Doi:10.1016/j.neuropsychologia.2006.01.037
Neuropsychologia 44 (2006) 1962–1977
Visual search deficits in Parkinson's disease are attenuated by
bottom-up target salience and top-down information
Todd S. Horowitz , Won Yung Choi , Jon C. Horvitz ,
Lucien J. Cˆot´e , Jennifer A. Mangels
a
Brigham & Women's Hospital and Harvard Medical School, MA, USA
b
Department of Psychology, Columbia University, NY, USA
c
Boston College, MA, USA
d
Columbia University Medical Center, NY, USA
Accepted 2 October 2005
Available online 31 March 2006
Patients with Parkinson's disease (PD), a degenerative disorder primarily affecting the nigrostriatal dopamine system, exhibit deficits in selecting
task-relevant stimuli in the presence of irrelevant stimuli, such as in visual search tasks. However, results from previous studies suggest that thesedeficits may vary as a function of whether selection must rely primarily on the "bottom-up" salience of the target relative to background stimuli, orwhether "top-down" information about the identity of the target is available to bias selection. In the present study, moderate-to-severe medicated PDpatients and age-matched controls were tested on six visual search tasks that systematically varied the relationship between bottom-up target salience(feature search, noisy feature search, conjunction search) and top-down target knowledge (Target Known versus Target Unknown). Comparisonof slope and intercepts of the RT × set size function provided information about the efficiency of search and non-search (e.g., decision, response)components, respectively. Patients exhibited higher intercepts than controls as bottom-up target salience decreased, however these deficits weredisproportionately larger under Target Unknown compared to Target Known conditions. Slope differences between PD and controls were limited tothe Target Unknown Conjunction condition, where patients exhibited a shallower slope in the target absent condition, indicating that they terminatedsearch earlier. These results suggest that under conditions of high background noise, medicated PD patients were primarily impaired in decisionand/or response processes downstream from the target search itself, and that the deficit was attenuated when top-down information was availableto guide selection of the target signal.
2006 Elsevier Ltd. All rights reserved.
Keywords: Attention; Dopamine; Striatum; Feature search; Conjunction search
DA depletion may extend to the cells of the ventral tegmen-tal area, site of origin for the mesolimbocortical pathway (
1.1. Overview
which comprises the dopamineneurons in the ventral tegmental area (VTA) and their projec-
Parkinson's disease (PD) is characterized by progressive
tions to the nucleus accumbens (NAc), amygdala, PFC, and other
degeneration of dopamine (DA) neurons in the substantia nigra
forebrain regions (
pars compacta (This degeneration leads
Although PD is primarily characterized as a disorder of motor
to DA depletion within the dorsal striatum, reducing the ability
control, a growing number of studies have found that PD patients
of this region to effectively process corticostriatal inputs and dis-
also demonstrate impairment in various cognitive abilities, par-
rupting information flow through the corticostriatal basal ganglia
ticularly the control of attention and memory (for a review, see
loops (As the disease progresses,
Of particular relevance to the present studyare recent findings implicating DA in set-switching (Gilbert, selective
∗ Corresponding author. Tel.: +1 212 854 7560; fax: +1 212 854 3609.
E-mail address: (J.A. Mangels).
0028-3932/$ – see front matter 2006 Elsevier Ltd. All rights reserved.
doi:
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
sensory gating (These find-ings are consistent with evidence that DA plays a key role inthe selection of task-relevant striatal inputs by amplifying theactivity of striatal cells receiving strong glutamate excitationwhile attenuating the impact of those receiving weaker inputs(Thus, in information-processing terms,DA may boost signal-to-noise ratios (promoting the selection of salient target stimulifor processing by response systems (
Given that salience-based models also have been highly suc-
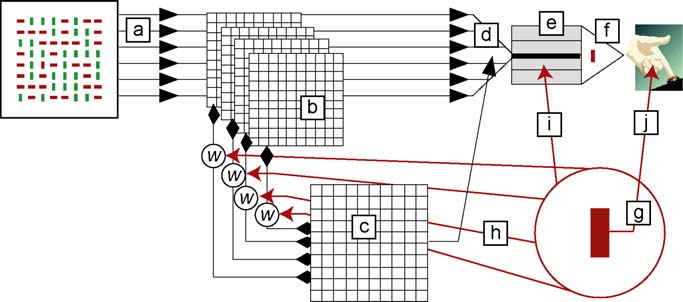
Fig. 1. Model of information flow in visual search. This example illustrates a
cessful in explaining visual search performance (
conjunction search in which the subject is instructed (top-down information) tosearch for a red (dark bar) vertical target in a field of red horizontal and green
the present study examines the
(light bar) vertical distractors. The front-end early visual system (a) analyzes
relationship between PD, selective attention, and salience using
visual input into a hierarchy of feature maps (b). Information from the feature
a visual search paradigm that allows manipulation of both the
maps is abstracted into a salience map, (c) which directs the focus of attention (d).
bottom-up salience of the target stimulus relative to the dis-
Attended items enter visual short-term working memory (VSTM, e) for further
tractor field and the participant's top-down knowledge of the
analysis. Items in VSTM compete for access to decision and response processes(f) that control behavior. Top-down information (g) influences processing in
target identity. Previous studies of visual search in PD have
three ways: by weighting the input from feature maps to the salience map (h);
exclusively manipulated bottom-up factors such as the num-
by biasing competition in VSTM (i); by adjusting decision thresholds (j). (For
ber and composition of distractors in the search array (
interpretation of the references to color in this figure legend, the reader is referred
to the web version of the article.)
However, knowing which target to expect may be critical
from various feature maps into a single representation in visual
in determining whether search deficits will be apparent in PD
short term memory (VSTM, (
patients. This type of top-down information may allow patients
where object rep-
to facilitate target processing at multiple stages (see Section
resentations are stored and consolidated
thus reducing interference from irrelevant bottom-up informa-
tion. By systematically varying top-down and bottom-up factors,
VSTM then compete for access to decision and response mech-
our study clarifies and extends previous findings by showing that
search performance in PD patients is disproportionately affected
Only a single object (or perceptual grouping) can be attended
when target salience is reduced both by a lack of salient features
at one time, keeping information from different objects segre-
and the absence of top-down guidance for stimulus selection.
gated. In visual search tasks, attention serves as a bottleneck,restricting processing rate. Attentional deployments are con-
1.2. A model of visual search: the influence of bottom-up
trolled by a salience map
salience and top-down information
which is a representation of "inter-esting" locations in the visual field. In this context, "interesting"
Visual search refers to a wide range of tasks in which
means "likely to contain a target". Functionally, the salience
observers must search for a target item (whose location is
map serves to restrict attention to the most likely target items,
uncertain) in the presence of one or more distractor items. In
reducing the effective set size.
this section, we outline a model of information processing in
The salience map is derived from two separate sources
visual search based on Treisman's Feature Integration Theory
of information, bottom-up and top-down. Bottom-up informa-
tion consists of differences between neighboring items (
Guided Search Theory (
along a limited set of dimensions (for a recent review see
We introduce the model not as a computational
The second source of salience is top-
simulation of search deficits in PD, but as a framework for under-
down information (which here we will take to mean
standing the different ways in which bottom-up stimulus salience
"knowing what you are looking for"
and top-down information influence visual search behavior. Dif-
For example, par-
ferent ideas about the effects of PD on information processing
ticipants will be relatively slow to find a red vertical target among
lead to different sets of predictions (see Section
green horizontal distractors in a block of trials where targets
In this model (see a visual stimulus, such as a search
could be unpredictably red, blue, orange, or purple, compared
array, is analyzed by the massively parallel front-end of the
to when they are informed in advance that the target on the
visual system (into a hierarchical set of feature maps
upcoming trial will be red vertical (
(which encode a set of properties ranging from color
The effect of top-down information is to
to 3D shape Although these prop-
increase the weight on information coming from objects hav-
erties are distributed across multiple, independent processing
ing desired features Of course, the effects of knowing
streams in early vision, behavior is directed to unitary objects.
what to look for are not restricted to weighting the salience map.
