Microsoft word - 100710.docx
Oncotarget, Advance Publications 2015
Cerdulatinib, a novel dual SYK/JAK kinase inhibitor, has broad
anti-tumor activity in both ABC and GCB types of diffuse large B
cell lymphoma
Jiao Ma1, Wei Xing2, Greg Coffey3, Karen Dresser2, Kellie Lu4, Ailin Guo5, Gordana
Raca6, Anjali Pandey3, Pamela Conley3, Hongbo Yu2, Y. Lynn Wang5
1Department of Pathology and Laboratory Medicine, Weill Cornell Medical College, New York, NY
2Department of Pathology, University of Massachusetts Memorial Medical Center and Medical School, Worcester, MA
3Department of Biology, Portola Pharmaceuticals, Inc., South San Francisco, CA
4University of Chicago Laboratory School, Chicago, IL
5Department of Pathology, Division of Genomic and Molecular Pathology, University of Chicago, Chicago, IL
6Department of Medicine, University of Chicago, IL
Correspondence to: Y. Lynn Wang,
e-mail: [email protected]Keywords: diffuse large B cell lymphoma, cerdulatinib, SYK, JAK-STAT, molecularly targeted therapy
Received: May 27, 2015 Accepted:
October 23, 2015 Published: November 05, 2015
B-cell receptor (BCR) and JAK/STAT pathways play critical roles in diffuse large
B-cell lymphoma (DLBCL). Herein, we investigated the anti-lymphoma activity of
cerdulatinib, a novel compound that dually targets SYK and JAK/STAT pathways. On a
tissue microarray of 62 primary DLBCL tumors, 58% expressed either phosphorylated
SYK or STAT3 or both. SYK and STAT3 are also phosphorylated in a panel of eleven
DLBCL cell lines although ABC and GCB subtypes exhibited different JAK/STAT
and BCR signaling profiles. In both ABC and GCB cell lines, cerdulatinib induced
apoptosis that was associated with caspase-3 and PARP cleavage. The compound
also blocked G1/S transition and caused cell cycle arrest, accompanied by inhibition
of RB phosphorylation and down-regulation of cyclin E. Phosphorylation of BCR
components and STAT3 was sensitive to cerdulatinib in both ABC and GCB cell lines
under stimulated conditions. Importantly, JAK/STAT and BCR signaling can be blocked
by cerdulatinib in primary GCB and non-GCB DLBCL tumor cells that were accompanied
by cell death. Our work provides mechanistic insights into the actions of cerdulatinib,
suggesting that the drug has a broad anti-tumor activity in both ABC and GCB DLBCL,
at least in part by inhibiting SYK and JAK pathways.
demand for the development of more effective therapies
based on the understanding of molecular pathogenesis.
Diffuse large B-cell lymphoma (DLBCL) is the
Aberrant B-cell receptor (BCR) signaling is
most common type of non-Hodgkin lymphoma (NHL)
implicated in B-cell malignancies including DLBCL.
and accounts for approximately 40% of all NHL cases.
The BCR complex consists of surface immunoglobulins
The tumor progresses rapidly and treatment is normally
(sIg) that bind antigen in association with disulfide-linked
initiated immediately after a patient is diagnosed with the
heterodimer CD79A and CD79B proteins [1]. Upon antigen
disease. The standard chemoimmunoregimen, rituximab,
binding, the conformational change of sIg transduces
cyclophosphamide, doxorubicin, vincristine and
the signal to the cytoplasmic portions of CD79A/B and
prednisone (R-CHOP), is effective in 60% of patients,
results in the phosphorylation of the immunoreceptor
but nearly 50% of patients treated with R-CHOP will
tyrosine-based activation motif (ITAM) by SRC-family
eventually progress and relapse. The death rate of DLBCL
protein tyrosine kinase LYN. Phosphorylation of ITAMs
remains at approximately 30%. Thus, there is an urgent
then recruits cytosolic tyrosine kinase SYK and causes
its phosphorylation and activation. SYK then triggers
From a clinical perspective, targeting the BCR
activation of the PI3K-AKT and BTK-PLCγ2 pathways
pathway, however, has met with limited success in
with the subsequent generation of inositol triphosphate
DLBCL patients compared to patients with other types
(IP ) and diacylglycerol (DAG). This event is followed by
of NHL and chronic lymphocytic leukemia (CLL).
activation of multiple distal signaling pathways for B-cell
Fostamatinib, a SYK inhibitor, produced an objective
activation, such as RAS-MAPK pathway, PKC activation
response in 5 of 23 (22%) relapsed/refractory (R/R)
and formation of CARD11/BCL10/MALT1 complex, and
DLBCL patients in a phase I/II study [15]. Enzastaurin,
subsequent NFκB activation [1].
a PKCβ inhibitor, produced 3 complete responses and
The first evidence that dysregulated BCR activation
1 stable disease in 55 R/R patients [16]. As to ibrutinib,
is a major contributor to DLBCL pathogenesis came from
a BTK inhibitor, in a phase II study of 80 R/R DLBCL,
gene expression profiling analysis. Based on relatedness of
only 37% ABC and 5% GCB subtypes were responsive
gene expression profiles to normal B-cell subsets, DLBCL
and the response rate of all DLBCL tumors was 19% [17].
were classified into three cell-of-origin subtypes: germinal
Genetic analysis revealed that mutation in MYD88 can
center B-cell (GCB) subtype, activated B-cell (ABC) subtype
nullify the effect of BCR signaling blockade via Toll-like-
and primary mediastinal B-cell lymphoma (PMBL) [2–5].
receptor (TLR) signaling pathway leading to downstream
Subsequent studies revealed that different signaling
NF-κB activation [17]. In addition, gain-of-function
pathways are involved in these distinct subtypes of DLBCL
mutations in downstream CARD11 and A20 can drive the
[6]. The main molecular and genetic abnormalities in
constitutive activation of the NF-κB pathway irrespective
GCB DLBCL include activation of PI3K/AKT/mTOR
of upstream BTK blockade. The complexity of molecular
pathway, BCL2 translocations, and BCL6 rearrangements
and genomic alterations in DLBCL has severely limited
and overexpression, MYC rearrangements, and EZH2
the effectiveness of single-targeted therapy, thus the
mutations; while ABC DLBCL is featured with the
combination of targeted agents or multi-targeted agents
activation of BCR, NF-κB and JAK-STAT pathways
have greater appeals in improving treatment response in
with associated mutations in genes including
CD79A/B,
CARD11, TNFAIP3 (A20) and
MYD88. For PMBL DLBCL,
The Janus kinase and Signal Transducer and
key molecular abnormalities include CIITA translocations,
Activator of Transcription (JAK-STAT) pathway
amplification of REL, amplification of chromosome
represents another important signaling pathway in the
region 9p24 containing PD-L1, PD-L2 and JAK2 loci, and
pathogenesis of DLBCL. One mechanism of STAT3
activation of NF-κB pathways [3, 7, 8]. These molecular
activation in ABC DLBCL (or non-GCB) has been defined.
subtypes are clinically relevant as patient outcomes and
STAT3 is both overexpressed and activated primarily as
responses to chemoimmunotherapeutic regimens are
a function of autocrine secretion of IL-6/IL-10 by tumor
different: GCB DLBCL has much higher response rate than
cells [18], a survival mechanism that can be promoted in
ABC subtype to R-CHOP, while most of PMBL DLBCL
the context of mutations that drive NF-κB activation and
can be cured with DA-EPOCH-R regimen [6].
subsequent NF-κB-mediated cytokine expression [19]. In
Additional information regarding the molecular
addition, it is possible that non-tumor cells of the tumor
features of DLBCL was revealed by analysis of gene
microenvironment promote malignant cell survival in
expression profiles with consensus clustering which
part via paracrine cytokine secretion [20, 21]. Clinically,
groups lymphomas by functional relatedness of genes
STAT3 activation, as reflected by STAT3 phosphorylation
[9]. This analysis also identified three groups: BCR/
has been associated with worse survival in patients treated
proliferation, OxPhos, and Host response. The BCR group
with R-CHOP [22].
In vitro inhibition of STAT3 activity
is featured with high expression of SYK mRNA along with
with either JAK inhibitors or STAT3 knockdown results
mRNA of other BCR pathway components. Concordance
in decreased cell proliferation and increased apoptosis in
between the cell-of-origin and consensus clustering
ABC tumor cell lines [18, 23]. Moreover, early clinical
schemes, however, is poor. A common molecular feature
studies suggest that targeting JAK/STAT pathways using
identified is the increased BCR signaling in a significant
small molecule JAK inhibition [24], STAT3 knock down
fraction of DLBCL patients, ABC-DLBCL by cell-of-
(Hong DS, et al. 2013 ASCO annual meeting abstract
origin scheme and BCR-DLBCL by consensus clustering
#8523), or a neutralizing antibody specific for IL-6 [25]
scheme. These studies provide a strong rationale to target
may be beneficial for patients with B-cell malignancies.
BCR signaling in DLBCL.
