing
through the paper (it's meant to show as a distractor). Once the patient begins to draw,
no further assistance is allowed.
Scoring guidelines and examples are below and on the next page. Some of the CLOX
drawings may not much resemble a clock, but they may have a score greater than zero.
Person completing form: Record the name of the examiner who administered and
scored the tests on this form.
After the Administration
Re-staple all 18 pages back together. Review the test scoring for completeness. If for some reason a test was not administered, write a brief explanation on that part of the form. If you have any questions about test administration or scoring, the easiest way to contact the SPS3 neuropsychologist is by e-mail at [email protected].
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Figure resembles a clock
Circular face present
Dimensions > 1-inch
All numbers inside perimeter
No sectoring or tic marks
12,6,3,& 9 placed first
Only Arabic numerals
Only #s 1-12 among numerals present
Sequence 1-12 intact, no omissions or
Only 2 hands present
All hands represented as arrows
Hour between 1 and 2 o'clock
Minute hand obviously longer than hour hand
None of the following#
SCORE FOR DRAWING ABOVE
unable to score without observing patient # hand pointing to 4 or 5 o'clock, "1:45" present, any other notation (e.g. "9:00", any arrows pointing inward; intrusions from "hand" or "face" present; any letters/words/pictures; any intrusion from circle below
ManOp section XXV - 07/01/07
CLOX Scoring Guidelines
Figure resembles a clock
Score if position of number(s) or hand(s) suggests a clock.
Do not score if both numbers and hands are absent.
Circular face present
Score if perimeter of shape drawn approximates a circle even if traced.
Do not score if no boundary, e.g. patient uses circle from below but doesn't trace it. (Also, do not score if patient turns paper back over and uses pre-drawn circle.)
Dimensions > 1-inch
Score if largest clock dimension is at least 1 inch wide even if traced.
Do not score if largest dimension is smaller than 1 inch.
All numbers inside perimeter
Score if all numbers present are inside boundary of the shape.
Do not score if no numbers are present; do not score if figure drawn does not have a perimeter drawn or traced.
No sectoring or tic marks
Score if no lines dividing up the shape or its perimeter.
Do not score if dividing marks are present around perimeter; do not score if lines divide up the shape. Be aware that line(s) without arrow(s) may represent sectoring.
12,6,3 & 9 placed first
Score if all 4 of these numbers are present and written in any order before
Do not score if any of these 4 numbers are absent from figure; do not score
any other numbers are written.
if any other number is placed before any of these numbers is placed.
Only Arabic numerals present
Score if all of numbers written are standard numbers. Number may be
Do not score if no numbers are present; do not score if Roman or other
written backwards/mirror-imaged.
language numerals written.
Spacing intact (symmetry on either sides of 12 and 6
Score if numbers 1-12 are roughly spaced equally and numbers 12 and 6
Do not score if any of numbers 1-12 are absent; do not score if more
are opposite each other and roughly top and bottom. Numbers may be
numbers are on one side than other; do not score if spacing is grossly
inside or outside of perimeter.
unequal between numbers.
Only #s 1-12 among numerals present (ignore notation)
Score if whatever numbers present on drawing are 1-12 (not all of 1-12
Do not score if no numbers are present on figure; do not score if additional
need to be drawn)
numbers besides 1-12 are on drawing, e.g. "45".
Sequence 1-12 intact (no omissions or intrusions)
Score if all of numbers 1-12 are in order in a clockwise direction on the
Do not score if any number is omitted or out of sequence; do not score if
drawing and no additional numbers are present.
any number is mirror imaged/backward; do not score if additional numbers are present.
Only 2 hands present (ignore sectoring/tic marks)
Score if exactly 2 hands are present.
Do not score if no hands or 1 hand is present.
All hands represented as arrows
Score if each hand, regardless of number of hands, is drawn as a line with
Do not score if no hands are present; do not score if one or more hands
a < or > somewhere along its length.
does not have an < or > somewhere along its length. Be aware that lines without arrows may represent sectoring rather than hands particularly if more than 2 lines present.
Hour hand between 1 & 2 o'clock
Score if a hand drawn is between the numbers 1 and 2 even if 1 and 2 are
Do not score if no hand or only hand between 1 & 2 o'clock is the
in the incorrect position or if the hand between 1 and 2 o'clock is the same
obviously longer hand. Do not score if numbers 1 and 2 are not both on
length as the other hand.
drawing or if it is not clear through tics and numbers present where the 1 and 2 positions are.
Minute hand obviously longer than hour hand
Score if hand not between 1 and 2 o'clock is obviously longer than the
Do not score if none or one hand is drawn. Do not score if both hands
other as indicated by arrows, or length of lines if no arrows. Score if both
equal in length based on arrow position, or if no arrows, by length of lines.
hands between 1 and 2 o'clock and one obviously longer than other.
None of the following present:
Score if all of these are absent.
Do not score if any are present:
- any hand pointing to 4 or 5 o'clock
i.e. either hand points to the 4 or 5
i.e. 1:45 written as part of the clock or outside of the clock
- any other notations (e.g. 9:00)
i.e clock time is other than 1:45
- any arrows pointing inward
i.e. arrow points toward center of clock rather than outside perimeter
- intrusions from "hand" or face" present
i.e. patient draws part of a hand or face
- any letters/words/pictures,
e.g. smiling face in center of clock
- any intrusion from circle below
i.e. traces or uses in any way the circle that is faint through the paper
*** Do not score any points for a strictly digital clock unless a circular face is present, in which case, a point for "circular face" , "dimension > 1 inch", and "resembles a clock" may be possible.
ManOp section XXV - 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XXVI. TRAINING MATERIALS: Randomization & Data Transfer System
The SPS3 Data Entry System (DES) is used to randomize patients into the study, edit
data, and print labels, data collection forms and reports. If you require further
assistance beyond the material related to the DES in this chapter, please contact the
UAB SPS3 Statistical Center.
Section XXVI Contents
Introduction . 2
System Requirements . 3
Installing the SPS3 DE System . 3
Login and password . 9
Randomizing a Patient . 9
Entering and Viewing Data . 11
A. Entering data . 11
B. Navigating through a form . 12
C. Locking a form . 14
D. Viewing and updating a form . 15
Entering Ineligible Subjects' Screening Forms . 15
Printing Patient ID Labels . 16
Printing Forms . 17
IP Address Notification Form . 22
Login and Password Request Form . 23
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
The SPS3 Data Entry System (DES) is used to randomize patients, enter data and print
labels, reports (including follow-up schedules) and data forms. If you cannot find an
answer to a DES-related question in this section, please contact the Statistical Center
(SC) via telephone toll free at 866.304.5010, or via email at [email protected] . The
members of the SC are readily available, during normal working hours/central standard
time, to assist with technical troubleshooting issues.