Thus, focal attention (is required to unify information
Advance knowledge of the target may serve to bias competition
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
the dorsal striatum reduces the responsiveness of striatal neu-
or at decision/response stages
rons to auditory, visual, and tactile stimuli
In the present study, total number of items in the display (set
Extending these findings to the context of visual search, DA
size) was varied from trial to trial, allowing us to derive the func-
depletion in the dorsal striatum may result in a poorer signal-to-
tion relating reaction time (RT) to set size. This RT × set size
noise ratio, making it more difficult to filter out task-irrelevant
function partitions RT into slope and intercept. The slope mea-
responses and/or stimuli. This reduction could result either from
sures the cost for adding additional items to the display, while
a decrease in the strength of inputs from the "target" signal and/or
the intercept is the theoretical RT that would be observed if there
an increase in the strength of the "noise" from weak distractors.
were no search stage, but all other stages had to be completed.
As such, patients with Parkinson's disease may have particu-
Slope is often interpreted as "search efficiency", with steeper
lar difficulty detecting targets in conjunction displays, where
slopes indicating slower, less efficient search. In the model, two
bottom-up salience of the target is low. On the other hand, detec-
factors influence slope: the quality of information on the salience
tion of targets with sufficiently high bottom-up salience, such
map (how well can it distinguish the target from the distrac-
as in "pop-out" displays may be less impaired. Indeed, even
tors), and the rate of attentional shifting (how long does it take
when DA in the nigrostriatal system is compromised, it appears
to change the locus of selection). In contrast, factors that influ-
that the organism can still select and respond to highly salient
ence early visual processes, VSTM competition, or decision and
and novel information in the environment
response processes will contribute to the intercept. From a neu-
In accordance with this notion, PD
ropsychological point of view, then, we can expect that damage
patients have been observed to show surprisingly intact ability to
to salience mechanisms or to the ability to shift attention will
locomote in response to a loud fire alarm or salient lines drawn
increase the slope (thus slowing search), while damage to other
stages of processing will show up in increased intercepts.
As previously mentioned, however, salience in visual search
is derived not only from bottom-up properties of the stimu-
1.3. Dopamine and salience
lus array, but also from top-down information that can serveto bias a particular feature or response. Several studies have
From our cognitive model for the relationship between
demonstrated that PD patients' motor performance on tasks such
salience and attention in visual search, we now turn to a pharma-
as reach-to-grasp movement sequen-
cological model of DA's role in detection and response to salient
tial button pressing (and finger-tapping
environmental stimuli. Studies in animal models have shown
(can significantly improve when provided with
that midbrain DA neurons show phasic activation in response
external cues that specify a particular response. These data pro-
to salient environmental change For example,
vide some indirect support for the view that PD patients might
single-unit recordings show that salient auditory and visual stim-
benefit disproportionately from top-down information about the
uli of high intensity and rapid onset produce excitation of DA
identity of the target. For PD patients, top-down information
neurons in both the ventral tegmental area (
may be an effective means of increasing target salience, even
and substantia nigra
to the point of helping them compensate for what may be
These midbrain DA neurons are also activated by less intense
greater impairments in detecting stimuli with low bottom-up
stimuli with primary or conditioned reward properties
by novel stimuli (Similarly,
1.4. Visual search and Parkinson's disease
that novel events (i.e.,infrequently presented visual distractors), produce increased
In one of the first investigations of search performance in PD,
activation in the nucleus accumbens, a forebrain region which
Troscianko and colleagues (found
receives particularly dense VTA DA innervation.
that medicated PD patients exhibited an unusual non-zero slope
Functionally, the strong DA response to salient events may
on an easy feature search for a vertical bar among horizontal
modulate activity at striatal neurons receiving strong glutamate
bars, but showed no difference from controls in the slope of a
input from regions involved in stimulus and/or response process-
conjunction search task (They concluded
ing. Rather than producing a general excitation or inhibition,
that mechanisms to detect salience were so impaired as to render
DA appears to amplify the activity of striatal neurons receiv-
"bottom-up" salience information useless, leaving the patient to
ing strong cortical glutamate input, and filter out activity at
resort to a serial search. However, based on the model put forth in
weakly activated synapses
Section to fundamental salience mechanisms would
be expected to increase the slope of both feature and conjunction
Thus, a disruption in striatal DA activity may interfere
search functions.
with corticostriatal information processing either by disrupting
Furthermore, these findings were not replicated by
the transmission of strong input signals, or by permitting the
found no impairment in the slope of the search func-
transmission of weak signals that would not normally be permit-
tion of medicated PD patients on either feature or conjunction
ted to compete for basal ganglia processing beyond the level of
search. The only difference between PD patients and controls
the striatum. Consistent with this notion, DA depletion within
was an increased intercept for a subgroup of PD patients identi-
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
fied as "frontally impaired" based on their poor Wisconsin CardSort Test (WCST) performance. Intercepts for this subgroupwere increased on both feature and conjunction search relativeto controls and non-frontally impaired PD patients. Althoughthe authors interpreted this finding as evidence of non-specificimpairment associated with frontal lobe dysfunction, increasedintercepts in the absence of slope effects could stem from bot-
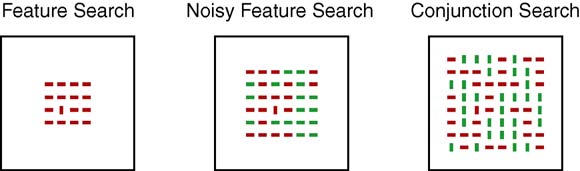
Fig. 2. Examples of stimuli from feature search (left panel), noisy feature search
tleneck effects at the decision/response stage, which is past the
(middle panel), and conjunction search (right panel) displays. For illustration
point at which the effects of set size would exert an influence.
purposes, stimuli in the figure are depicted on a white background (actual stimuliwere presented against a black computer screen) and color values have been
also failed to repli-
adjusted so that red stimuli appear as darker bars and green stimuli appear as
cate the feature search deficit.
lighter bars. (For interpretation of the references to color in this figure legend,
In contrast, tested search behavior in
the reader is referred to the web version of the article.)