Thus, literature evidence provides a strong rationale
Previously, several groups including ours have
to target both BCR and JAK-STAT pathway in DLBCL.
explored the potential of inhibiting BCR pathways in
Cerdulatinib (previously known as PRT062070) is a novel
DLBCL cell lines and primary tumor cells [10–14]. We
orally available small-molecule ATP-competitive inhibitor
showed that targeting LYN [12] or SYK [13] inhibits BCR
that demonstrates inhibition of SYK, JAK1, JAK2, JAK3,
signaling and cell proliferation in a subset of DLBCL.
and TYK2 in a biochemical assay [26] (Table 1). However,
Shipp's group further demonstrated the cells responding to
at the cellular level, cerdulatinib demonstrates specificity
SYK inhibition carry the BCR signature with either high
towards JAK1/JAK3 and TYK2, but not JAK2-mediated
or low NFκB activity [14].
responses. The specificity of cerdulatinib was also
Table 1: Activity of cerdulatinib against selected kinases, and their expression in normal LN and
IC50 (nM)
Expression in normal lymph node*
Expression in primary lymphoma tissue*
Medium to High
Medium to High
Mostly High
Low to Medium
Low to Medium
Low to Medium
Medium to High
Mostly High
Medium to High
Mostly High
ND: Not detected. N/A: Not Available.
*Tissue microarray data adapted from the human protein atlas website at: http://www.proteinatlas.org. IC data were
derived from Coffey G, et al. [26].
demonstrated by its lack of inhibition of T cell receptor
refractory CLL and B cell non-Hodgkin lymphoma (NHL;
signaling or protein kinase C signaling in whole blood
NCT01994382). Initial clinical results have demonstrated
[26]. In animal models, the agent reduces inflammation
good tolerability, significant inhibition of SYK and JAK,
in a rat model of autoimmune disease, and blocks B-cell
and greater than 50% target tumor reductions in patients
activation and alleviates splenomegaly induced by chronic
with CLL and NHL (Flinn I, et al. 2015 ASCO annual
BCR stimulation in mice [26]. Notably, in primary CLL
meeting Abstract #8531). Herein, we further characterize
cells with the BTKC481S mutation, cerdulatinib is able to
antitumor activities of cerdulatinib in subtypes of DLBCL
overcome ibrutinib resistance by completely blocking the
cell lines and primary tumor cells. The results suggest
proliferation of the resistant cells [27–29]. Cerdulatinib
cerdulatinib exerts broad anti-tumor activity in both ABC
is currently under investigation as a single orally
and GCB DLBCL including cells with resistance to BCR-
administered agent in a dose escalation study in relapsed/
targeted therapy.
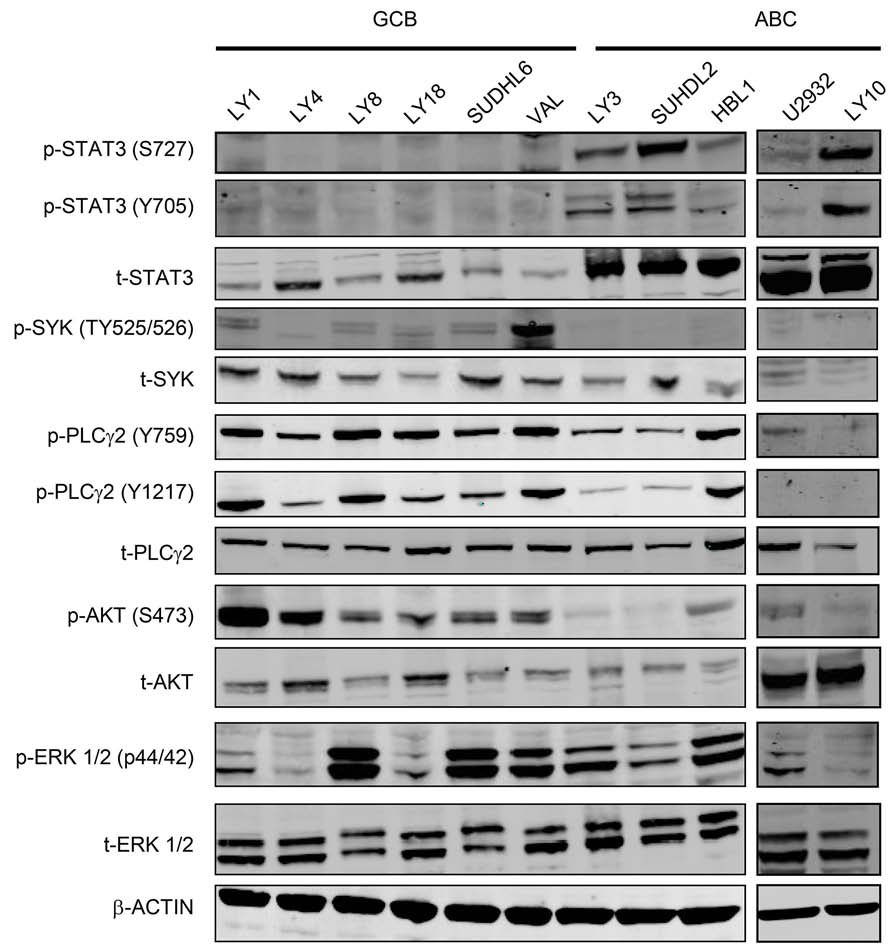
sites (LY3, DHL2 and LY10). In contrast, little total or
phosphorylated STAT3 proteins were detected in the six
GCB cell lines. The immunoblotting also revealed some
STAT3 and SYK are active in an array of
interesting findings regarding all the GCB and HBL1 cell
primary DLBCL tissues of both GCB and
lines. Although the expression and phosphorylation of
SYK, PLCγ2 and ERK and total AKT levels are variable
among the cell lines, in general, all GCB appear to
To determine whether simultaneous targeting of
express higher levels of p-SYK, p-PLCγ2 (at both Y759
both JAK/STAT and SYK is relevant in DLBCL, we
and Y1217) and p-AKT suggesting downstream BCR
examined the expression of p-STAT3 (Y705) and p-SYK
activity is more active in these cell lines. Among five
(Y525/526) on a tissue microarray of 62 DLBCL primary
ABC cell lines, HBL1 cell line appears to be the only one
tumors, including 41 germinal center-like (GCB) and 21
that expresses high levels of p-PLCγ2 (Figure 2). Taken
non-germinal-center-like (non-GCB) tumors classified
together with Figure 1, active forms of SYK and JAK are
using Han's algorithm [30] (Figure 1). p-STAT3 exhibits
expressed in a wider range of DLBCL tumors than each of
a characteristic nuclear staining pattern in DLBCL cases
the single molecule alone.
(Figure 1A). Patterns other than nuclear were excluded as
positive staining. p-STAT3 staining in tonsil is included
Both ABC and GCB subtypes of DLBCL are
as control (Figure 1 A-d). A total of 26 (26/62, 42%)
sensitive to dual SYK/JAK inhibition with
stained positive for nuclear p-STAT3; 16 were GCB type
(16/41, 39%) and 10 were non-GCB type (10/21, 48%,
Figure 1B). p-SYK expression was detected in 29 (29/62,
Cerdulatinib is an orally available ATP-competitive
47%) cases with a characteristic peri-membrane staining
small molecule kinase inhibitor currently in clinical
pattern (Figures 1A and 1B). Patterns other than peri-
development for the treatment of B-cell malignancies
membrane were excluded as positive staining. p-SYK
(NCT01994382). Early clinical results (Flinn I, et al. 2014
staining in tonsil is included as control (Figure 1A-h).
ASCO annual meeting Abstract #2619) suggest that high
While occasionally germinal centers were found to contain
level of SYK and JAK inhibition is achieved in treated
a few scattered p-SYK positive cells, most of the germinal
patients, with an acceptable safety profile. In biochemical
centers in the tonsil are completely negative for p-SYK. Of
assays, cerdulatinib demonstrated inhibitory activity
these 29 p-SYK positive cases, 17 were GCB type (17/41,
against 24 kinases with IC 's < 200 nM (Table 1). Most
41%) and 12 were non-GCB type (12/21, 57%, Figure
of the kinases affected by cerdulatinib are not expressed at
1B). Interestingly, there are 19 cases (19/62, 31%) among
all, or expressed at very low levels in normal lymph nodes
the total 62 cases with reactivity for both p-SYK and
and primary lymphoma tissues. Seven of these affected
p-STAT3, of which, 11 were GCB type (11/41, 27%) and
kinases are notably expressed including AMPK, JAK2,
8 were non-GCB type (8/21, 38%, See Figure 1B for case
TBK1, JAK1, RSK4, SYK, and FLT3 (Bold in Table 1).
numbers and Figure 1C for percentage breakdown). These
Cerdulatinib inhibits SYK and JAK1 with IC 's of 32 nM
data are consistent with the single stain results published
and 12 nM, respectively. A recent publication detailing the
previously on p-STAT3 by Huang et al [22] and on p-SYK
translation of biochemical assays to cellular potency and
by our group [13]. Together, these findings demonstrated
selectivity revealed that cerdulatinib principally acted as a
that SYK and STAT3 are active in a significant number of
SYK, JAK1/3, and TYK2 inhibitor, lacking cellular activity
DLBCL cases (Figure 1C).
against JAK2, against the SRC family members LCK
and LYN, as well as the structural homolog ZAP70 [26].
STAT3 and SYK are active in a panel of DLBCL
Potency against AMPK, TBK1, RSK family members, and
cell lines of both GCB and ABC subtypes
FLT3 has not been determined in cellular assays.