The SPS3 DES has been designed to be "user friendly." We have attempted to make
navigating the DES as easy as possible. An attempt has been made to have the DES
screens look very similar to the SPS3 data collection forms. A required programming
difference in the electronic and paper forms accounts for a variety of monitor
dimensions. A page from the paper forms is divided in half and listed in two columns on
the screen in the DES, with a line on the screen dividing the columns.
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Computer System Requirements
Please ensure your computer has the following requirements before attempting to install
the SPS3 DES:
• Windows XP (with latest service pack) or Windows 2000
(With the latest service pack included)
• Minimum screen resolution of 800 x 600 pixels • Minimum 256MB RAM • Pentium III or higher processor • At least 200MB disk space for installation • Antivirus package with scheduled updates to the scanning engine • Desktop fire wall software (the free version of ZoneAlarm is a good choice) • IP Address must be static and visible on the internet • High speed internet access (cable modem, DSL, T1 LAN, T3 LAN, etc.)
Installing the SPS3 Data Entry System (DES)
Report your IP Address
Before starting to use the CD-ROM to install the SPS3 DES, please make sure your IP
address has been allowed access to the UAB's computers. The firewall at the SC
blocks all IP addresses except those of sites participating in the study. If you have not
reported your IP address to the Statistical Center, please complete the IP address form
located at the back of this ManOp section and fax it to 205.975.2540. You may also
email your computer's IP address to [email protected] , including a written request to add
the IP address to the SPS3 UAB firewall.
Accessing your computer's IP address
Log on the IP address website: http://www.soph.uab.edu/soph_catalog/ipaddress.asp .
As soon as the website opens in a new window, the information will be displayed,
indicating your IP address
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Software installation
After you have received clearance from the Statistical Center, you may begin the
installation of the SPS3 DES. Go to the software installation website, at
http://www.soph.uab.edu/sps3/ to install 1) the Microsoft SOAP toolkit and 2) the SPS3
DE software. Please follow the prompts, as the website instructs you to perform specific
actions during the installation.
Installation Trouble Shooting
Do you have Service Pak 1?
You can see which service pack you're currently running by clicking on Start Æ Settings
Æ Control Panel Æ System. The General tab will say "Service Pack "underneath the
"Windows 2000" or "Windows XP" if your Windows has been updated. It needs to be at
least service pack 1 for Windows XP, or service pack 1 for Windows 2000.
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
How to update my Service Pak?
• Close all other programs. You will need to restart your computer after the
software download.
• Go to the SPS3 web-site: http://images.main.uab.edu/isoph/sps3/servicepack • Double click on the correct service pack (SP) to download • Windows 2000: Windows 2000 SP3 • Windows
• Choose Open (or Run on some versions of Windows and Internet Explorer). You
may be prompted again whether or not you want to run the program, depending
on your browser security settings.
• Close this browser window • Click
• Choose "I agree" and click "Next" • Click
• Choose "Yes" to restart Windows. If the install window prompted you the "the
specified service already exists", click "ok".
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Login and Password
Logins and passwords are assigned by the Statistical Center. These are given at the
time of IP address notification or by submitting a Login and Password request form
(form at back of this ManOp section). After entering a correct login, password, and
clicking the Log On button, you are in the SPS3 DE System at the Main Menu and you
are ready to randomize a patient, enter data or perform other tasks.
Randomizing a Patient
Have the patient's completed Eligibility/Randomization form in front of you. You will
enter the data from this form as part of the randomization process. Data will be
validated as you perform data entry and only eligible patients may be randomized.
Remember that randomizing a patient is an irreversible action.
Log onto the system
• At the main menu, select the first button, "Randomize a New Patient". • On the next screen, again click on "Randomize a New Patient".
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
• The screen before you contains
all the questions from page 1 of
the Eligibility/Randomization form.
Question 1, Date of
randomization, has automatically
been completed for you with the
current date. Starting at Question
2, Date of Qualifying Event, enter
the data recorded on the form.
• After you have entered the data
for Page 1, click on "Next Page" at
the bottom, and enter the data for Page 2 of the form.
• When you have finished entering the data, click on "Randomize Subject Into
• You are now prompted that once the patient is randomized there can be no
changes made to the Eligibility/Randomization form for the subject. "Are you
sure you're ready to randomize the subject?" Click on "yes" if you are ready.
• A pop-up box
will appear with
assignment. Record the patient id and BP target assignment on the
Eligibility/Randomization form (questions 33 and 34). Click "OK".
The randomization process for this patient is now complete. You may either randomize
a new patient at this point (click on "Randomize a New Patient") or exit to the main
menu (click on "Exit").
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Additional information
Unlike most forms in the SPS3 DE system, when entering data on the
Eligibility/Randomization screen
you cannot partially enter the form
and save the data. If the form is
exited before you randomize the
patient, entered data are lost and
need to be entirely re-entered. If the
randomization button is depressed
before all fields meet the inclusion criteria or are within valid range, a message box will
appear saying, "The subject can not be randomized into the study because: …"; this
message will list all fields prohibiting the patient from being randomized.
Entering and Viewing Data
A. Entering Data
After a patient has been randomized other forms for that patient may be entered.
Log onto the system
At the main menu, select the second button, "Enter/Update/ Review Forms for a
Randomized Patient".
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Choose the Patient ID from the pull down menu.
After clicking on the ID, the window will be populated with a list of forms. Click on the
form name or number to select it. Click "Add Form", and a blank form of that type will
appear on the screen ready for data entry. The patient id will appear above the page
Enter the values as recorded on
the form.
To exit the form, click on the "Exit"
button in the top RH corner. You
will be asked whether or not you wish to save your changes. Click "Yes" to save, "No"
to exit without saving, and "Cancel" to return to the form.
B. Navigating Through a Form
In general, questions on one page of a data form appear on one data screen for entry.
Enter the values as recorded on the form. When entering a text field, a <Tab> or
<Enter> key may be used to progress the cursor to the next field. You may also use the
mouse to select a value from the drop down box. When a field is full, the cursor
automatically progress to the next field.
After all data are entered from one page, the system automatically progresses to the
next page or the "Next Page" button is highlighted by the cursor (Use the TAB or
ENTER key to change the page). The MOUSE may also be used to change pages by
left clicking on the top of the page you want displayed.
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
At the bottom of each data entry screen are the buttons: NEXT PAGE, SAVE, CLEAR
CHANGES, and LOCK FORM.
• NEXT PAGE - Takes the screen to the next page of the data form.
• SAVE - Saves the current data entered. This allows forms to be entered, saved,
and updates or finished at later time. Note: Data for Eligibility/ Randomization
forms and Event Notification forms may not be partially entered and saved - the
process of entering and locking these forms must be done all at once.