PD patients with an adaptive staircase procedure, found resultssuggestive of a deficit in early vision. They tested four differ-ent search tasks. Only one task yielded a reliable impairment
In summary, there is little agreement across previous visual
in medicated PD patientsThe critical task was search for a
search studies regarding the stage(s) of information processing
patch of oriented line segments against a background of spa-
impaired in PD patients. These studies have variously impli-
tially filtered, vertically oriented noise, in which the angle of
cated upstream early vision (and salience map
the oriented line segments was adjusted by the staircase to find
processes, as well as downstream
the 62.5% threshold. The patients required a larger orientation
decision/responses processes (Yet, they con-
difference in order to reach threshold performance, which Lieb
verge with respect to two findings: (a) there is little evidence
et al. took as evidence that PD impaired preattentive orientation
for a deficit in the speed of shifting attention between items
(which would be evident as selectively increased slopes in an
Yet, examining Lieb et al.'s data with the importance of
effortful/conjunction search), and (b) they are more likely to
bottom-up salience and top-down information in mind reveals
demonstrate impaired performance when there is uncertainty
that the orientation texture condition differs in two critical ways
about the identity of the target and selection must be guided
from the other feature search tasks tested. First, due to the
exclusively by "bottom-up" factors
Gaussian noise of the background, the salience of the texture
patch was relatively low. Second, participants did not knowfrom trial-to-trial exactly what the target stimulus would look
1.5. The present experiment
like, because the orientation of the target patch was controlledby the staircase method. Thus, instead of (or in addition to)
We compared medicated moderate-to-severe PD patients to
demonstrating a weakness in preattentive orientation processing,
matched controls on visual search tasks in which both bottom-up
these data might indicate that PD patients are at a disadvantage
salience and top-down knowledge were manipulated to produce
when targets are both unpredictable from trial-to-trial and of low
higher or lower levels of stimulus salience. We chose to test
patients while "on" medication in order to allow for direct com-
The sensitivity of PD patients to unpredictable trial struc-
parison with previous studies of visual search in PD. Although
ture has also been demonstrated in studies of set-shifting. For
the use of medicated patients has implications for conclusions
example, wed that,
about the direct contribution of DA to any observed dysfunction,
while patients and controls performed similarly on a consistent-
we note that medication often fails to fully restore DA func-
mapping search task for novel shapes (
tion in moderate-to-severe patients (
patients were disproportionately slowed in a varied-
mapping version of the task, relative to controls. A similar
and will return to this issue in
finding was obtained by
the discussion. Bottom-up salience was varied across task by
using a modified odd-man out task, a type of search task. In
increasing distractor heterogeneity and target-distractor simi-
addition, that while both medicated
larity (see In the feature search task, distractors were
and unmedicated PD patients were impaired on visual discrim-
homogenous (e.g., red horizontal bars), such that the target (e.g.,
ination learning when an extra-dimensional shift was required,
red vertical bar) would easily pop-out from the background. In
they were more or less unimpaired on varied-mapping visual
the noisy feature search task, there were two types of distrac-
tors, but the target differed on a basic feature dimension (e.g.,red vertical target with red and green horizontal distractors). Inthe conjunction search task, there were two types of distractors,
1 Patients in the study by tested twice on each task.
each sharing one feature with the target (e.g., a red vertical target
Deficits in detecting targets in filtered noise were present at both first and second
with red horizontal and green vertical distractors). The availabil-
test (unlike deficits in texton detection, which were found only at first test).
ity of top-down information was manipulated by including both
Although at the time of the first test, 6 of the 16 patients were de novo and hadnot yet begun their medication regimen, at the time of the second test, all patients
Target Known and Target Unknown target conditions. In Target
were receiving l-Dopa and/or dopaminergic agonists.
Known conditions, participants were informed that the target
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
(red vertical or green horizontal) would be held constant for a
2. Method
block of 36 trials. In Target Unknown conditions, the target var-ied randomly from trial to trial, and participants had to find the
2.1. Participants
A replication of previous studies of feature search (
Ten patients with Parkinson's disease and 10 age- and education-matched
elderly controls participated in the experiment. All patients had received a diag-
nosis of idiopathic PD from neurologist and author L.C. (i.e., at least two of the
and conjunction search
following signs with progressive onset: akinesia, resting tremor, rigidity, or pos-
is thus embedded in our design. However, the addition
tural instability, and the absence of any other condition that may produce signs
of the noisy feature search condition allows us an intermediate
of Parkinsonism, including Progressive Supranuclear Palsy [PSP]). Patients had
level of salience between standard feature search and conjunc-
no known history of other significant medical disease such as diabetes, thyroiddisease or major psychiatric disorder, substance abuse or additional neurological
tion search. Finally, the Target Known versus Target Unknown
events (e.g., head injury, stroke, tumor). PD patients were in the moderate-to-
manipulation allows us to observe the effect of top-down infor-
severe range of the disease, as confirmed by the Hoehn & Yahr (range 2–4,
mation on both intercept and slope measures across these varying
M = 3.1, S.D. = 0.16) and the total score on the UPDRS (Unified Parkinson's Dis-
levels of salience.
ease Rating Scale) taken while patients were "on" medication (range = 10–60;
We can anticipate five possible outcomes from this experi-
M = 35.9, S.D. = 4.94). Mean duration of illness at the time of testing was 8.0years (range: 2–13 years).
ment, depending on where PD disrupts the flow of information
All patients were also tested in the visual search tasks while "on" medication,
processing. Outcomes are presented in order from low-level to
1–2 h after daily dose, which means that they were tested at approximately
their peak medication level. This ensured that the level of motor impairmentexperienced during the task was representative of the level indicated by theUPDRS, which was conducted at a similar medication level during their most
1. There may be no PD-specific effect. Since all previous studies
recent office visit to physician L.C. Nine patients were taking l-Dopa medication
have obtained some effect of PD on search (for at least a
(Sinemet). The one patient who was not taking Sinemet was taking Selegiline and
subset of patients), we rate this outcome as unlikely.
the anticholinergic medication Norflex. Removal of this patient did not changethe pattern of the data. Seven other patients were also taking Selegiline, and six
2. The salience map may be impaired in PD. In this
were additionally taking a DA agonist (Permax [n = 2], Requip [n = 1], Mirapax
case, we should see increases in search slope for all con-
[n = 4]). One other patient was taking Norflex.
ditions. Again, non-zero slopes in the feature search con-
Patients and elderly controls were screened for dementia using a modi-
dition would be strong evidence for this scenario. Addi-
fied version of the Mini-Mental State Examination (mMMSE cut-off = 50/57,
tionally, effects on RT should be proportional to salience.
and for depression using the Beck Depression Inventory-II (BDI-II cut-off = 17,
This outcome would be compatible with the conclusions of
There were no significant differences between the
Troscianko and his colleagues (
elderly controls and patients on the mMMSE (t[9] < 1). Although a significant
difference on the BDI-II was found between the patients and elderly controls
3. Shifts of attention may be slowed (In this case,
(t[9] = 5.63, p < .0005), the average scores of both groups were well within the
we would expect that feature searches would remain effi-
normal range (see participants also demonstrated normal visual acu-ity and contrast sensitivity as tested by a computerized version of the Freiburg
cient (i.e., near zero slopes), but conjunction search should
Visual Acuity Test
be noticeably slowed. Another way to describe this would
All participants gave informed consent before participating in the experi-
be as a multiplier on the slope, relative to the elderly con-
mental session in accordance with Columbia University Medical Center and
trols. This would be a surprising outcome, given previous
Morningside Institutional Review Board regulations.