We next investigated the activity of cerdulatinib in
We then determined the basal expression levels
the panel of DLBCL cell lines. A cell-based MTT assay
of BCR signaling and JAK/STAT signaling molecules
that reflects cellular metabolic activity was performed
and their phosphorylation status in a panel of GCB and
at 72 h following treatment with cerdulatinib at varying
ABC DLBCL cell lines using immunoblotting analysis.
concentrations (Figure 3A). All cell lines demonstrated
The cell lines studied include six GCB cell lines: OCI-
sensitivity to cerdulatinib with IC at or below 2 μM
LY1 (LY1), OCI-LY4 (LY4), OCI-LY8 (LY8), OCI-LY18
(data compiled in Figure 3B). Notably, ABC cell lines
(LY18), SUDHL6 (DHL6) and VAL, and 5 ABC cell
with relatively higher total and phosphorylated STAT3
lines: OCI-LY3 (LY3), SUDHL2 (DHL2), HBL1, U2932
(Figure 2) displayed good sensitivity to the drug with
and OCI-LY10 (LY10). As shown in Figure 2, three of
IC ranging from 0.29 to 1.80 μM (Figure 3B). Of these
the five ABC cell lines exhibited very high levels of
ABC cell lines, LY3 and DHL2 were insensitive to a
total and phosphorylated STAT3 at both Y705 and S727
SYK selective inhibitor, PRT060318, as demonstrated
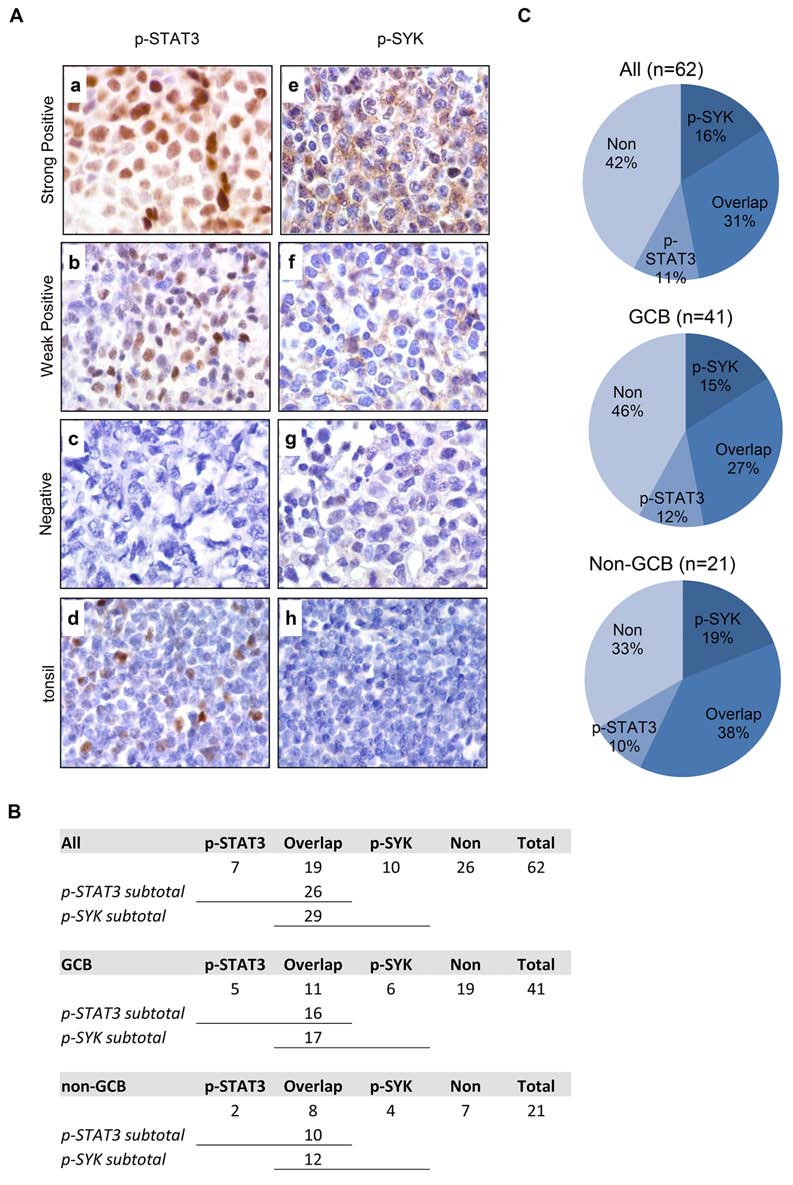
Figure 1: Expression of p-STAT3 (Y705) and p-SYK (Y525/526) in normal tonsil and primary DLBCL tissues on tissue
microarray. A. Expression of p-STAT3 (a-d) and p-SYK (e-h) was examined by immunohistochemistry on paraffin-embedded sections
from DLBCLs (a-c and e-g) and normal tonsil tissue (d and h). a. A representative example of strongly positive p-STAT3 staining (60–90%
lymphoma cells). b. A representative example of weakly positive p-STAT3 staining (30–50% lymphoma cells). c. A representative example
of negative p-STAT3 staining (<30% lymphoma cells). d. Basal level of p-STAT3 expression in normal tonsil tissue with scattered positive
cells in germinal center. e. A representative example of strongly positive p-SYK staining (60–90% lymphoma cells). f. A representative
example of weakly positive p-SYK staining (30–50% lymphoma cells). g. A representative example of negative p-SYK staining (<30%
lymphoma cells). h. Absence of p-SYK positivity in normal tonsil tissue. (a-h, x1,000). B. Table showing the number breakdown of p-SYK
and p-STAT3 staining in all, GCB or non-GCB cases. C. Pie charts showing the percent breakdown of p-SYK and p-STAT3 staining in all,
GCB or non-GCB cases.
in our previous study [13]. LY4, a GCB cell line, was
and LY18) and three ABC (HBL1, SUDHL2 and LY3) cell
also resistant to the selective SYK inhibition due to lack
lines, which were characterized further below.
of surface immunoglobulins [13]. The cell line was,
however, sensitive to dual inhibition by cerdulatinib with
Cerdulatinib induces apoptosis in both GCB and
an IC of 2.1 μM. Early clinical results suggest that these
ABC subtypes of DLBCL cell lines via caspase 3
concentrations can be safely achieved in the plasma of
and PARP cleavage
treated patients (Flinn I, et al. 2014 ASCO annual meeting
Abstract #2619). Overall, the results demonstrate potent
The effects of cerdulatinib on cellular metabolism
and broad activity of cerdulatinib in DLBCL cell lines.
(MTT) can result from either cell death or cell cycle
Sensitivity to cerdulatinib was confirmed in an independent
inhibition or both. We therefore analyzed the effects of
cell growth assay (Figure 3C) utilizing two GCB (SUDHL6
cerdulatinib on DLBCL cell viability. Using annexin V
Figure 2: Distinct signaling pathways in GCB-DLBCL vs ABC-DLBCL cell lines. Western blotting analysis of the basal
levels of total and phosphorylated STAT3, SYK, PLCγ2, AKT and ERK in GCB and ABC DLBCL cell lines. Phosphorylated residues for
each protein are indicated. β-actin was included as a loading control.
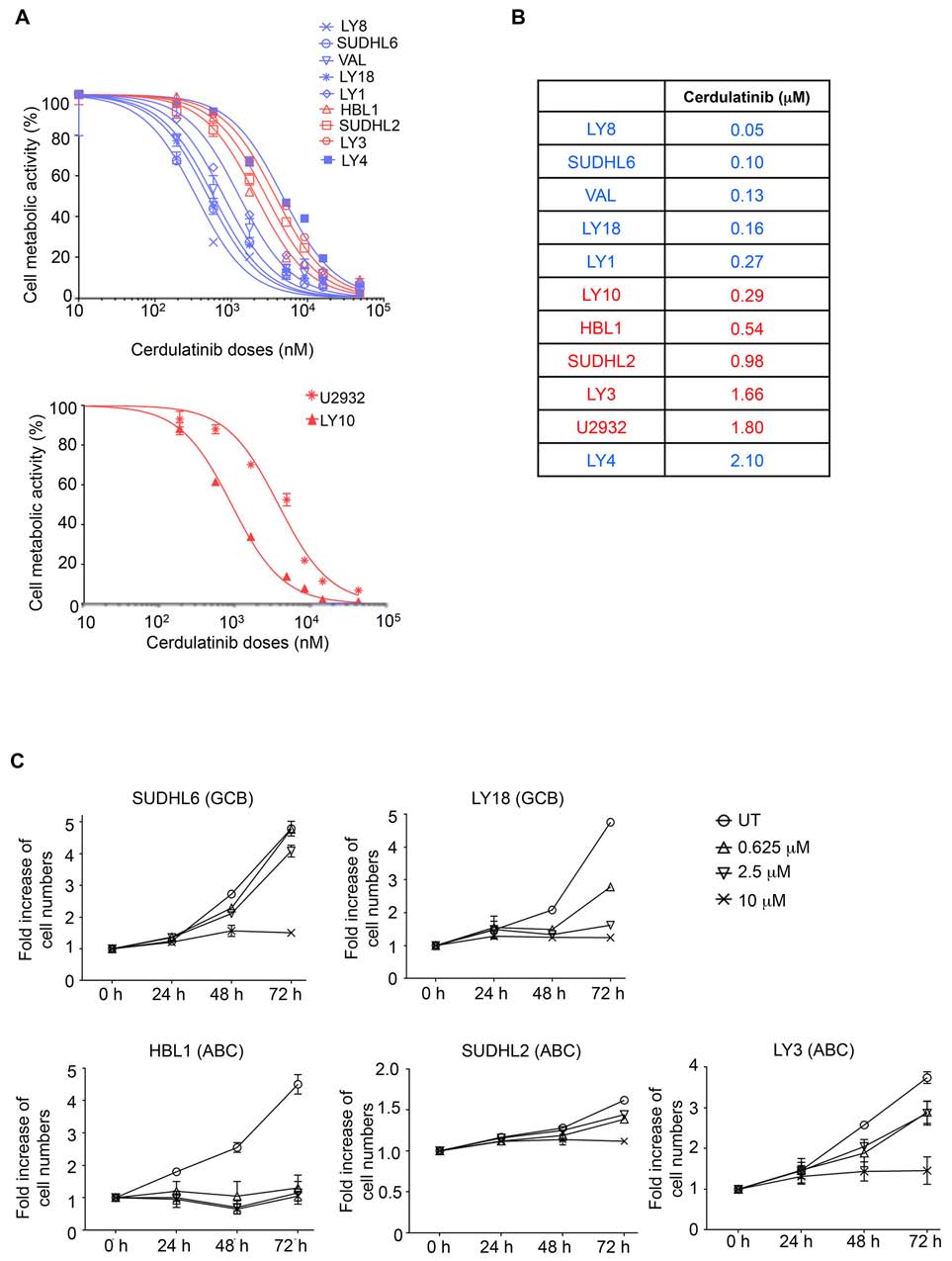
Figure 3: Both ABC and GCB subtypes of DLBCL are sensitive to dual SYK/JAK inhibition with cerdulatinib. A.