• CLEAR CHANGES - Clears any changes made to the screen since the last
• LOCK FORM - Locks the form. When locked, changes cannot be made to the
data entered. This option should be used when you are confident that all
outstanding issues have been addressed on the specific form. A form cannot be
locked or closed if any fields are highlighted yellow on the screen. Contact the
SC if you have incorrect data on a locked form
Fields are color-coded to show their status:
• PINK - indicates the current data entry field
• WHITE - indicates the entered value meets all validation rules
• YELLOW - indicates value is incomplete or inconsistent
• LIGHT/AQUA BLUE - indicates value does not meet validation rule but data has
been approved by PI
After a value has been entered in a field, its validity is checked. If the entry does not
meet a criterion, an Invalid Value message box appears explaining the reason and your
options: Change the Value, Ignore for Now, or This Value Has Been Approved By a PI.
Change the Value – Returns you to the field to correct the value entered
Ignore for Now - Skips you to the next field and leaves current field yellow
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
This Value Has Been Approved By a PI – Confirms value entered is "unusual but
correct". It is anticipated that this option will be seldom used. Frequency of its use will
be closely monitored to ensure that invalid data are not being routinely approved. Use
of this option on the Eligibility/Randomization form 12 requires approval by Oscar
Benavente and a one time use password.
If an Invalid Value message box pops up, and you don't understand why, contact the
Study Coordinator at the CC or Data Manager at the SC. Data-entry Screens are also
color-coded to show their status:
• SEA GREEN - indicates an unlocked form. Data may be entered, updated or
• BLUE - indicates a locked form. Data may only be viewed.
C. Locking a Form
A form may be locked when all entered data meet the validation rules. Eligibility/
Randomization forms and Event Notification forms are automatically locked after
completion. All other forms may be manually locked using the "Lock Form" button at
the bottom of the DE screen. A locked form may only be viewed - no changes can be
made to the data. All data editing must take place before the form is locked, and only
locked forms are considered
complete for site reimbursement and
data analysis.
Select the "Lock Form" button.
You will be prompted to confirm that
you wish to lock that form. Click
"Yes" to lock. Click "No" to return to
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
D. Viewing and Updating a Form:
• Log on to the system. At the main menu, select the second button,
"Enter/Update/ Review Forms for a Randomized Patient".
• Choose the Patient ID from the pull down menu. • After clicking on the ID, the window will be populated with a list of forms. Click on
the form name or number to select it. Click on the date of the relevant form.
Click "View Form".
• If the form is not locked, data may be updated and saved.
Entering Ineligible Subjects' Screening Forms
The Screening form is the only form entered for those subjects who were screened and
identified as ineligible for the study. After a patient is identified as ineligible:
Click the third button on the main
menu, "Enter Screening Form for
Ineligible Patient"
The window will open and you will
have the option of Adding a New
Ineligible Subject or
Viewing/Editing a previously
entered subject. After Clicking
"Add New Ineligible Subject", the
system will prompt you to make
sure you want to enter the subject
as ineligible. An ineligible subject
cannot be changed to eligible status. The same Screening form will appear for data to
be entered as with a randomized subject. The only difference is that there is no
randomized ID field and answers to questions 13 and 14 will be accepted as "Yes" or
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Printing Patient ID Labels
Patient ID labels may be printed for any randomized patient. The format is for Avery
5267 labels (0.5" X 1.75", 80 labels per sheet).
• At the main menu, select the bottom button, "Labels/Forms/Reports".
• Select "Labels" from the next
• Select the patient id from the pull
• Insert a sheet a labels in your
printer, and click "Print".
• You will receive a prompt asking do
want to print labels for the selected
ID and to make sure Avery 5267
labels are in the printer.
• To print additional labels, select
"Print More Labels". Click "Exit" to
return to the main menu.
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Printing Forms
The system can be used to print forms for SPS3 randomized patients. There are 33
forms that can be printed from the data entry system; two of which are not entered into
the data entry system, Form 03: Patient Contact and Form 11: Current Medications.
• Select the last button from the Main Menu "Labels/ Forms/ Reports" • On the next screen, select "Print Labels and Forms" • The next screen that will open lists all of the forms in numerical that can be
• Select the form which you would like printed. • The "Choose Printer" button on this screen will allow you to select the printer with
which you want to print the forms or labels.
• Click on "Open Form" • The Form will open in a PDF format for printing • A full form set can be printed by clicking "Full set"
A set of forms specific for a particular
visit or event type can also be printed: a
BP check, 3 month follow-up, annual
follow-up, an event, etc. Click on the
visit type to print those forms.
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
The SPS3 Data Entry system produces multiple reports for your site including: a
quarterly follow-up schedule for a patient, a list of patients due for a quarterly follow-up,
a list of patients overdue for a quarterly follow-up and a report of overdue forms.
Quarterly Follow-Up Schedule
• Select the last button on the Main Menu, "Labels/Forms/Reports" • On the next screen select "Reports" • On the next screen click on "Patient Follow-up Schedule" • On the next screen, at the prompt, "Choose the patient's randomized ID for the
subject you want to print a follow-up schedule:", select a randomized ID from the
• A message box will pop up and prompt "Do you want to print a SPS3 follow-up
exam schedule for <patient id selected>?" Click "Yes", if correct ID chosen or
Click "No" to pick
another randomized
Sample patient's
follow-up
List of Patients Expected for Quarterly Follow-up Visits
This report lists patients due for a quarterly follow-up visit over a specified time period.
Participants are listed by weeks along with the type of quarterly follow-up visit due and
their initial date of randomization. To produce this report:
Select the last button on the Main Menu, "Labels/ Forms/ Reports"
• On the next screen select "Reports" • On the next screen click on the Patients Expected in Clinic
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
• On the next screen enter the dates for which you want to produce the list of
expected quarterly visits. For example: 06/23/03 to 7/03/03
• After two dates are entered, you will be prompted by a pop up box: "Do you want
to print a list of patients due for quarterly follow-ups during the period 07/07/2003
to 7/18/2003?" Click "Yes" to produce the report or Click "No" to change the
• Patients will appear on the list based on the beginning of the follow-up window.
Remember that a visit is considered on time in the two week period on either
Missing Quarterly Follow-up Visits
This report lists patients who have a Follow-up Form missing. A follow-up form is
missing if data entry has not been initiated within one week following the end of the
follow-up window. This report is always produced for "Today's date". Enter forms as
quickly as possible to keep this list at a minimum.