4. PD patients may have a deficit in VSTM processing or at the
2.2. Apparatus and stimuli
decision and response stages and f). These effectswould be present in the intercepts, rather than the slopes of
The experiment was run on an Apple Powerbook G3 laptop computer with
a 21.6 cm × 28.6 cm LCD screen. Screen resolution was set to 1024 × 768 pix-
the search functions. However, since these stages are after
els, color depth to 24 bits. The refresh rate was 60 Hz. Stimulus presentation
the computation of salience (and c), we would expect
and response collection were controlled by Matlab 5.2.1 software (MathWorks)
their deficits to be proportional to salience.
using routines from the Psychophysics Toolbox Participantsresponded using a PsyScope three-button box.
Stimuli were presented on a black background. The fixation cross was a
It is of course possible that the effects of PD would manifest
plus sign drawn in 48-point Arial font and measuring roughly 1.12◦ × 1.12◦.
at more than one level, producing more complicated patterns
Search stimuli consisted of red and green bars, which could be either vertical
in the data. However, the key features to look for are non-zero feature slopes, changes in conjunction slopes relative to
elderly controls, and changes in intercept that are independent
Participant demographics
of, or proportional to, salience. The conflicting results of the
existing literature make it difficult to predict an exact pattern,
however they suggest that there may be an interaction with thepresence of top-down information: when participants do not
know which target to expect, patients should be more severely
Education (years)
impaired relative to elderly controls than when the target is
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
(.42◦ visual angle [◦] × 1.12◦) or horizontal (1.12◦ × .42◦). Display density was
and green horizontal bars, or red horizontal and green horizontal bars. In the
controlled by presenting search arrays on 4 × 4, 6 × 6, and 8 × 8 grids for set
conjunction search condition, there were two types of distractors, each sharing
sizes of 16, 36, and 64 items, respectively. Inter-item center-to-center distance
a different feature with the target. Again with a red vertical target, distractors
was 1.68◦. The 4 × 4 grid thus subtended 6.73◦ × 6.73◦, the 6 × 6 grid subtended
would be red horizontal and green vertical. Note that the same distractors would
10.09◦ × 10.09◦, and the 8 × 8 grid subtended 13.45◦ × 13.45◦. The 8 × 8 grid
be used for a conjunction search with a green horizontal target.
defined the possible stimulus area. Smaller grids were displaced randomly within
In the Target Known sessions, each block began with the target for that block
the larger grid. Thus, targets could appear at any location within the display area,
(e.g., a red vertical bar or a green horizontal bar) presented in the center of the
independent of set size. This allowed us to hold the range of target eccentricity
screen along with the text "This will be your target". Participants then advanced
from initial fixation constant across set size.
to practice trials by pressing the middle button on the button box. When a blockof trials was complete and the target changed, a new example was shown.
2.3. Protocol
In the Target Unknown sessions, participants were told that on some trials
there would a single bar in the display that was different from all the rest ofthe bars and that this was the "oddball" target. Their task was to determine, as
Participants were tested individually. The majority of patients were tested
quickly and accurately as possible, whether an "oddball" was present or not.
in their homes, as it was often difficult for them to travel to the laboratory.
In the Target Known sessions, the first block within each set of feature
Elderly controls were tested either in their homes or in the laboratory, depend-
search and noisy feature search trials was an orientation search, and the sec-
ing on which was more convenient. After consent, but prior to testing on the
ond block was a color search. In the Target Unknown sessions, however, targets
visual search task, participants were given the mMMSE, BDI, and asked some
varied randomly from trial to trial, so any trial in a feature or noisy feature
questions about their mental and physical health.
block could be color or orientation search with equal likelihood. In these ses-
Target Known and Target Unknown conditions were tested in a counterbal-
sions, distractors did not provide any information about the target. That is,
anced order. Six of the elderly controls and two of the patients performed both
within an "unknown" feature search block, green horizontal distractors could
conditions on the same day. The remaining participants performed these tasks
mean a search for a green vertical target or a search for a red horizontal target
in separate test sessions, conducted at least 10 days apart for the elderly controls
with equal likelihood. Targets and distractors in the two blocks of conjunc-
and patients. When testing was done in separate sessions it was to minimize
tion search were identical under both Target Known and Target Unknown
fatigue reported by the participant. In addition, for patients, separate sessions
ensured that the start time of the tasks was controlled with regard to time since
Each trial began with a screen instructing the participant to hold down the
last dose of medication.
yellow middle button on the button box. Controls were asked to use the indexfinger of their dominant hand. Patients used the index finger on the side that
2.4. Design and procedure
was first or most impaired. Once this button was pressed, the fixation cross waspresented at the center of the screen for 833 ms, followed immediately by the
Examples of the displays used in the feature, noisy feature and conjunction
search array. At this point, the computer checked to make sure that the participant
search tasks are shown in
was still holding down the center key. If not, the trial was aborted.
There were four stimuli: red horizontal bars, red vertical bars, green hor-
Participants responded by moving their index finger to the green button on
izontal bars, and green vertical bars. Each stimulus could potentially serve as
the right of the button box to indicate that they saw a target, or the red button on
target or distractor. In order to reduce the number of possible combinations, each
the left to indicate that they did not see a target. As soon as the response button
participant was assigned two stimuli as targets; all stimuli served as distractors
was pressed, the search array was replaced with a feedback display, indicating
at one point or another for all participants. Targets were randomly assigned such
trial number, RT in ms, and whether or not the response was correct. If there
that half of the participants in each group searched for red vertical and green hor-
was no response within 10 s, the trial was declared a time out. Participants were
izontal bars, while the other half searched for red horizontal and green vertical
informed if the trial was aborted or if they did not respond before the time out
The experiment consisted of two sessions, the Target Known session and
Any errors, time outs, or aborted trials were recycled, so that the same number
the Target Unknown session. Each session consisted of 12 blocks of 36 trials
of correct responses was obtained for each condition, regardless of the error rate
preceded by 6 practice trials, for a total of 1008 correct trials in the experiment.
in that cell. Participants were informed that if they made an error, that trial
Each session was in turn divided into two sets of six blocks each. In the Target
would be repeated. We added this feature in anticipation of the possibility that
Known condition, the red bar (vertical or horizontal, depending on which tar-
there might be differences in the errors, time outs or aborted trials across the
gets had been assigned to that participant) was the target for the first set of six
three groups, either overall or as a function of condition. Given that RT was our
blocks, and the green bar (horizontal or vertical) for the second set. In the Target
primary measure, this recycling reduced the possibility that group differences
Unknown condition, targets were picked randomly from trial to trial in both
in RT would be influenced by greater variability stemming from fewer trials in
sets. Within each set, there were two blocks of each search type (feature, noisy
groups with higher error rates. Thus, there were always 24 correct trials per cell
feature, and conjunction). There were six possible orders of the three search
for all groups. We also recorded error rates.
types, counterbalanced across participants in each grouporder in the sec-ond set within each session was always the reverse of the order in the first set.