DLBCL cell lines were treated with various concentrations of cerdulatinib for 72 h followed by MTT assay. GCB are highlighted in blue
and ABC in red. B. IC values were calculated using GraphPad Prism 5 software (GraphPad, La Jolla, CA). C. DLBCL cell lines were
treated with indicated concentrations of cerdulatinib for up to 72 h. Cell numbers were determined at indicated time points and normalized
to vehicle treated control. Error bars represent the standard error of the mean (SEM) from three independent experiments.
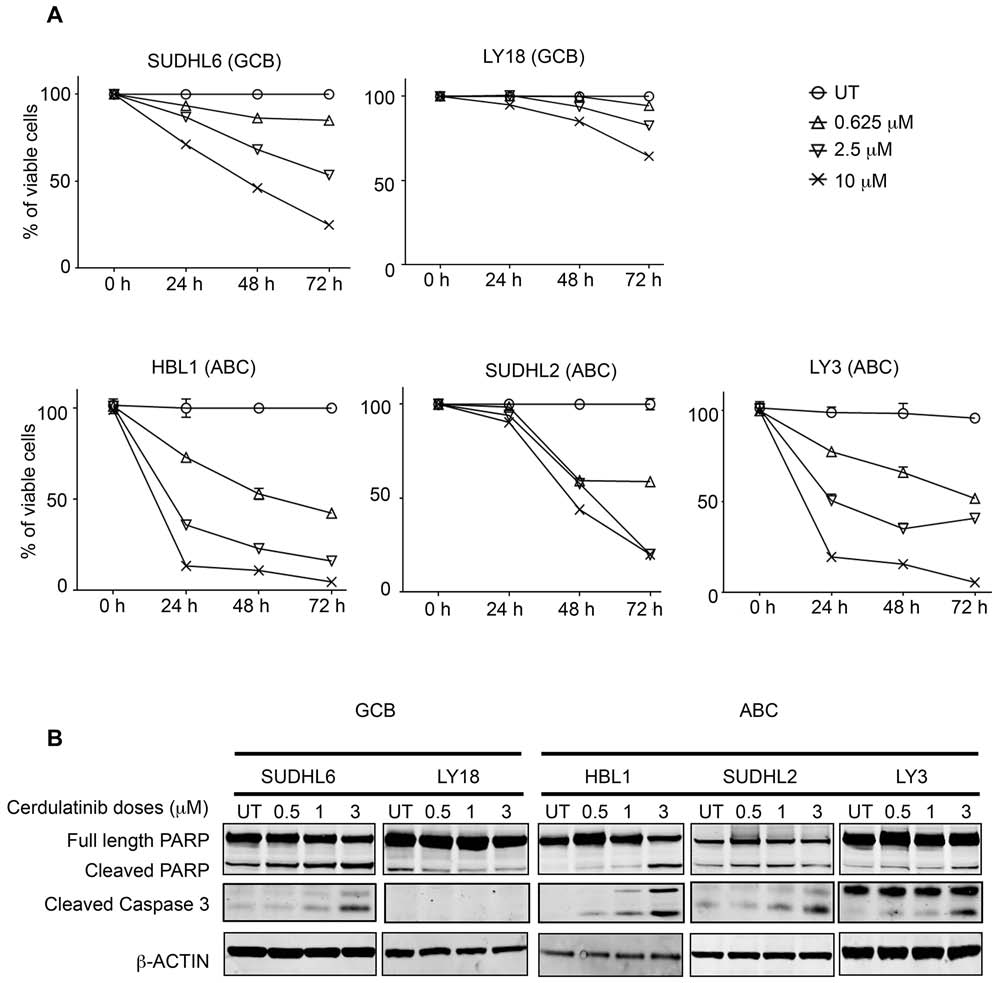
and 7-AAD double staining, we demonstrated that with
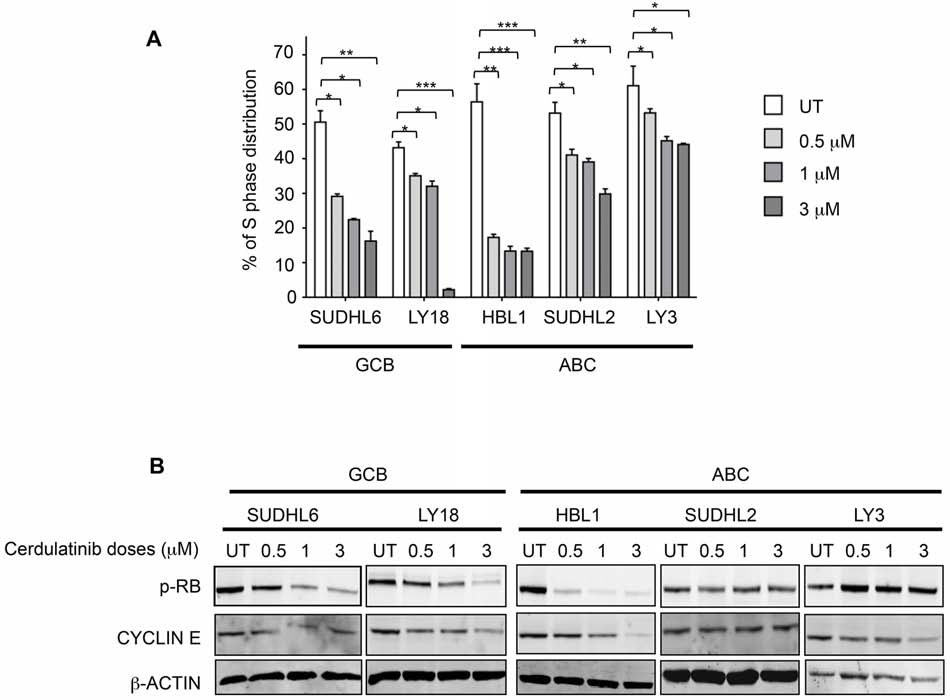
Cell cycle arrest by cerdulatinib is associated
the exception of LY18, viability of all cell lines was
with inhibition of RB phosphorylation and
reduced by cerdulatinib treatment in a concentration- and
down-regulation of cyclin E
time-dependent manner (Figure 4A). Apoptosis induction
with increasing concentrations of cerdulatinib in the four
After establishing apoptosis induction as one of the
responding cell lines was accompanied by both PARP and
drug's actions, we then studied the effect of cerdulatinib
caspase 3 cleavage whereas little changes were observed
on cell cycle progression using BrdU incorporation. As
in LY18 (Figure 4B).
shown in Figure 5A, DHL6, LY18 and HBL1 appeared
Figure 4: Cerdulatinib induces apoptosis in both GCB and ABC subtypes of DLBCL cell lines via caspase 3 and PARP
cleavage. A. Viability of DLBCL cells treated with various doses of cerdulatinib for up to 72 h. Cells were collected at indicated time
points, and stained with annexin V and 7-AAD. Cell viability was measured using flow cytometry, and shown as the percent viable cells
(annexin V-/7-AAD-) relative to vehicle treated control. Error bars represent the SEM from three independent experiments. B. DLBCL cells
were treated with indicated doses of cerdulatinib for 48 h. Western blotting was performed using antibodies against PARP and caspase 3.
β-actin was included as a normalization control.