Select the last button on the Main Menu, "Labels/ Forms/ Reports"
On the next screen select "Reports"
On the next screen click on the button "Missing Follow-ups"
You will be prompted by a pop up box: "Do you want to print a report of SPS3 patients
missing their most recent follow-up form?" Click "Yes" to print the report and click "No"
to take you back to the screen to select a type of report
Sample Patients Missing Most Recent Quarterly Follow-Up Report:
Randomization Date
Date of Last Follow-up
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Data Completeness Report
The Data Completeness Report, previously available via the DES, serves as the basis
to determine if patient information is overdue at the Statistical Center for any patient
visit. As data are received and processed, the SPS3 database is automatically updated
to indicate the receipt of data for an individual patient for a specific visit. These overdue
notices, as illustrated by the report, are cumulative, so that indications that data are
missing remain on the report until the data are provided. This report aids in illustrating
any discrepancies discovered in the database for the clinical coordinator's attention for
further verification and correction. The SPS3 Data Completeness Report is updated
weekly and available by Monday afternoon via the following link to the password-
protected website: http://138.26.144.9/sps3_report/login.asp . Coordinators will receive
an email alerting them to check the website for an updated report. If you experience
any problems accessing the report, or need access to your login name and password,
please contact the SC.
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Login and Password Request
Please use this form to request a login and password for additional data entry personnel. Fax the completed form to the Statistical
Center (205-975-2540). You will receive verification of receipt and your login and password via the e-mail address you give below
within 24 hours.
Date:
SPS3 Clinic:
SPS3 Site PI:
Clinic Coordinator:
E-mail address:
List name(s) in which SPS3 data entry system login and
password are requested.
Any additional information:
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
IP Address Notification Form
Please use this form to notify the Statistical Center of the IP address of the computer in which you are going to perform SPS3 data
entry. You will receive verification of receipt and your login and password via the e-mail address you give below within 48 hours.
Date:
SPS3 Clinic:
SPS3 Site PI:
Clinic Coordinator:
E-mail address:
IP Address:
DCHP Enabled?
Computer System Contact:
List any other names of people at your site who are
trained and will be performing data entry for SPS3.
Please fax this form to the Statistical Center (205-975-2540
ManOp section XXVI – 07/01/07
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XXVII. TRAINING MATERIALS: Use of Colin BP Device
The Press-Mate Model 8800 series of monitors have been factory calibrated for all of
the test modes by the manufacturer. The standards follow the recommendations of the
AAMI, the Academy of Advancing Medical Instrumentation. Each machine will be
shipped annually to the manufacturer for calibration/tune-up.
Following is a brief summary of operating instructions. A more detailed manual
accompanies the machine.
• On the rear panel, turn the switch to "ADULT/PEDIATRIC" position, turn the printer
switch to "ON", and the display switch to "ON".
• Connect one end of the grey hose to the front panel of monitor and the other end to
the proper size cuff.
• Connect the machine to power. • Press the "ON" switch in the lower RH corner of front panel of monitor. BP
measurement may begin when ‘0' is displayed in the "MEAN DISPLAY" window.
• Wrap the proper size BP cuff on the patient's bare arm. • To initiate measurements at intervals, press the "INTERVAL" switch. Press again to
set interval, e.g. ‘2' = 2 minutes. (Omit this step if single BP is desired.)
• Press the "START/STOP" button to start the measurement. It may take up to 3
inflations to obtain a systolic measurement. Systolic and diastolic pressures and
pulse rate will be displayed after the measurement.
• If interval measurements were specified, the next measurement will automatically
begin. If single measurements, then press the "START/STOP" button to begin the
next measurement.
• "START/STOP" may be pressed at anytime to interrupt a measurement.
ManOp section XXVII - 04/15/03
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
XXVIII. TRAINING MATERIALS: Use of Ambulatory BP Device
Ambulatory blood pressure (ABP) measurement will be performed on all patients to better
characterize blood pressure while patients are engaging in usual activities and to classify
patients as dippers/non-dippers. ABP monitoring will be carried out on:
• All patients once they are stable on anti-hypertensive medications (if being prescribed)
and are "In Target" (BP falling within their assigned BP target group) at 2 or more
consecutive visits. This includes patients who are not taking anti-hypertensive
medications whose BP < 130 mmHg, regardless of assigned target.
To provide some consistency to the data collection, please schedule the monitor
placement at 6 to 12 months post-randomization at the time of a quarterly follow-up or
BP Check. This clinic measurement of blood pressure will serve as a comparison to the
ABP readings. If you have planned to perform the ABP measurement, but the patient's
clinic blood pressure reading that day is outside the assigned target, please go ahead
with the procedure as long as the SBP is within 10 mmHg of the target and no
adjustments are being made to the medications. If medications need to be adjusted or
SBP is more than 10 mmHg away from the target, postpone the measurement until the
patient is again in target.
• Selected patients with difficult hypertensive management or in the situation where a
"white coat" syndrome may be suspected of influencing blood pressure
• Patients who are declared FAAT
The ABP monitors will be assigned to selected SPS3 sites based on their ability to perform the
ABP measurements in order to maximize ABP data collection. The ABP package will be
shipped from the Coordinating Center and will contain the monitor, cuffs and a few other
accessories, software for installing the system on your computer, a video which will guide you
through the set-up as well as a part that can be shown to patients, and a CD from Spacelabs
that contains information for troubleshooting the monitor.
Steps for 24-hour ABP Monitoring
1. Install and login to the ABP Report Management System program
2. Program with the SPS3 protocol
3. Initialize the monitor
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
4. Instruct the patient
5. Complete the 24-hour monitoring procedure
6. Upload the monitor
7. Record the required information on Form 13 (24 HR AMBULATORY BP)
8. Save file as a pdf or copy the data to a CD (labeled with patient IDs) and send to the
Coordinating Center
1. Install and Login to the ABP Report Management System program
• Start Microsoft Windows
• Insert the ABP Report Management System CD – program will automatically load
• Choose the express install which will provide you with all the features you need to run
• Open the Spacelabs ABP Client Application program
You will be required to enter an initial password and user information (user ID can be your name – select 123456 for this initial password as you will be required to change password the next time you log on to the program and then you can select whatever password you wish). Almost all sites have had an occasion where they have forgotten their password for the ABP software. Unfortunately the version of the software that we have does not come with a default password and the only option to be able to access the software is to uninstall and reinstall (which is a pain and also means that you will lose all procedures that you have performed to date). Rather than selecting your own username and password, you can also use the following generic username and password: Username: SPS3
Password: lacune (all lowercase)
• When you login, you will be required to select a profile and a role. Please select
‘administrator' (this will allow you to change the monitor set-up) and select
‘physician' (need to select physician to initialize the monitor).
• On the right side under ‘password control', changing the password after so many
weeks is checked as the default. So that you do not need to be regularly changing your
password, please disable this function.
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
2. Program monitor with SPS3 set-up
• After opening program and entering username and password, you will come to the
Main Menu
• Select View/Change System Settings
• Under Report Format, please leave ‘use standard report' as the default
• Select Statistics Tab
• When the statistics tab is opened, the Factory Default setting, as seen below,
To configure the SPS3 statistical setting:
• Click New/Edit and a box called Statistical Settings will pop up
• Where it asks for Item, enter SPS3, click ADD and then click CLOSE
• The Auto Edit Limits boxes define the edit limits for data analysis. You can leave these
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
• Indicate the start hour as 8 in the first row and the start time as 20 in the second row.