2.5. Data analysis
For example, if a participant experienced two blocks of feature search, followedby noisy feature search, followed by conjunction search in the first set, in the
RT is typically measured from stimulus onset to the depression of the
second set she would start with conjunction search, then noisy feature search,
response button. We partitioned the total RT into response initiation time (RTi)
and finish with feature search.
and movement time (MT), where RTi was the time from stimulus onset to the
Within a block, there were six trials at each level of set size and target
release of the start button, and MT the remainder. We used RTi as our primary
presence, randomly intermixed. Since there were two blocks of each search type
dependent measure, rather than RTtotal, on the assumption that RTi is a more
per set, collapsing across sets there were 24 trials per cell.
direct measure of cognitive processing time, eliminating variability associated
In feature search, all distractors were identical. For example, if the target was
with motor implementation. There is also some suggestion in the literature that
a red vertical bar, distractors might be all green vertical bars (color search) or all
movement times in this population do not accurately reflect processing times
red horizontal bars (orientation search). In noisy feature search, there were two
However, we did analyze RTtotal, and the statistical con-
types of distractors, but the target was distinct from both distractors along a single
clusions were identical.
dimension. Thus, distractors for a red vertical target might be green vertical
All analysis of variance (ANOVA) results were subjected to Mauchly's
Test of Sphericity. The Huynh-Feldt correction was applied when violationsof sphericity were observed. In these cases, the corrected degrees of freedom
2 Two PD patients initially included in the counterbalancing scheme were later
are reported. Where appropriate, we also report partial eta-squared (ˆη2) as a
found not to meet our inclusion criteria.
measure of effect size.
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
Both slope and intercept of the RT × set size functions were computed based
on the medians of correct responses after responses faster than 250 ms hadbeen eliminated as anticipations. Planned comparisons tested slopes againstzero using one-tailed t-tests.
Error data were converted to the signal detection measures d� and c, repre-
senting sensitivity and criterion, respectively. High d� values indicate a betterability to discriminate between the presence of the target and its absence. Posi-tive c values denote a more conservative criterion (more likely to say the targetis absent), negative scores a more liberal criterion (more likely to say the targetis present) Sensitivity and criterion are theo-retically independent quantities.
3. Results
3.1. Overview
the mean of the median RT data for both
groups as a function of search task (feature, noisy feature, andconjunction), Target Known versus Unknown, target presence
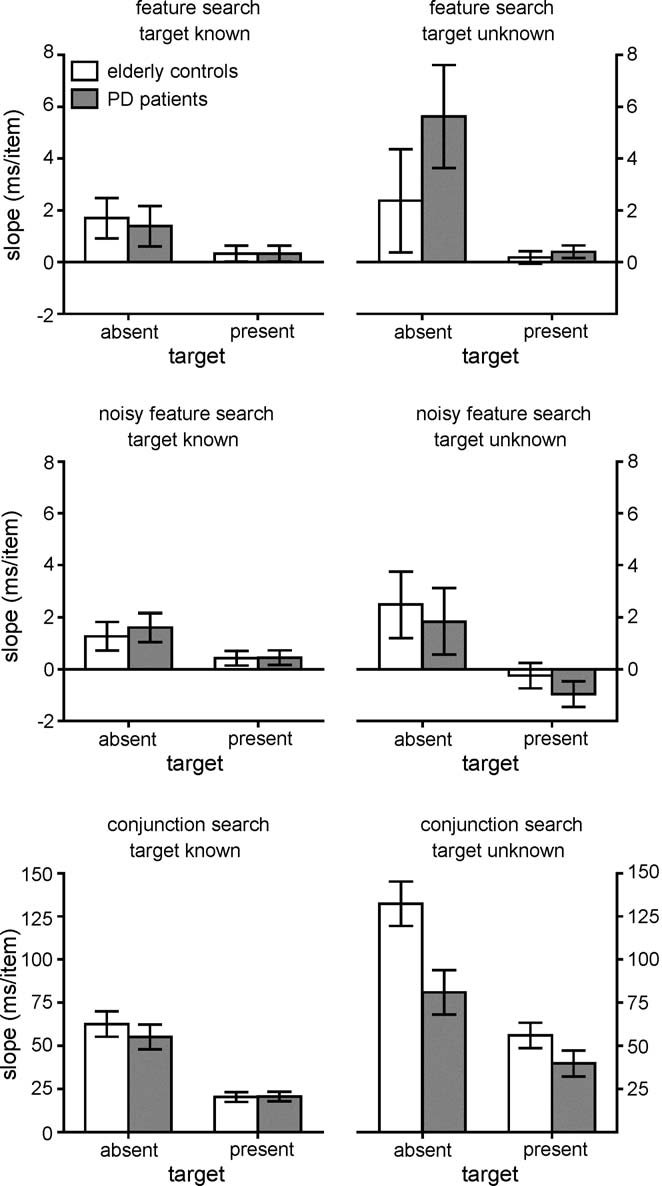
Fig. 4. Slopes of the median RT × set size functions. Open bars denote elderlycontrol data, and dark gray bars denote PD patient data. Left-hand panels plotdata from the Target Known conditions, while right-hand panels plot data fromthe Target Unknown conditions. Top panels show data from the feature searchcondition, middle panels show data from the noisy feature search condition, andbottom panels show data from the conjunction search condition. Note that thevertical scale is different for the conjunction data.
versus absence, and set size (16, 36, and 64). From these datawe extracted slopes of the RT × set size functions, which areshown in intercepts, which are shown in Thefirst analysis (Section compares our results directly withthose of previous studies of search behavior in PD (
Fig. 3. Median RT by set size. Data from elderly control participants are plottedas triangles (�), data from PD patients as squares (�). Open symbols denote
this analysis involves only the Target Known data for feature and
target-absent conditions, filled symbols target-present. Left-hand panels plot
conjunction search (top left and bottom left panels of
data from the Target Known conditions, while right-hand panels plot data from
Next, we analyze the effect of known versus unknown targets
the Target Unknown conditions. Top panels show data from the feature search
on the two groups across all three search types, looking first at
condition, middle panels show data from the noisy feature search condition,
slope (Section and then at intercept (Section Next,
and bottom panels show data from the conjunction search condition. Note thatthe vertical scale is different for the conjunction data. Error bars in this and all
since PD patients are known to have troubles with set-shifting,
subsequent figures denote the standard error of the mean.
we tested whether they would be more impaired than controls in
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
3.1.1. Comparison to previous studies of search in PD(Target Known)
The first question is whether our PD patients demonstrated
a pattern of results that replicate the impaired feature searchperformance found by Troscianko and colleagues or the intact featuresearch observed by The known target con-ditions of feature search and conjunction search correspond tothe experiments conducted by these two groups.
For feature search, slopes for both groups were flat. Target-
present slopes did not differ from 0.0 for either the PD patients(t[9] < 1) or the elderly controls (t[9] = 1.1, p > .10). Target-absent feature slopes were greater than 0.0 for the elderlycontrols (t[9] = 3.3, p < .01) and marginally greater for the PDpatients (t[9] = 2.1, p = .06). Nonetheless, both slopes were stillquite shallow (1.7 ms/item and 5.6 ms/item, respectively). Asexpected, conjunction search slopes for both groups all differedfrom 0.0 (all p < .00001).
When slope data were entered into a three-way mixed
ANOVA with Group (elderly controls versus PD patients),search type (feature versus conjunction) and target present ver-sus absent as factors, there was no main effect of Group, nor didGroup interact with any other factor (all F[1,18] < 1, p < .05).