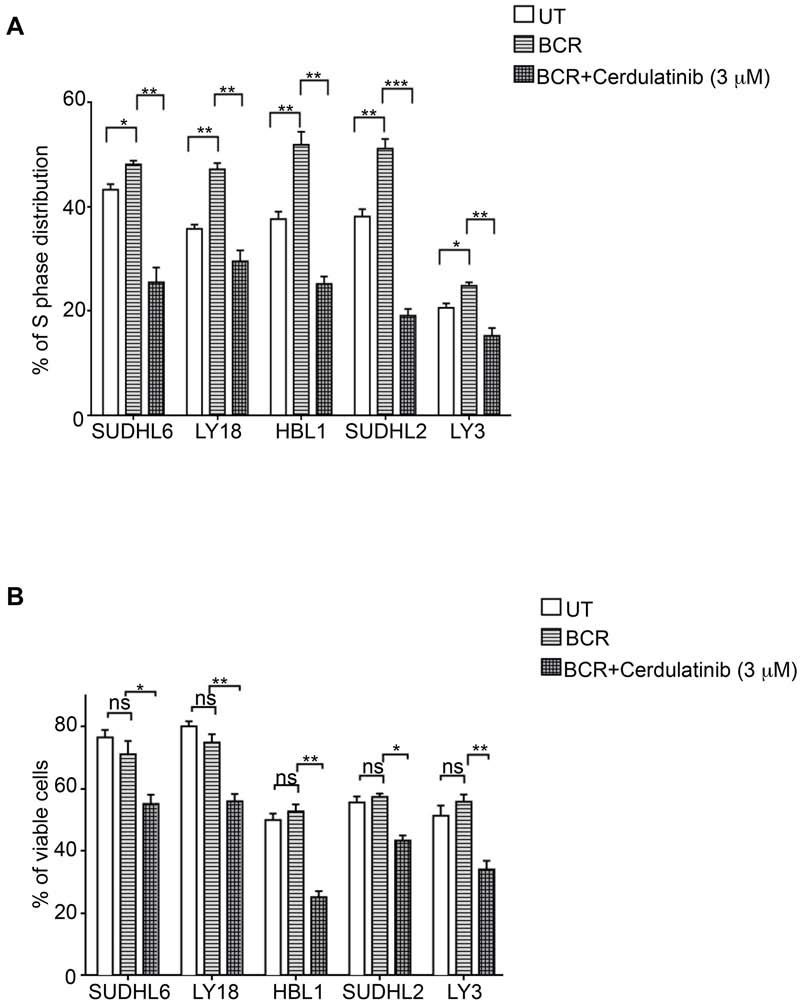
to be particularly sensitive to cerdulatinib treatment with
cerdulatinib to inhibit cell cycle and induce apoptosis
significant dose-dependent reduction in S phase fraction,
under the condition of BCR stimulation. Figure 6A shows
while the effects of the drug on DHL2 and LY3 were
that BCR stimulation with anti-IgM and anti-IgG drove
modest in comparison. The dose-dependent S-phase
more cells into S-phase in all five cell lines regardless of
reductions caused by the drug are largely consistent with
subtypes and these stimulated tumor cells were sensitive
its inhibition of phosphorylated RB and with decrease in
to cerdulatinib treatment. Similarly, the viability of
cyclin E expression (Figure 5B): While bigger changes
stimulated DLBCL cells were reduced by cerdulatinib in
were observed in HBL1, SUDHL6 and LY18, little
all cell lines tested (Figure 6B). Taken together with the
or no changes were observed in SUDHL2 and LY3.
results under the resting conditions (Figure 4A and 5A),
Collectively, our data on apoptosis (Figure 4) and on cell
we conclude that cerdulatinib achieves its anti-tumor
cycle progression (Figure 5) suggests that cerdulatinib
effects in ABC and GCB DLBCL cell lines via induction
achieves its inhibitory effects in different DLBCL cell
of apoptosis and cell cycle arrest with or without external
lines (Figure 3A and 3B, MTT assay) via different cellular
processes, either induction of apoptosis or induction of
cell cycle arrest.
Cerdulatinib blocks JAK/STAT and BCR
signaling in both ABC and GCB DLBCL cell
Cerdulatinib induces apoptosis and cell cycle
arrest in BCR-stimulated DLBCL cells
To determine whether cerdulatinib inhibits BCR
Since the BCR pathway may be chronically active
and JAK-STAT signaling pathways, we first measured
in many DLBCL, we next examined the capability of
the phosphorylation of SYK, PLCγ2, AKT and ERK in
Figure 5: Cerdulatinib blocks cell cycle in both ABC and GCB subtypes of DLBCL via inhibition of RB phosphorylation
and down-regulation of cyclin E. A. DLBCL cells were treated with indicated doses of cerdulatinib for 48 h. Cells were labeled with
10 μM BrdU for 2 h, followed by double staining with BrdU antibody and 7-AAD prior to flow cytometry analysis. Percentage of cells at S
phase was statistically analyzed using one-way ANOVA test and graphed using prism 5 GraphPad. Error bars represent the SEM from three
independent experiments. *p < 0.05; **p < 0.01; ***p < 0.005. B. DLBCL cells were treated with indicated concentrations of cerdulatinib.
The whole cell lysates were prepared at 48 h following treatment. Immunoblotting was performed using p-RB and cyclin E antibodies.
β-actin was included as a loading control.
BCR-stimulated DLBCL cells treated with or without
was observed: SYK, PLCγ2, AKT and ERK in DHL6;
cerdulatinib (Figure 7A). Immunoblotting analyses
SYK, PLCγ2 and AKT in LY18; SYK and AKT in HBL1,
revealed a significant reduction of p-SYK in all cell lines,
SYK in DHL2; and SYK, AKT and ERK in LY3. Thus,
reduction of p-PLCγ2 in the two GCB cell lines (DHL6
despite the variability, at least one signaling component of
and LY18), reduction of p-AKT in four of the five cell
the BCR pathway was effectively inhibited by cerdulatinib
lines (except DHL2) and reduction of p-ERK in two of the
in each individual cell line.
five cell lines (DHL6 and LY3). When analyzing data by
We also determined whether cerdulatinib inhibits
individual cell lines, reduction in protein phosphorylation
the JAK-STAT signaling pathways under the condition of
Figure 6: Cerdulatinib induces cell cycle arrest and apoptosis under the condition of BCR stimulation in all DLBCL
cell lines. A. DLBCL cells were treated with 3 μM of cerdulatinib for 48 h and labeled with 10 μM BrdU for 2 h, followed by double
staining with BrdU antibody and 7-AAD prior to flow cytometry analysis. B. Following 48 hr drug treatment, cells were stained with
annexin V and 7-AAD. Percentage of viable cell relative to vehicle control or cells at S phase was statistically analyzed using one-way
ANOVA test and graphed using prism 5 GraphPad. Error bars represent the SEM from three independent experiments. *p < 0.05; **p <
0.01; ***p < 0.001.
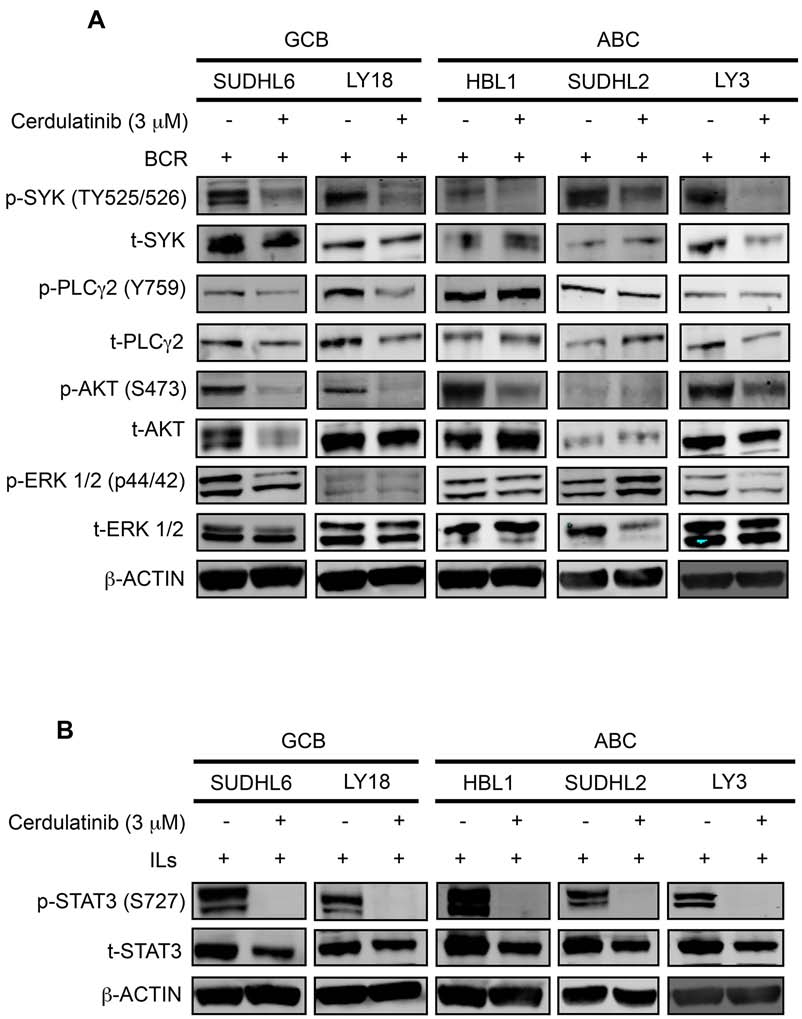
cytokine stimulation. Cells stimulated with IL-6 and IL-10
cellular toxicity in terms of cell survival and proliferation
were treated with cerdulatinib or vehicle, phosphorylation
of STAT3 at Y705 was measured. As shown in Figure
7B, cytokine stimulated cells expressed very high levels
Cerdulatinib induces cell death in primary
of p-STAT3 regardless of subtype designation. Addition
human DLBCL samples
of cerdulatinib completely blocked this phosphorylation
event to a level that goes below the detection level. Thus,
To confirm whether data obtained with the cell lines
we conclude that modulation of both BCR and JAK-STAT
are reproducible with primary patient tumors, we tested
signaling pathways by cerdulatinib correlated with its
several human DLBCL samples, including 3 GCB, 2 non-
Figure 7: Cerdulatinib blocks JAK/STAT and BCR signaling in both ABC and GCB DLBCL cell lines. DLBCL cells were
treated with 3 μM of cerdulatinib for 30 min, and then stimulated with either anti IgM/IgG or IL6/IL10 for 24 h before cell collection and
lysate preparation. Immunoblotting analysis of A. p-SYK, p-PLCγ2, p-AKT, p-ERK expressions and B. p-STAT3 expression in selected
DLBCL cell lines. β-actin was included as a loading control.