You can designate the time starting at 20 as Sleep. Designating the night hours as
‘sleep' is needed for calculating the percent of ‘dipping'.
• The systolic and diastolic pressures in this section can be left as seen above (140/90
for 0800 to 2000 and 120/80 for 2000 to 0800). These boundaries are used in the
summary report and the percentage of readings that are above the values entered here
will be calculated.Select Monitor tab and you will see the following displayed
To configure the SPS3 initialization setting:
• Click New/Edit and a box called Monitor Initialization List will pop up
• Where it asks for Item, enter SPS3, click ADD and then click CLOSE
• Under Wake periods, enter start time as 8, enter 2 readings / hour, and remove the
check mark from the Tone box (so that it will not beep each time the pressure is
• Under Sleep periods, enter start time as 20 and enter 1 reading / hour
• There are 3 boxes in the upper left hand corner that you, in most case, do not want
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
Show results of reading check box is selected, the reading results will
appear on the ABP monitor display after each BP reading.
Clinical verification setup check box is selected, the monitor will detect
blood pressure levels as low as 40 mmHg (the monitor will always bleed to 40 mmHg).
Display Cuff Pressure check box is selected, the cuff pressure will
appear on the ABP monitor display while the cuff is pumping and bleeding.
Select OK to save these settings.
You should have to input the SPS3 program only once. Regardless of the time that you start the
24-hour procedure with your patients, there is a built-in clock in the monitor and the data will be
summarized as per the protocol above.
3. Initialize the Monitor
• Prior to initializing monitor, ensure that data from previous patient has been down-
loaded to your computer (monitor has been uploaded or read)
• Insert a new set of batteries for each 24-hour monitoring session (monitor takes 3 AA
• Attach the monitor to the computer serial port
• Turn monitor power on
• Under Main menu, select Initialize Monitor
• Enter the patient's demographic information
Patient ID is a required format – enter SPS3 ID in this format (01-001-7)
First and Last name are required fields – as these reports are sent here, you
should NOT enter the patient's name and can enter either their acrostic in both the First and Last name fields
Physician field is required and because you have logged in with this role, you
will just select yourself from the pull-down list
All of the other fields are optional and you don't need to enter patient's date of
birth, race, and gender as this can be captured from the database.
• Scroll to the next window and you should see the SPS3 configuration. If you do not,
select it from the pull-down window
• Scroll to the next window
• Click Initialize Monitor to complete the initialization process
• Once the monitor is initialized, turn it off
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
The monitor is now initialized, meaning that it is ready for the 24-hour monitoring procedure on
your next patient whose information you have just entered. You can initialize the monitor up to
several days in advance of the 24-hour monitoring session.
4. Patient Instructions
Prepare the patient for the 24-hour monitoring. Provide them with the following instructions
before coming to clinic to be connected to the monitor:
• Wear loose clothing and bring a belt for attaching the monitor
• Come to clinic accompanied by someone who can drive patient home
• Avoid bathing and showering during 24-hour monitoring
• Avoid strenuous activities during 24-hour monitoring
• Return to clinic after 24 hours to remove monitor
The day that the patient comes to clinic to wear the monitor, the following points should be
• Explanation of the procedure and the important reasons for undergoing it
• Provide a demonstration of attaching the cuff and monitor, re-attaching the cuff as it
may loosen with activity during the 24-hour period (can place a mark on the arm for
cuff re-attachment), manual inflation and deflation
• Explain the frequency of inflation and deflation
• Instructions about keeping arm steady during measurement
• Ability to engage in normal activities between measurements
• Keeping monitor attached at night
• Instructions to take all medications as prescribed, in particular their anti-hypertensive
In the video that you will receive with the ABP package, there is a section which has been
designed specifically for patient instruction and this can be shown to the patient, if you wish, in
addition to the teaching that you have provided.
5. Complete the 24-Hour Monitoring Procedure
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
• Turn the monitor on
• Attach the cuff to the ‘selected' arm and the monitor to the patient
• Manually take the patient's BP at least 2 to 3 times, a few minutes apart, so that the
patient can appreciate how it feels
• Compare with manual reading
• Organize to have patient return to clinic in about 23.5 hours
• Inform patient that if she/he does not arrive to clinic on time that the monitor will
continue taking her/his BP and to NOT TAKE OFF the machine
• Provide the patient with the SPS3 diary for obtaining specific information during the 24-
hour monitoring period and instructions on how to complete it
• Answer any last questions that the patient may have
• Provide patient with a number where they can reach someone over the next 24 hours
• When patient returns to clinic, manually take the last BP measurement
• Obtain the diary from the patient
• Turn the machine off
• When you get back to your computer, read the monitor
• After reading monitor and turn the machine off
6. Upload the Monitor (down-loading patient information from the monitor to store on your
• Attach monitor to the computer serial port
• Turn monitor power on
• At the Main Menu, select Upload Monitor
• Click Acquire Monitor Data
• When the download is completed, click Next – you will see Demographic Data and can
make changes if you wish
• Click Next – on this field you will see Select Statistical Setting - to apply the SPS3
summary periods to the data, select SPS3
• Click Next – on this field you can enter additional patient information if you wish.
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
• Click Finish
• Patient information opens and you can scroll through this using the Next and Prev
buttons seen in the upper left hand corner.
• To close the patient file select Close
7. Record the required information on Form 13
• Under Main menu, select Review / Edit Patient Test
• Select specific patient you wish to review and double-click on patient to open the file
• You can scroll through the file to see the raw data and the statistical summaries
• Information to complete Form 13 can be found on the first screen entitled Patient
Information and the second screen which provides the summary data
• The only thing that is not provided is scan end date and time. You can obtain this
information in two different ways: below scan start date and time (bottom left hand
corner), you will see an entry called duration – to get scan end time, you can add this
on to the scan start time to get the end time. The other way to do this is to scroll
forward to the screen entitled ‘ABP Raw Data Tabular'. Scroll to the last entry in the
table and record the time the last measurement was taken.
To print patient data:
• Under the Print menu, can print the whole report or select specific pages to print
• This can be given to the HTN MD to review and to the PCP (if desired)
8. To Save ABP Report to File and Send to Coordinating Center
• From the Main Menu, select Review / Edit Patient Test
• Highlight the patient file to be saved
• Under the File menu, select Save As
• Under ‘Page to Export', select ‘Complete patient Data(PDF format)'
• Choose where you wish to store the file (the most convenient is to create a folder
under My Documents to save all ABP files)
• Click Finish and file will be saved
• New window will pop up confirming where you want to save the file
ManOp section XXVIII - 08/15/09
Secondary Prevention of
Manual of Operations
Small Subcortical Strokes
• Please send this file to the Coordinating Center as an email attachment
Spacelabs Operations Manual
For the most part, the information that you require for setting up the system and monitoring a
patient is found in the material that is provided here (Section XXVIII). However, should you wish
to read further about the monitor and ABP program, additional information is available in the
Operations Manual published by Spacelabs. This manual can be found under the Help Menu
(choose Help Topics). It will provide you with information about the operation of the monitor, the
event codes that you may see displayed on the monitor and what they mean, troubleshooting,
and information on the ABP Report Management System (the program that you download on
ManOp section XXVIII - 08/15/09
The portable automatic blood pressure unit that
you are wearing will record your blood pressure
Ambulatory Blood
and heart rate every 30 minutes during the day
and every hour during the night.