3.1.2. Target Known versus Unknown: slopes
Overall, slopes were marginally greater for the patients
than for controls (F[1,18] = 6.9, p = .06, ˆη2 = .18). Looking atit is clear that patients and controls were producingvery similar slopes in the feature and noisy feature conditions,but something quite different was going on for the conjunc-tion condition. This impression is supported by a four-wayinteraction (Group × Target Known versus Unknown × SearchType × Target-present versus Target–absent: F[1.2,22.1] = 8.0,p < .01, ˆη2 = .31), and by separate ANOVAs on each searchtype.
In the feature and noisy feature search conditions, there were
no slope differences between patients and elderly controls (all
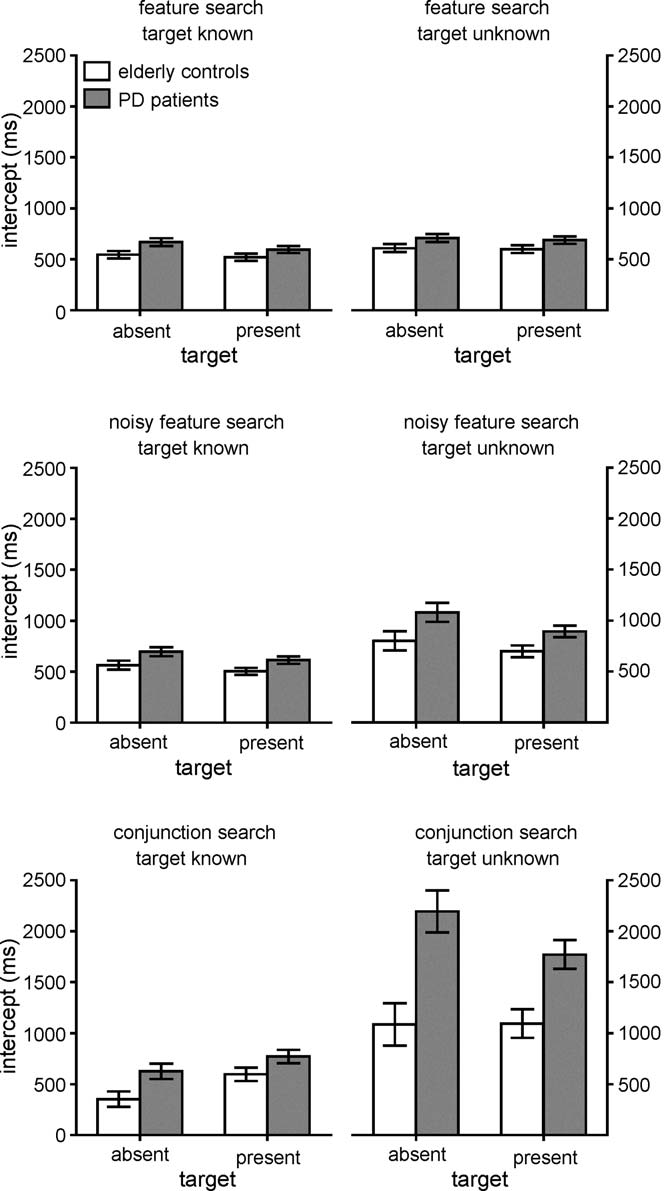
Fig. 5. Intercepts of the median RT × set size functions. Open bars denote elderly
F[1,18] < 1). Nor did Group interact significantly with any other
control data, and dark gray bars denote PD patient data. Left-hand panels plot
variable (all p > .10, ˆη2 < .15).
data from the Target Known conditions, while right-hand panels plot data fromthe Target Unknown conditions. Top panels show data from the feature search
In conjunction search, however, PD patients produced
condition, middle panels show data from the noisy feature search condition, and
marginally shallower slopes overall than elderly controls
bottom panels show data from the conjunction search condition.
(F[1,18] = 4.4, p = .05, ˆη2 = .20), and Group interacted withboth Known versus Unknown (F[1,18] = 7.1, p < .05, ˆη2 = .28),
the Target Unknown conditions when the target changed from
and present versus absent (F[1,18] = 5.2, p < .05, ˆη2 = .22).
trial to trial (Section
However, these effects were subsumed under a significant three-
Throughout these analyses, we found many within-subject
way interaction (F[1,18] = 7.1, p < .05, ˆη2 = .28). In the Target
effects of search type and condition that were expected and
Known case, slopes were nearly identical for the two groups,
easily predictable from the literature (e.g. Specif-
but when the target was unknown, the elderly controls produced
ically, target-absent trials produced steeper slopes and higher
steeper slopes than the patients, particularly on target-absent tri-
intercepts than target-present trials, and conjunction search pro-
als. This unexpected result will be discussed further in Section
duced steeper slopes and higher intercepts than feature search
trials. When the target was known, search was more efficient andresponses were faster than when it was unknown. Therefore, in
3.1.3. Target Known versus Unknown: intercepts
the interest of simplifying presentation of our results and given
Intercepts are shown in were higher overall
the specific emphasis of the current report on the effects of PD on
for patients than for elderly controls (F[1,18] = 20.7, p < 0005,
search behavior, we report below only the effects that involved
ˆη2 = .54). This was true for both the Known (F[1,18] = 7.6,
the group variable.
p < .05, ˆη2 = .30) and Unknown (F[1,18] = 22.5, p < .0005,
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
ˆη2 = .56) conditions, consistent with the expectation that more
not significant (F[1,18] = 2.5, p = 0.13, ˆη2 = .12). There was
advanced PD patients will show motor slowing even when med-
a significant interaction for conjunction search (F[1,18] = 10.1,
icated. More interestingly, however, the data in
p < .01, ˆη2 = .36), indicating that not knowing the target caused
that the difference between Known and Unknown conditions
a substantial increase in these intercepts for the patient group,
increased from feature to noisy feature to conjunction search,
relative to the elderly controls.
and that this effect was more dramatic for patients than forcontrols. This impression is supported by a Group × Known
3.1.4. Target Unknown: repeated target analysis
versus Unknown × Search type interaction (F[1.3,23.4] = 7.3,
Given the possibility that the increased switching demands
p < .01, ˆη2 = .29), and further confirmed by separate analyses of
in the Target Unknown condition might have contributed to the
the Group × Known versus Unknown interaction for the three
observed intercept increases in our medicated PD patients, we
search types.
sorted target-present trials on the basis of whether the target on
The difference between known and unknown targets did not
the previous target-present trial had been the same or different,
vary between groups for feature search (F[1,18] = 0.0, ˆη2 =
then submitted median repeated and unrepeated RTs for each
0.00). Although for noisy feature search, the cost for unknown
search type to a three-way mixed ANOVA. Although we did
targets was somewhat greater for the patients, the effect was
observe a sizeable advantage for repeated trials (F[1,18] = 13.6,p < .005, ˆη2 = .43), Group did not enter into any interactions (allp > .10, all ˆη2 < .05). Thus, these data do not point to switchingdeficits in our PD patients.
3.2. Error data
d� Scores (see were analyzed using a four-way
ANOVA with Group, Target Known versus Target Unknown,search type, and set size as factors. PD patients showedmarginally worse discriminability overall than elderly con-trols (d� = 3.5 versus 3.7, respectively; F[1,18] = 4.3, p = .05,
ˆη2 = .20). However, Group did not interact with any of the
within-subject factors (all p > .10, ˆη2 < .15). In the criterionanalysis, participants were just slightly conservative (more likelyto say that the target was absent than present, c = .07). Criteriondid not differ by subject group, nor did Group interact with anywithin-subject variables (all F < 1, ˆη2 < .10).