GCB and one unclassified (patient information shown
and JAK inhibition (Table 1), the data presented herein
in Supplemental Table 1), for their apoptotic response
with DLBCL cell lines and primary tumors are at least
to 0.5 μM and 1 μM cerdulatinib. As shown in Figure
consistent with the hypothesis that SYK and JAK
8A, all primary DLBCL cells responded to cerdulatinib
inhibition contributes to the antitumor activities of the
in a concentration-dependent manner with variable
drug. Target selectivity of cerdulatinib was demonstrated
previously in different cell types of normal human whole
We next studied the inhibition of p-STAT3, p-ERK
blood as well. The compound potently inhibit BCR and
and p-AKT in response to cerdulatinib treatment in these
FcR-induced SYK activation and cytokine receptor-
primary patient cells stimulated with anti-BCR. Phospho-
induced JAK1/3 and JAK1/TYK2 activation in B cells,
flow assay demonstrated a marked and simultaneous
T cells and monocytes while it does not inhibit protein
down-regulation of p-STAT3, p-AKT and p-ERK in
kinase C-mediated PMA signaling or LCK and ZAP70–
response to increasing concentrations of cerdulatinib in
mediated T cell antigen receptor signaling, or JAK2-
all six primary DLBCL cells regardless of their subtype
mediated GM-CSF signaling. [26]
designation (Figures 8B, 8C and 8D), but inhibition of the
Cerdulatinib demonstrated broader anti-tumor
individual protein phosphorylation was highly variable
activity relative to several BCR-specific inhibitors
among these primary samples. For instance, DLBCL3
we have evaluated. Previously, we have shown that
showed no significant inhibition in p-STAT3 in response
dasatinib (targets mainly SRC family kinases at low
to increasing concentrations of cerdulatinib, presumably
concentrations) and PRT318 (highly specific for SYK)
due to its very low baseline p-STAT3 level in untreated
exert anti-tumor activity primarily by affecting cell cycle
cells compared to the other patient samples (Figure 8B).
with minimal impact on cell viability [12, 13]. However,
DLBCL1 and DLBCL4 had little to no suppression of
with cerdulatinib, we observed apoptosis induction in
p-ERK activity upon cerdulatinib treatment (Figure 8D).
addition to cell cycle inhibition (Figures 4–6) although
These results are similar to those observed in cell lines
the specific cellular effect varied from cell line to cell
(Figure 7) suggesting individual patient samples may
line. These data suggest that simultaneous inhibition of
rely upon different signaling pathways. Interestingly,
multiple therapeutically relevant targets, such as combined
a significant linear correlation was found between the
SYK and JAK, may represent a more effective approach
degree of p-ERK inhibition and the extent of cell death
compared to single target-directed agent.
response (Figure 8E, left, p = 0.0117). Meanwhile, no
It is also noteworthy that many cell lines resistant
significant linear correlation was identified between the
to BCR-targeted inhibitors were sensitive to cerdulatinib.
extent of p-AKT inhibition and the cell death response
Among the cell lines studied, LY3 carries an activating
(Figure 8F, right, p = 0.2142). The results suggest that
mutation in CARD11 [31] (Supplemental Table 2); LY3,
indirect inhibition of downstream ERK phosphorylation
LY10, U2932 (ABC) along with LY8, VAL, and SUDHL2
by cerdulatinib may play a role in the final cellular
(GCB) carry inactivating mutations or hemizygous
outcome, and ERK inhibition may mediate the therapeutic
deletion in TNFAIP3 (A20), which negatively regulates
effects of cerdulatinib.
NFκB activity. Loss of A20 function results in constitutive
activation of NFκB that promotes tumor growth and
survival. ABC cell lines, HBL1, LY3, LY10 and SUDHL2,
along with GCB cell line SUDHL6 all carry activating
Available clinical data indicate that BCR-directed
MYD88 mutations, which promote IRAK1/4 and TRAF6
inhibitors such as SYK (fostmatinib), BTK (ibrutinib)
dimerization and subsequent NFκB activation.
and PKCβ (enzastaurin) are active in ≤ 20% of DLBCL
The ultimate activation of NFκB, resulting from
patients. This highlights the need to continue exploring
these BCR-associated or MYD88 mutations, leads
novel agents with expanded activities in this disease.
to increased IL6 and IL10 autocrine secretion that
In this study, we first demonstrated the presence of
subsequently activates JAK-STAT3 pathway to enhance
active SYK and JAK in their phosphorylated forms in
cell survival and proliferation. This perhaps represents
a significant fraction of DLBCL primary tumor tissues
one of several mechanisms rendering tumor cells resistant
and cell lines. We then showed that cerdulatinib, a
to inhibitors of early BCR components. As a matter
potent inhibitor of SYK and JAK, has broad anti-tumor
of fact, high levels of baseline phosphorylated STAT3
activity as demonstrated by inhibition of 1) cellular
exhibited in the ABC cell lines (Figure 2) may be a result
metabolic function, 2) cell viability, 3) cell cycling, 4)
of the BCR/MYD88 mutations they carry. Cerdulatinib,
signal transduction through SYK-PLCγ2-AKT or ERK,
with its ability to inhibit p-STAT3 in both cell lines and
and 5) signal transduction through JAK-STAT. We
primary tumors, provides another point of blockade after
further demonstrated that cerdulatinib induced cell death
NFκB activation. Antitumor activities were achieved by
in primary DLBCL cells and the degree of cell death
cerdulatinib in all cell lines carrying mutations including
significantly correlated with decreased p-ERK.
ABC cell lines HBL1 (MYD88), LY3 (CARD11, A20 and
While the broad activity of cerdulatinib in DLBCL
MYD88), LY10 (A20 and MYD88), SUDHL2 (A20 and
cell lines may be ascribed to mechanisms beyond SYK
MYD88), and U2932 (A20) as well as GCB cell lines LY8
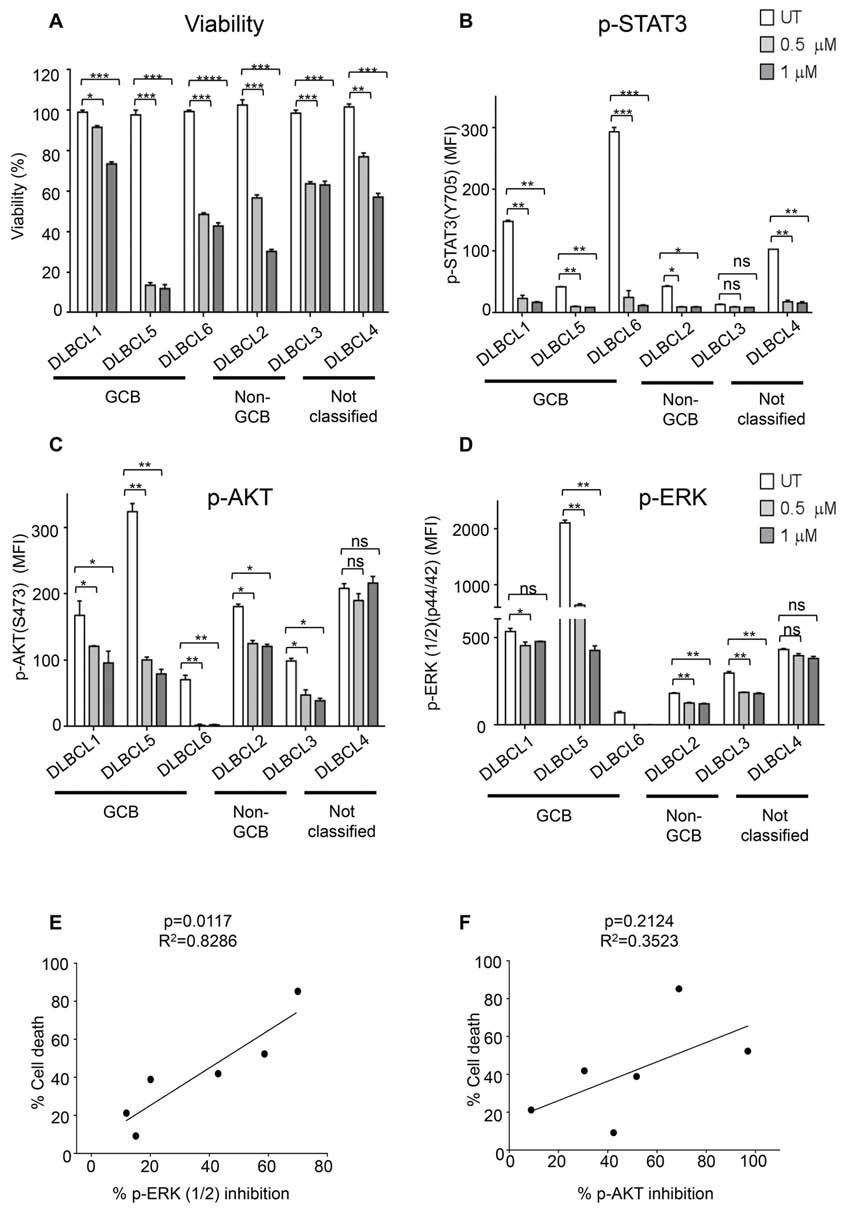
Figure 8: Primary DLBCL cells are sensitive to dual SYK/JAK inhibition. A. Six primary human DLBCL samples were
treated with 0.5 μM or 1 μM cerdulatinib for 72 h. Cell viability was measured by MTT assay, and normalized to vehicle control. Primary
human DLBCL samples were treated with 0.5 μM or 1 μM cerdulatinib for 6 h followed by stimulation with 5 μg/ml IgM/IgG for 15 min
at 37°C. Phospho-flow assays were performed to determine the levels of p-STAT3 in B, p-AKT in C and p-ERK in D in the primary cells.