Pressure
9 Be sure that the monitor is comfortably
Monitoring
positioned before you leave the clinic
9 Be sure that you have learned how to re-
position the cuff in case it becomes loose
Patient Diary
during the next 24 hours
9 Go about your normal activities but avoid
Patient ID _
vigorous activities such as going to the gym or
jogging
9 Take all medications including blood pressure
Reason for Test
pills as usual during the next 24 hours
9 When you feel the cuff beginning to
Please return to clinic tomorrow to have the last blood
inflate….STOP all movement and RELAX your
arm during the cuff inflation. KEEP your arm
pressure measurement taken and to have the monitor
still while the monitor is taking a
removed.
reading….once the cuff deflates, resume
Tomorrow's appointment ( ) is at
normal activity
9 Wear the monitor continuously for the next 24
hours….DO NOT REMOVE during the night
9 DO NOT REMOVE the blood pressure monitor
If you have any questions or have any difficulties
from the carrying case
with the ambulatory blood pressure monitor over the next
9 DO NOT get the monitor wet – should it get wet
24 hours, please contact:
there is no electrical shock hazard from the
Complete the diary on the next two pages and
bring it with you to clinic tomorrow
Activities while Wearing the Monitor
PLEASE RECORD TIME INTERVALS YOU PARTICIPATED IN THESE ACTIVITIES
House/Yard Work
Shopping
Watching TV/Reading
Childcare
Other Activities
Please describe work:
Please describe other activities:
How did you sleep during the night? (circle one)
Excellent
What time did you start your longest sleep?
What time did you wake from it?
Did you take your blood pressure pills as prescribed? (circle one)
Each time you take a blood pressure pill, please record the name of it and the time of day:
Name of BP medication and time of day
Name of BP medication and time of day
Name of BP medication and time of day
Name of BP medication and time of day
Name of BP medication and time of day
Name of BP medication and time of day
Name of BP medication and time of day
Name of BP medication and time of day
Name of BP medication and time of day
Manual of Operations
Small Subcortical Strokes
XXIX. TRAINING MATERIALS: Sending Imaging Files on CD
Imaging files or reprints of scans are sent to the Coordinating Center for central reading
of the following:
• Eligibility MRI scan documenting the qualifying stroke lesion (scan date referenced
in question 7 of Qualifying Stroke - 06 form) and MRA done at study entry (date
referenced on Intracranial Vascular Imaging - 15 form)
• Repeat MRI at 3-6 mo post-randomization, if applicable (see Repeat MRI - 38 form) • After a stroke or TIA during the course of the trial (scan date referenced in question
18 of Ischemic Stroke/TIA -21 form or question 8 of CNS Hemorrhage - 22 form;
scan date referenced in question 16 of BEA Ischemic Stroke /TIA - 31 form or
question 13 of BEA CNS Hemorrhage Event - 32 form)
• Following a major cognitive decline during the course of the trial
Most sites have the capability to send imaging files on CD rather than reprints of scans
to the Coordinating Center, and this method is usually less costly, easier to obtain, and
the preferred method. When sending scans on CD, please be sure that the files are
in DICOM format and that the date(s) of the studies included matches that
reported on the relevant forms. Other formats, e.g. JPEG, TIF, are not acceptable.
Please include studies for only one patient on a CD, including a completed label
on the CD case.
ID - - Acrostic
Event Date / /
MRI Date / / N/A
MRA Date / / N/A
Baseline
ManOp section XXIX - 06/06/05
Manual of Operations
Small Subcortical Strokes
XXX. TRAINING MATERIALS: Substudies
ManOp section XXX - 06/15/04
XXXI. STUDY CLOSURE
SPS3 is expected to end on April 30, 2012 and prior to that, all randomized subjects will
need to be brought back for an End-of-Study Visit, regardless of whether or not they are
actively receiving assigned SPS3 intervention (s). The maintenance of accurate contact
details for all participants is therefore of prime importance. If you have difficulty locating
a patient, please contact the SPS3 Coordinating Center before designating that person
"lost-to follow-up". All available resources should be used to locate these people in
order to establish their vital status at the end of the study.
To help ensure a successful close-out of SPS3, we ask study sites to ensure timely
subject evaluation and data entry and to respond promptly to data queries
The procedures will not differ significantly from those used for the Premature Study
Termination and will include the following:
1. Recording current medications (Form 04)
2. Assessing Post-Stroke Disability (Form 07)
3. Performing Neuro-psych Testing* (Form 09)
4. Obtaining Quality of Life Information (HTN & Stroke) (Forms 10 & 14)
5. Obtaining Follow-up Information (Form 16)
6. Obtaining End of Study Information (Form 37)
There is no need to complete Form 90 for participants who are beyond 5 years in
There are no new forms for the End of Study visit. Forms 16 and 37 have been
revised slightly to capture End-of-Study information.
No labs will be required.
ManOp section XXXI – 03/17/11
* Neuropsych testing (09) is only necessary for participants who are 5 years or less in
the study. It does not have to be repeated if performed in the previous 6 months. If the
participant refuses cognitive testing, an attempt should be made to perform the CASI, at
In addition to the above-referenced procedures, patients should be provided with a letter
of appreciation which will also include information pertaining to the unblinding process,
when and how trial results will be available and other frequently asked questions. A
letter should also be sent to the Healthcare Provider regarding the patient's participation
in SPS3. Sample letters are included in this section.
Please note that Event counting for individual subjects continues until the
day of their end-study visit.
To recap, please complete the following forms at the End of Study Visit: 04, 07, 09, 10,
14, 16 & 37.
For additional details regarding the closeout of SPS3, please refer to the End of Study
Plan.
ManOp section XXXI – 03/17/11
<physician address>
RE: <patient name>
On behalf of the Secondary Prevention of Small Subcortical Strokes (SPS3) study team, we
would like to thank you for supporting your patient, _, throughout
their involvement in this important trial. The treatment phase has now been completed and
your patient wil be transitioned back to regular medical care. We are committed to making
this as smooth a transition as possible.
Patients were randomized to receive 1) either aspirin alone or aspirin plus clopidogrel
and 2) to a systolic blood pressure control target, either 130-149 or below 130.