4.1. Overview
When target identity was made explicit and consistent from
trial to trial (Target Known), medicated PD patients and age-matched controls performed similarly on all three visual searchtasks (i.e., feature, noisy feature, conjunction). However, impor-tant differences between patients and controls emerged in theTarget Unknown conditions. While both groups paid some cost(in intercept terms) for not knowing the target in advance, thiscost was significantly elevated for patients compared to elderlycontrols. These differences were exacerbated by increasing lev-els of bottom-up noise.
In terms of the hypotheses presented in Section we
can decisively rule out the first hypothesis—that PD and aged-
Fig. 6. Sensitivity. d� ("d prime"), a signal detection theory measure of sensi-
matched controls show identical performance across conditions.
tivity computed from the error data, is plotted against set size. Greater d� valuesindicate higher sensitivity to target presence/absence. Data from elderly control
Flat search functions in the feature and noisy feature condi-
participants are plotted as triangles (�), data from PD patients as squares (�).
tions are also sufficient to eliminate the strong form of the
Left-hand panels plot data from the Target Known conditions, while right-hand
second hypothesis (damage to the salience map). Finally, given
panels plot data from the Target Unknown conditions. Top panels show data
that there was no increase in the conjunction search slope, we
from the feature search condition, middle panels show data from the noisy fea-
can also eliminate the third hypothesis (impaired attentional
ture search condition, and bottom panels show data from the conjunction searchcondition.
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
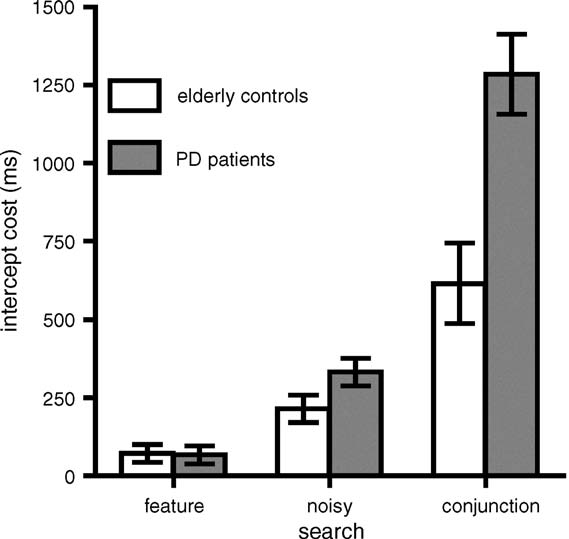
parts are slowed by 332 ms. In conjunction search, the cost is616 ms for the elderly controls, compared to 1285 ms for the PDpatients.
How do we interpret this effect? As noted in the introduction,
intercept is typically held to index "non-search" aspects of thetask. Here "search" is metonymy for the cognitive processes spe-cific to the visual search task: computing salience and shiftingattentional focus accordingly. We can think of information pro-cessing as flowing "downstream" from early vision to action,as in Intercept effects can occur either upstream fromsearch, in the massively parallel system we have abbreviated as"early vision", or downstream, after the attentional bottleneck.
There is some evidence in the literature suggesting that PD
impairs processing upstream from search. For example, ed an impairment of preattentive orientationprocessing in PD. If so, we should have seen steeper slopesin the feature search conditions due to impaired pop-out for
Fig. 7. Cost of unknown targets. Unknown target intercept–known target inter-
orientation targets, which we did not observe. Previously (see
cept plotted as a function of group and search task. Open bars denote elderly
Section we re-interpreted the Lieb et al. finding as a prod-
control data, and dark gray bars denote PD patient data.
uct of PD patients' difficulties with unknown targets, since theirstaircase procedure changed the target on each trial. However,
We are left with the fourth hypothesis. There were significant
even if there were some impairment to early visual processing
changes in intercept, and these varied systematically according
that did not disrupt salience computations (imagine, for exam-
to stimulus salience, strongly suggesting that the locus of the
ple, that orientation information was not degraded, but merely
effect was after salience computation. We conclude that search
arrived late), this could not explain why the patients were more
behavior of these medicated moderate-to-severe PD patients
impaired with unknown targets than with known targets. The
is influenced by a deficit at either the VSTM or decision and
stimulus, and therefore the input from early visual processing,
response processing stages, although further experimentation
is exactly the same in these two conditions, but the magnitude
will be necessary to more precisely identify the source of the
of the intercept depends on top-down information. Furthermore,
deficit. In any case, it is clear that without target foreknowl-
as we have noted, both the general PD impairment and the cost
edge, patients were at a severe disadvantage, particularly as
of not knowing the target in advance vary inversely with target
bottom-up information decreased in salience. Moreover, these
salience. An effect that is modulated by salience must logically
results indicate that PD patients greatly benefit from the ability
be downstream from the salience n other words, it is what
to constrain and facilitate decision/response selection using top-
observers do with the output of the search process that differs
down information, consistent with the view that external cues
between patients or controls.
or other information that serves to limit decision or response
The output of the search process (i.e., the candidate target
options can serve to compensate for performance deficits in this
representation) enters VSTM, then is passed to decision and
response processes. Any of these stages may be the source ofthe increased intercepts that we observed. As we noted in the
4.2. The cost of unknown targets
Introduction (see Section top-down information can mod-ulate processing at the level of the salience map, at the level of
Our findings in the slope domain were primarily negative,
competition in VSTM, or at the decision/response level. Fur-
however a robust and systematic pattern of effects emerged
thermore, if we think of DA as serving to modulate the flow of
in the intercept analyses. First, intercepts increased as salience
information through corticostriatal basal ganglia circuits, boost-
decreased. This was true for both groups (see However,
ing task-relevant signals and inhibiting task-irrelevant noise
this pattern was greatly exaggerated in PD patients relative to
then we might speculate that DA is playing
elderly controls. Specifically, the intercept difference between
a role in biasing competition in VSTM for access to decision
conjunction and feature search for PD patients is three times
and response mechanisms (This
what it is for the elderly controls. Second, this pattern was aggra-
would predict that PD patients should show a greater second-
vated in the Target Unknown, compared to the Target Known
target impairment in the attentional blink paradigm (
case. We can measure this effect in terms of the cost of not
knowing the target in advance (see In the feature searchcondition, elderly controls are slower by about 72 ms in theTarget Unknown condition, relative to the Target Known con-
3 Of course, salience computations may feedback onto early "preattentive"
dition. For the PD patients, the cost is a nearly identical 67 ms.
In the noisy feature condition, the elderly controls are slowed
However, if re-entrant processes were impaired in PD patients, we would
by 215 ms when the target is unknown, while their PD counter-
again expect effects on slope, rather than intercept.