Error bars represent the SEM from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. E. Relationship
between cell death following treatment with 1 μM cerdulatinib and percent inhibition of ERK1/2 was analyzed by Spearman correlation.
F. Relationship between cell death and percentage of inhibition of AKT was statistically analyzed using one-way ANOVA test. Percent
inhibition of phosphorylation was calculated using the formula: [(MFI
(A20), SUDHL6 (MYD88) and VAL (A20) (Supplemental
Table 2), likely by blocking the up-regulation of STAT3
activity induced by the sequential events of BCR/MYD88
The study included 62 DLBCL samples in two tissue
microarrays from 2000–2011. Twenty-five cases were
STAT. In particular, not only high levels of baseline
from nodal sites and 37 from extranodal sites. Only tumors
phosphorylated STAT3 exhibited in the ABC cell lines
with enough tissues were included. Immunohistochemical
render these cells very sensitive to cerdulatinib inhibition,
stains of p-STAT3 (Y705, rabbit monoclonal Ab, clone
but also cytokine-induced STAT3 phosphorylation in
D3A7, Cell Signaling) and p-SYK (Y525/526; rabbit
GCB cell lines (Figure 7B). Notably, some of these cell
polyclonal Ab; Cell Signaling) were performed and the
lines, LY3 in particular, was shown to be highly resistant
results were evaluated by proportion of lymphoma cells
to inhibition of SRC, SYK and BTK [10]. In the clinical
that were stained. The antibody reactivity in ≥ 30% of
trial setting, patients with mutations in CARD11, A20
the lymphoma cells was considered positive. Paraffin-
and MYD88 indeed demonstrated resistance to ibrutinib,
embedded normal tonsil tissue was included as a baseline
a BTK inhibitor [17]. In addition to BCR inhibitor-
expression level for p-STAT3 and p-SYK.
resistant DLBCL, cerdulatinib (PRT2070) also has the
capability to overcome ibrutinib resistance in CLL cells
Immunoblotting assays and antibodies
carrying BTKC481S mutation [28, 29]. These data suggest
that simultaneous inhibition of multiple therapeutically
Immunoblotting were performed as previously
relevant targets, such as combined SYK and JAK, may
described [32]. Antibodies including total STAT3,
represent a more effective approach for the treatment of
SYK, PLCγ2, AKT, ERK1/2, PARP, caspase 3, and
B-cell lymphoma.
phosphorylated STAT3 (S727), STAT3 (Y705), SYK
(Y525/526), PLCγ2 (Y759), PLCγ2 (Y1217), AKT (S473),
ERK1/2 (Y204) and RB (S807/811) were purchased from
MATERIALS AND METHODS
Cell Signaling (Danvers, MA). Cyclin E antibody was
purchased from Santa Cruz Biotechnology (Dallas, TX),
and β-actin antibody was purchased from Sigma-Aldrich
Cell lines, primary cells and culture conditions
(St Louis, MO). Antibodies used in phosphoflow assay,
GCB cell lines OCI-LY1, OCI-LY4, OCI-LY8,
including phosphorylated STAT3 (S727), SYK (Y525/526),
OCI-LY18, SUDHL6 and VAL were cultured in Iscove's
PLCγ2 (Y759), AKT (S473), and ERK1/2 (Y204) were
Modified Dulbecco's Medium (IMDM) supplemented
purchased from BD Biosciences (San Jose, CA).
with 20% Fetal Bovine Serum (FBS) and 100 μg/mL
penicillin/streptomycin. ABC cell lines SUDHL2 and
Cell metabolic activity, cell growth and viability
HBL1 were cultured in RPMI1640 supplemented with
20% FBS and 100 μg/mL penicillin/streptomycin. OCI-
LY3 cells were grown in RPMI1640 supplemented
DLBCL cell lines were treated with various
with 20% FBS, 100 μg/ml penicillin/streptomycin
concentrations of cerdulatinib for up to 72 h. The
and HEPES. ABC cell lines OCI-LY10 was cultured
metabolic activities of cells were determined with MTT
in Iscove's Modified Dulbecco's Medium (IMDM)
assay according to manufacturer's instruction (Roche
supplemented with 20% human serum and 100 μg/
Applied Science, Indianapolis, IN) at 72 h time point.
mL penicillin/streptomycin. ABC cell line U2932 was
IC was calculated using the Sigma Plot generated with
cultured in high glucose RPMI1640 supplemented
GraphPad Prism 6 software (GraphPad, La Jolla, CA).
with 20% FBS, 100 μg/ml penicillin/streptomycin.
Cell growth was measured at every 24 h counting live
All cells were maintained in a humidified 37°C/5%
cells with flow cytometry as previously described [12].
CO incubator. Frozen primary DLBCL cells were
Cells were collected every 24 h and cell viability was
obtained from the tumor bank of the Department of
determined by the PE Annexin V Apoptosis Detection Kit
Pathology at Weill Cornell Medical College after
I (BD Biosciences).
Institutional Review Board review and approval. All
primary cells were thawed as previously described
Cell cycle analysis
[32] and cultured in above conditions at 37°C for 6 h
DLBCL cells were treated with various
in the presence or absence of cerdulatinib followed by
concentrations of cerdulatinib for 48 h. Cells were incubated
BCR stimulations with 5 μg/mL of IgM and IgG. The
with 10 μM BrdU (BD Biosciences, San Jose, CA) at 37°C
clinical characteristics of these patients are listed in
for 2 h, and stained with PE conjugated anti-BrdU antibody
the supplemental Table 1. Cerdulatinib [26] was kindly
(BD Biosciences) according to the supplier's manual. The
provided by Portola Pharmaceuticals Inc. (South San
percentage of cell cycle distribution was analyzed with
Francisco, CA) and stored as 10 mM stock at −20°C.
FlowJo (Tree Star Inc. Ashland, OR).
Intracellular phosphospecific flow
4. Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A and
Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lym-
DLBCL cells were treated with cerdulatinib for 6
phoma. Proceedings of the National Academy of Sciences
h followed by stimulation with 5 μg/mL of goat F (ab')2
of the United States of America. 2003; 100:9991–9996.
anti-human IgM and IgG antibodies (Southern Biotech,
5. Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P,
Birmingham, AL) at 37°C for 15 min. Cells were fixed
Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner
in 4% formaldehyde at room temperature for 10 min, and
TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO,
permeabilized with 100% methanol on ice for 20 min
Smeland EB, Kvaloy S, et al. Molecular diagnosis of pri-
before flow cytometric analyses.
mary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma
related to Hodgkin lymphoma. The Journal of experimental medicine. 2003; 198:851–862.
A One-way ANOVA test was performed to compare
the percentage of S phase distribution or the MFI fold
6. Roschewski M, Staudt LM and Wilson WH. Diffuse large
changes between the treated and untreated DLBCL
B-cell lymphoma-treatment approaches in the molecular
cell lines/primary cells. The relationships between the
era. Nature reviews Clinical oncology. 2014; 11:12–23.
percentage of cell death response and inhibition of
7. Davis RE, Brown KD, Siebenlist U and Staudt LM.
ERK1/2 or AKT phosphorylation were analyzed by
Constitutive nuclear factor kappaB activity is required for
Spearman correlation
survival of activated B cell-like diffuse large B cell lym-phoma cells. The Journal of experimental medicine. 2001;
CONFLICTS OF INTEREST
8. Compagno M, Lim WK, Grunn A, Nandula SV,
GC, AP and PC are employees of Portola
Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra
Pharmaceuticals, Inc. The research was partially sponsored
M, Califano A, Bhagat G, Chadburn A, Dalla-Favera R and
through a research grant from Portola. There are no other
Pasqualucci L. Mutations of multiple genes cause dereg-
competing financial interests to declare.
ulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009; 459:717–721.
9. Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P,
Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RC,
YLW and GC formed the hypothesis. JM developed
Dal Cin P, Ladd C, Pinkus GS, Salles G, Harris NL, Dalla-
the assays, designed and performed the experiments,
Favera R, et al. Molecular profiling of diffuse large B-cell
solved technical problems, analyzed the data and wrote
lymphoma identifies robust subtypes including one char-
part of the manuscript; WX, KD, KL, AL, GR, HY
acterized by host inflammatory response. Blood. 2005;
performed some experiments and analyzed the data; GC,
AP and PC contributed useful discussions and suggestions.
10. Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser
YLW directed and coordinated the project designed the
PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, Xu W,
experiments, analyzed the data and wrote the manuscript.
Shaffer AL, Wright G, Xiao W, Powell J, Jiang JK, et al. Chronic active B-cell-receptor signalling in diffuse large
B-cell lymphoma. Nature. 2010; 463:88–92.
11. Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig
1. Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-
TE, Habermann TM, Kutok JL and Shipp MA. SYK-
Bernard AE and Cambier J. B cell antigen receptor signal-
dependent tonic B-cell receptor signaling is a rational treat-
ing 101. Molecular immunology. 2004; 41:599–613.
ment target in diffuse large B-cell lymphoma. Blood. 2008;
2. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS,
Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell
12. Yang C, Lu P, Lee FY, Chadburn A, Barrientos JC, Leonard
JI, Yang L, Marti GE, Moore T, Hudson J, Jr., Lu L, et al.
JP, Ye F, Zhang D, Knowles DM and Wang YL. Tyrosine
Distinct types of diffuse large B-cell lymphoma identified
kinase inhibition in diffuse large B-cell lymphoma: molecu-
by gene expression profiling. Nature. 2000; 403:503–511.
lar basis for antitumor activity and drug resistance of dasat-
3. Rosenwald A, Wright G, Chan WC, Connors JM, Campo E,
inib. Leukemia. 2008; 22:1755–1766.
Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland
13. Cheng S, Coffey G, Zhang XH, Shaknovich R, Song Z, Lu
EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L,
P, Pandey A, Melnick AM, Sinha U and Wang YL. SYK
Wilson WH, Jaffe ES, et al. The use of molecular profil-
inhibition and response prediction in diffuse large B-cell
ing to predict survival after chemotherapy for diffuse large-
lymphoma. Blood. 2011; 118:6342–6352.
B-cell lymphoma. The New England journal of medicine.
14. Chen L, Monti S, Juszczynski P, Ouyang J, Chapuy B,
Neuberg D, Doench JG, Bogusz AM, Habermann TM,
Dogan A, Witzig TE, Kutok JL, Rodig SJ, Golub T and
activated B-cell subtype of diffuse large B-cell lymphomas.
Shipp MA. SYK inhibition modulates distinct PI3K/AKT-
Blood. 2008; 111:1515–1523.
dependent survival pathways and cholesterol biosynthe-
24. Younes A, Romaguera J, Fanale M, McLaughlin P,
sis in diffuse large B cell lymphomas. Cancer cell. 2013;
Hagemeister F, Copeland A, Neelapu S, Kwak L, Shah J,
de Castro Faria S, Hart S, Wood J, Jayaraman R, Ethirajulu
15. Friedberg JW, Sharman J, Sweetenham J, Johnston PB,
K and Zhu J. Phase I study of a novel oral Janus kinase
Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha
2 inhibitor, SB1518, in patients with relapsed lymphoma:
R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe
evidence of clinical and biologic activity in multiple lym-
AM, Levy R and Shipp MA. Inhibition of Syk with fosta-
phoma subtypes. Journal of clinical oncology : official jour-
matinib disodium has significant clinical activity in non-
nal of the American Society of Clinical Oncology. 2012;
Hodgkin lymphoma and chronic lymphocytic leukemia.
Blood. 2010; 115:2578–2585.
25. Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad
16. Robertson MJ, Kahl BS, Vose JM, de Vos S, Laughlin M,
L, Lonial S, Borghaei H, Jagannath S, Sokol L, Usmani
Flynn PJ, Rowland K, Cruz JC, Goldberg SL, Musib L,
SZ, van de Velde H, Qin X, Puchalski TA, Hall B, Reddy
Darstein C, Enas N, Kutok JL, Aster JC, Neuberg D, Savage
M, Qi M, et al. A phase I, open-label study of siltuximab,
KJ, et al. Phase II study of enzastaurin, a protein kinase C
an anti-IL-6 monoclonal antibody, in patients with B-cell
beta inhibitor, in patients with relapsed or refractory diffuse
non-Hodgkin lymphoma, multiple myeloma, or Castleman
large B-cell lymphoma. Journal of clinical oncology : offi-
disease. Clinical cancer research : an official journal of
cial journal of the American Society of Clinical Oncology.
the American Association for Cancer Research. 2013;
17. Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S,
26. Coffey G, Betz A, DeGuzman F, Pak Y, Inagaki M, Baker
Wright G, Lih CJ, Williams PM, Shaffer AL, Gerecitano
DC, Hollenbach SJ, Pandey A and Sinha U. The novel
J, de Vos S, Goy A, Kenkre VP, Barr PM, Blum KA,
kinase inhibitor PRT062070 (Cerdulatinib) demonstrates
Shustov A, et al. Targeting B cell receptor signaling with
efficacy in models of autoimmunity and B-cell cancer. J
ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;
Pharmacol Exp Ther. 2014; 351:538–548.
27. Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M,
18. Lam LT, Wright G, Davis RE, Lenz G, Farinha P, Dang
Liu M, Buggy JJ, Furman RR and Wang YL. BTK inhi-
L, Chan JW, Rosenwald A, Gascoyne RD and Staudt LM.
bition targets in vivo CLL proliferation through its effects
Cooperative signaling through the signal transducer and
on B-cell receptor signaling activity. Leukemia. 2014;
activator of transcription 3 and nuclear factor-{kappa}B
pathways in subtypes of diffuse large B-cell lymphoma.
28. Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A,
Blood. 2008; 111:3701–3713.
Racchumi J, Xu G, Wu H, Ma J, Steggerda SM, Coleman
19. Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim
M, Leslie C and Wang YL. Ibrutinib resistance in chronic
KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL,
lymphocytic leukemia. The New England journal of medi-
Romesser P, Wright G, Powell J, Rosenwald A, Muller-
cine. 2014; 370:2352–2354.
Hermelink HK, et al. Oncogenically active MYD88 muta-
29. Cheng S, Guo A, Lu P, Ma J, Coleman M and Wang YL.
tions in human lymphoma. Nature. 2011; 470:115–119.
Functional characterization of BTK mutation that confers
20. Gascoyne RDS, C. VII. The role of the microenviron-
ibrutinib resistance: exploration of alternative kinase inhibi-
ment in lymphoid cancers. Annals of Oncology. 2011; 22:
tors. Leukemia. 2014.
30. Hans CP, Weisenburger DD, Greiner TC, Gascoyne
21. Scott DW and Gascoyne RD. The tumour microenvi-
RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E,
ronment in B cell lymphomas. Nat Rev Cancer. 2014;
Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini
B, Banham AH, Rosenwald A, et al. Confirmation of the
22. Huang X, Meng B, Iqbal J, Ding BB, Perry AM, Cao W,
molecular classification of diffuse large B-cell lymphoma
Smith LM, Bi C, Jiang C, Greiner TC, Weisenburger DD,
by immunohistochemistry using a tissue microarray. Blood.
Rimsza L, Rosenwald A, Ott G, Delabie J, Campo E, et al.
Activation of the STAT3 signaling pathway is associated
31. Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D,
with poor survival in diffuse large B-cell lymphoma treated
Chiarenza A, Wells VA, Grunn A, Messina M, Elliot O,
with R-CHOP. Journal of clinical oncology : official jour-
Chan J, Bhagat G, Chadburn A, Gaidano G, Mullighan CG,
nal of the American Society of Clinical Oncology. 2013;
Rabadan R, et al. Analysis of the coding genome of diffuse
large B-cell lymphoma. Nature genetics. 2011; 43:830–837.
23. Ding BB, Yu JJ, Yu RY, Mendez LM, Shaknovich R,
32. Ma J, Lu P, Guo A, Cheng S, Zong H, Martin P, Coleman
Zhang Y, Cattoretti G and Ye BH. Constitutively activated
M and Wang YL. Characterization of ibrutinib-sensitive
STAT3 promotes cell proliferation and survival in the
and -resistant mantle lymphoma cells. British journal of haematology. 2014; 166:849–861.
Source: http://oncology.tv/Portals/16/Downloadables/Ma-DLBCL-Oncotarget-2015.pdf?timestamp=1455220367250
The new england journal of medicine established in 1812 Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Ramón Estruch, M.D., Ph.D., Emilio Ros, M.D., Ph.D., Jordi Salas-Salvadó, M.D., Ph.D., Maria-Isabel Covas, D.Pharm., Ph.D., Dolores Corella, D.Pharm., Ph.D., Fernando Arós, M.D., Ph.D., Enrique Gómez-Gracia, M.D., Ph.D., Valentina Ruiz-Gutiérrez, Ph.D., Miquel Fiol, M.D., Ph.D.,
Isabell Hensel, Gunther Teubner Matrix Reloaded. Critica dell'effetto orizzontale dei diritti fondamentali centrato sullo Stato sull'esempio del publication bias (errore sistematico di pubblicazione) Versione in tedesco: http://www.jura.uni-frankfurt.de/49069887/KJ_Teubner_Hensel.pdf Matrix Reloaded Critica dell'effetto orizzontale dei diritti fondamentali centrato sullo Stato sull'esempio del