Since the results of the study are not yet known, please feel free to manage the antiplatelet
and blood pressure therapy according to the current standard of care. Results of the SPS3
study are expected to be available soon.
In the meantime, we have discussed with your patient the fact that their medications may
need to be changed, particularly if cost is an issue. For the duration of the study, antiplatelet
and antihypertensive medications were provided free of charge. When possible, active
patients having difficulty paying for these medications have been given up to three month's
supply of aspirin and/or antihypertensive medications to aid in the transition.
If you have questions regarding your patient or any aspect of the study, please do not
hesitate to contact us at ( ) .
SPS3 Principal Investigator
SPS3 Study Coordinator
SPS3 Clinical Center
Dear <participant name> / (SPS3 ID number),
On behalf of the entire SPS3 Study team, we would like to inform you that the study ended
successfully as planned on April 30, 2012.
This was a very long and involved trial that required an incredible amount of commitment.
We are very grateful for your contribution to this important study, which could not have been
accomplished without you.
The results are currently being analyzed and wil be communicated to you soon. Only after
all of these analyses have been performed, will we be able to tell you what medication you
were receiving. Please let us know if you want to be informed of this.
Your participation has helped to improve the treatment of stroke and for that we are indebted
If you have questions, please do not hesitate to contact us at ( ) _.
SPS3 Principal Investigator
SPS3 Study Coordinator
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
Af ix Patient ID Label Here
1. DATE OF FOLLOW-UP:
2. TODAY'S SCHEDULED FOLLOW-UP: ( one)
3. TYPE OF FOLLOW-UP CONTACT: ( one)
1 Phone contact
2 Clinic visit, seen by MD
Verify patient contact information .If there been a
3 Clinic visit, seen by RN only
change of address or phone, update Patient
Information Form.
5 Phone contact with outside clinic BPs
1 Never smoked
2 Former smoker
3 Current smoker
If current, average number of cigaret es per day AND/OR 1 Pipe or cigar smoker
1 yes 2 no
Does patient regularly use alcohol in a week?
If yes, complete the fol owing:
Usual number of days per week patient drinks:
Usual number of drinks consumed in a week:
Maximum number of drinks on any one day:
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
6. IF ANNUAL FOLLOW-UP OR END OF STUDY, EMPLOYMENT STATUS IN LAST 3 MONTHS: ( one)
1 Employed outside the home 2 Not employed outside the home
If not, primary reason: (√ one) 1 retirement due to age 2 prior or qualifying stroke
3 other health reason, specify
4 non-health reason, specify
Number of days per week patient has sustained exercise for at least 10 min
exclusive of work and home activities (e.g. walking, swimming, exercise class…)
MEDICATIONS - RECORD ON MEDICATIONS FORM 8. Is patient currently prescribed BP lowering meds? 1 yes 2 no
If yes, time since last dose of any BP lowering med hrs
9. BP lowering medication compliance
1 excel ent (takes meds 99% of time)
2 good (misses a dose weekly)
3 poor (misses two or more doses of one or more meds weekly)
10. Side-ef ects possibly related to BP lowering meds
2 yes, mild, no med changes required
3 yes, change prompted in BP lowering meds
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
BLOOD PRESSURE MEASUREMENT 11. Time of first blood pressure:
: (24 hr clock)
SPS3 BP Measurement Technique…
Measure BP in the morning with morning BP
12. Sitting BPs in "Selected" Arm
medications held. If not possible, avoid
Arm measured: 1 Right
measurement within 3 hrs of taking BP
No caffeine or tobacco within prior 60 minutes
"Selected" arm relaxed and supported at level
of the heart after 15 minutes of sit ing quietly
Minimize physical contact during measurement
Three readings separated by > 2 minutes
If the measured BP is unexpectedly high or
low, re-check with a recently calibrated
2 Other (includes other electronic and manual)
(preferably mercury) sphygmomanometer
(after all sitting BPs done and after standing for 2 min)
After standing, does the patient report experiencing: 1 yes
light headedness
other symptoms, specify
HTN TRIAL 14. HTN trial status during follow-up period: ( one)
1 Active, including normotensives not taking BP lowering meds
2 Active, but FAAT
1 systolic BP 130-149
2 systolic BP < 130
16. Is the average systolic BP in assigned target range?
1 yes -> Check plan
1 1st BP in target; wil recheck within 1 mo 2 2nd BP in target, wil recheck at next quarterly fol ow-up 3 recheck at next quarterly follow-up 4 other (explain in progress note)
2 no -> Check plan
1 adjust meds now; recheck within 1 mo 2 no meds adjustment; recheck within 1 mo 3 no meds adjustment, declaring FAAT (-> complete FAAT-18 form) or already FAAT
4 other (explain in progress note)
5 normotensive on no meds
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
DURING THE LAST 3 MONTHS, HAVE YOU… 17. 1 yes 2 no
been bothered by flushing (redness) or sensation of warmth in the skin?
18. 1 yes 2 no
been troubled by indigestion?
19. 1 yes 2 no
had trouble fal ing asleep?
20. 1 yes 2 no
been bothered by dizziness?
21. 1 yes 2 no
been troubled by headaches?
22. 1 yes 2 no
had swollen ankles?
23. 1 yes 2 no
suffered from breathlessness?
24. 1 yes 2 no
had palpitations (abnormal heart beats)?
25. 1 yes 2 no
suffered from constipation?
26. 1 yes 2 no
had nightmares?
27. 1 yes 2 no
noticed a decrease in your sexual capacity?
28. 1 yes 2 no
been bothered by cold hands/feet?
29. 1 yes 2 no
suffered a dry cough?
30. 1 yes 2 no
had dif iculty concentrating or with memory?
31. 1 yes 2 no
had dizziness when standing up?
32. 1 yes 2 no
suffered from calf pain or cramps?
33. 1 yes 2 no
suffered new onset or change in chest pain?
34. 1 yes 2 no
passed out or blacked out?
35. 1 yes 2 no
Has your walking or balance changed?
36. 1 yes 2 no
Do you get up at night to urinate? If yes, number of times
DURING THE LAST 3 MONTHS, HAVE YOU BEEN BOTHERED BY… 37. 1 yes 2 no
38. 1 yes 2 no
39. 1 yes 2 no
40. 1 yes 2 no
41. 1 yes 2 no
42. 1 yes 2 no
Urinary tract bleeding
43. 1 yes 2 no
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
ANTIPLATELET TRIAL 44. Antiplatelet study status during fol ow-up period: ( one) 1 Active
Complete questions 45 - 48 if active.
45. 1 yes 2 no
Patient reports taking antiplatelet medication from both bot les as directed
46. BOTTLE # 1 – ASA
Date last bot le dispensed: / /
Number of pil s dispensed
Number of pil s returned
% compliance (computed by PC)
Type (prescription of non-study ASA requires CC pre-approval):
2 non-EC 325 mg
47. BOTTLE # 2 – clopidigrel vs. placebo
Date last bot le dispensed: / /
Number of pil s dispensed
Number of pil s returned
% compliance (computed by PC)
48. If compliance < 80% or > 105%, indicate reason(s):
1 surgery or medical reason
1 side ef ects
1 did not understand 1 forgetful or careless 1 uncooperative 1 other, specify
ANNUAL LABORATORY DATA (collect at indicated follow-ups only, not necessary at end of study) 49. Serum creatinine
Al annual follow-ups - within 3 mo prior or 1 wk after follow-up
50. HbA1C - diabetics
Al annual follow-ups - within 2 mo prior or 1 wk after follow-up
51. Lipid profile
Al annual follow-ups - within 3 mo prior or
1 wk after follow-up
total cholesterol mg/dL LDL
triglycerides mg/dL
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
ANNUAL PHYSICAL MEASUREMENT (TO BE COLLECTED AT END OF STUDY)
53. Waist circumference: cm or inches (12" = 1 foot)
SUBSTUDY DATA (collect at indicated follow-ups only) 54. LIMITS Blood draw
1 non-participant
Initial 1 year follow-up only
1 non-participant
Initial 6 mo follow-up and all annual follow-ups.
Ignore any < signs when recording values.
Round non-ratio numbers to nearest whole number.
(round any number below 1 to 1)
Enter all results available, but do not compute values.
albumin/creatinine ratio .
1 no unit 2 mg/g 3 other,
STROKE SYMPTOMS 56. 1 yes 2 no
In the past 3 months, have you had any sudden loss or major changes in speech?
57. 1 yes 2 no
In the past 3 months, have you had any sudden loss or blurring of vision in one eye, complete or
58. 1 yes 2 no In the past 3 months, have you had a sudden spel of double vision, that is, did you see two objects
side by side, or one on top of the other?
59. 1 yes 2 no
If you closed one eye, did the double vision go away?
60. 1 yes 2 no
In the past 3 months, have you had sudden numbness, tingling or loss of feeling on one side of
your body, including your face, arm and leg? (must be some combination of face and arm, or arm
and leg together, etc , in order to mark yes)
61. 1 yes 2 no
Did the feeling of numbness or tingling occur only when you kept your arms or legs in a certain
62. 1 yes 2 no
In the past 3 months, have you had any sudden episodes of paralysis or weakness on one side of
your body, including your face, arm, and leg?
If any of the circled-shaded boxes are checked AND a stroke/TIA has not been reported, complete the Stroke
Symptoms Questionnaire form immediately….
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
HEALTH SINCE LAST QUARTERLY FOLLOW-UP 63. How would you say your health is these days? In general would you say your health is: ( one)
64. 1 yes 2 no
Has the patient seen a doctor for an unscheduled visit for a new or active health problem? If yes, specify reason:
65. 1 yes 2 no
Hospitalization since last follow-up? If yes, specify reason:
66. 1 yes 2 no
Epileptic seizure since last fol ow-up? (If initial 3 mo fol ow-up, ask since qualifying stroke.)
67. 1 yes 2 no
Coronary angioplasty, coronary stent or CABG
Have any of the fol owing events occurred since the last quarterly follow-up? 68. 1 yes 2 no
69. 1 yes 2 no
If any yes, complete Event – 20 or
Status of Therapy – 36 form
71. 1 yes 2 no
Major non-CNS bleeding
72. 1 yes 2 no
Serious complication of Hypotension
73. 1 yes 2 no
Other SPS3-related SAE
74. 1 yes 2 no
Acute MI or other non-CNS thromboembolism
75. 1 yes 2 no
Discontinued or resumed SPS3 AP therapy or SPS3 BP management
76. 1 yes 2 no
Major cognitive decline
Auxil ary Forms Due by
*Required ONLY for patients who have not reached 5 years in the study, and have not had cognitive testing the last 6 months. A form 90 is not required.
Secondary Prevention of
Small Subcortical Strokes
FOLLOW-UP - 16
BP medications at Clinic Dismissal (list all)
name (generic preferred), formulation (i.e., extended-release)
Date of next appointment
Reminder: Send let er to PCP/family physician
SPS3 Study Progress note
RN and MD completing form
Secondary Prevention of
Small Subcortical Strokes
STUDY TERMINATION - 37
Af ix Patient ID Label Here
• Complete this form for al patients who terminate from the study regardless of the reason.
• Complete if a participant refuses further SPS3 involvement (i.e. early termination where the participants withdraws
consent for al contact including telephone), is truly lost-to-fol ow-up after vigorous ef orts to find him/her, is fol owed for 4.5 years and refuses to re-consent for further follow-up, and for participants who are fol owed until SPS3 study end.
• For early termination or lost to fol ow-up, SPS3 physician investigator should contact the Coordinating Center before
completing the narrative to discuss the case.
• In addition to this form, please complete Forms 4, 7, 9, 10, 14, and 16. If cognitive testing (Form 9) has been completed
within less than 6 months, do not repeat for the end study visit.
1. Date of termination:
2. Reason for end of SPS3 participation:
1 unable to locate patient (describe steps taken to locate patient in narrative)
2 patient withdrew consent (describe reason in narrative)
3 physician request for patient to withdraw
4 clinical site closed
6 patient refused to re-consent for extended fol ow-up
7 patient withdrew after re-consenting for extended follow-up
99 STUDY END
5 other reason,
3. Current living arrangements:
1 alone in own home or apartment 2 with spouse/relatives/others in home or apartment 3 assisted living facility or nursing home or other paid caregiver 4 unknown
Secondary Prevention of
Small Subcortical Strokes
STUDY TERMINATION - 37
Af ix Patient ID Label Here
4. Narrative summary: SPS3 physician investigator to complete after discussing the case with the Coordinator Center. Explain all efforts
undertaken to avoid study termination. (May be dictated and at ached)
5. Date CC contacted: / /
Initials of CC "approver":
(Not needed for End of Study Visit)
Person completing form
Source: http://med-sps3.sites.olt.ubc.ca/files/2012/06/manual_of_operations.pdf
Markopoulos C - PubMed - NCBI Display Settings: Abstract, 200 per page, Sorted by Pub Date Filter your results:All (109) Results: 109 c Lancet. 2011 Aug 27;378(9793):771-84. Epub 2011 Jul 28. 1. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R.
Periodontal Disease, Matrix Metalloproteinases andChemically Modified Tetracyclines From the Department of Periodontology, Faculty of Dentistry, University of Oslo, Oslo, Norway Correspondence to: Svein Steinsvoll, Sagvollveien 1, 2830 Raufoss, Norway. Tel.: �/ 47 61191481; Fax: �/4761191481; E-mail: [email protected] Microbial Ecology in Health and Disease 2004; 16: 1 �/7