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
PD patients may also have problems at the
Unknown conditions, participants entered a singleton detection
level of decision or response processing, either in addition to or
instead of at the VSTM competition level. Impaired response
to the feature search mode used in the Target Known conditions.
competition processing has been blamed for PD patients' set-
By this view, no active set switching would be required in the
shifting difficulties (Variations on
Target Unknown situation, as the attentional control mode would
the flanker task may serve to parse further these possibilities
remain constant throughout a session. The switching "costs" that
we did observe would then be reclassified as priming for repeatedtargets (see
4.3. Comparison to previous studies
that a frontally impaired (according
to the WCST) subset of Parkinson's patients produced greater
As discussed in the Introduction, most of the prior work on
intercepts in visual search, while the non-frontally impaired sub-
visual search in PD indicates that medicated patients produce
set performed similarly to age-matched controls. Based on this
slope data similar to those of age-matched controls in tasks
finding, they suggested that slowed RTs associated with PD
where the target is known in advance (
would be observed only when proper functioning frontal lobes
The exception is the work
was compromised by the disease. We did not acquire WCST data
of Troscianko and his colleagues (
on our patients. However, in Berry et al. the average UPDRS
who argued that PD patients were intact
score for the "non-frontal" subgroup was 15.0, compared to
on conjunction search but impaired at feature search. Our data
24.0 for the "frontal" subgroup, suggesting that motor dysfunc-
replicate Berry et al. rather than those of Troscianko and col-
tion was more advanced in the latter subgroup. By comparison,
leagues, in that when the target was known in advance, there
our patients had an average score of 35.9, making them more
were no significant differences in slope between PD patients
similar to the "frontal" subgroup with regard to the severity of
and elderly controls on either search type.
their motor symptoms. Thus, the intercept increases observed in
It is not obvious why we observe different results than Tros-
Berry et al.'s "frontal" PD subgroup in and in our moderate-to-
cianko and colleagues (
severe patients might both have resulted from DA dysfunction
One methodological difference between our study
in the dorsal striatum. Indeed, although the majority of patients
and those of Troscianko and colleagues is that we used RTi
in both studies were tested while "on" medication, by the time
rather than RTtotal as our primary dependent measure. If RTi
that the disease is more advanced, l-Dopa treatment may restore
was not a valid substitute for RTtotal, then our data might be sus-
DA in the cortex to levels comparable to age-matched controls
pect. We think this is unlikely for two reasons. First, the elderly
control group produced RT data that closely match what we
failing to restore normal DA functioning in the dorsal striatum
would expect from previous studies using RTtotal
(In contrast, the
less impaired "non-frontal" subgroup may have had DA levels in
the nigrostriatal pathway more fully restored by the medication.
ran the same experiment on a group of young controls (data not
We should also note that our patients were, on average,
shown) and obtained the standard results that have been obtained
more depressed than elderly controls. Clinically depressed
with these tasks numerous times before in the literature (see
patients have more difficulty with search for conjunctive
With only ten participants in each group in our study, it is
or low salience (targets. Although our patients'
also possible that we simply lacked the power to detect group
scores on the BDI were far from the threshold for clinical depres-
differences. However, the effect sizes of the group factor in the
sion, it is not implausible that subclinical individual differences
known target conditions were extremely small, both in terms
in depressive affect might be related to search performance.
of ˆη2 and in absolute terms; target-present slopes differed by
However, while self-reported depression and group membership
less than 0.5 ms/item. In addition, we should note that the
were somewhat confounded in our study, we do not think that this
whose results were similar to ours in this
should undermine our conclusions. First, the effects of depres-
respect, was methodologically more similar to the Troscianko
sion do not seem to be modulated by top-down information, as
our effects were; recall that we observed no differences between
had a larger sample size than either our study or Troscianko's.
groups when the target was known. Second, while
One effect that we expected to see in the unknown target
ed an increase in slopes for depressed patients as
condition, but did not, was some sort of elevated cost in the PD
search became less efficient, our inefficient conjunction condi-
group for switching targets. Since PD patients in other tasks
tion produced a slope advantage for patients (see next section).
have some difficulty switching sets (
Finally, an analysis of the patient group did not reveal any corre-
lations between BDI score and measures of search performance.
we pre-dicted that it would be more difficult for them to switch targets.
4.4. Explaining the conjunction search data
This was not the case. There were target-switching costs, pro-portional to target salience, but they did not differ between the
One unexpected finding of our study was that elderly controls
patients and elderly controls. One possibility is that, in the Target
actually had steeper target-absent slopes than the PD patients in
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
the Target Unknown conjunction condition. This effect was not
Given that we did not monitor eye movements or enforce
driven by a few outliers, as only three of the elderly controls had
fixation in any way, it is likely that participants made eye move-
Target Unknown conjunction target-absent slopes of less than
ments during search of large arrays in the conjunction condition.
100 ms/item, whereas only two of the PD patients had slopes
There is ample evidence that gaze control is affected by PD
greater than this value. Although this finding was not predicted,
some speculation on its source seems appropriate.
Interpretation is more difficult for target-absent data than for
differences in gaze patterns may have produced the patterns we
target-present data. Early theories of search assumed a simple
observe in the data. Of particular relevance to our result, there
exhaustive processing rule
is some evidence, for example, that PD patients show stronger
which predicts a 2:1 absent:present slope ratio. How-
inhibition of return (
ever, this assumes that the brain has some way of knowing which
a reduced tendency to revisit attended locations. This might have
items have been attended and which have not This
produced more efficient gaze strategies in the patients than con-
assumption no longer seems tenable, as the capacity for keep-
trols, allowing them to quit the search earlier.
ing track of attended items appears to be quite small (
In summary, the apparent advantage for PD patients in con-
junction search, at least for target-absent trials, is an intriguing
finding. Although our current data do not allow a definitive
thermore, slope ratios are often reliably greater than 2:1 (
explanation, clearly a number of avenues are open for subse-
Therefore, participants must set some sort of threshold
quent research.
(either in terms ofthe amount of time to search or the number of items to exam-
4.5. Conclusions
ine before concluding that no target is likely to be found. Thisthreshold is probably determined adaptively; participants slow
How does PD impair visual search behavior in patients?
down after errors and speed up after correct responses
Given the importance of DA in modulating salience, we might
expect that moderate-to-severe PD patients, who suffer from
Within this framework, one possibility is that elderly controls
compromised striatal DA transmission even when medicated
were using an excessively conservative threshold when they did
not know which target to look for, searching much longer than
ficulty using visual feature information to guide attention. As
necessary. We allowed up to 10 s for participants to respond,
a result, search tasks of varying difficulty would all be treated
and it is clear from the elderly controls were unwill-
by the PD visual system as inefficient searches. However, the
ing to commit to a target-absent response before most of that
few experiments on the issue have generally found that PD
window had elapsed. Against this argument is the fact that the
patients display the normal pattern of "pop-out" for an easy fea-
observed absent:present slope ratios (2.4 and 2.1 for elderly con-
ture search task. Here, using a set of highly controlled studies, we
trols and PD patients, respectively) are well within the normal
have replicated that finding. However, we suggest that the typi-
range for searches of this degree of difficulty (see Fig. 4 of
cal procedure of using a constant, known target throughout the
The slopes themselves are comparable to those
search task may mask patients' real difficulties with visual search
obtained by a similar task with an
when target salience is decreased by the presence of increasing
elderly population. Thus RTs > 7 s could simply be the result
distractor noise. We demonstrate that when target identity is
of slow search through a very large array. On the other hand,
unpredictable from trial to trial, patients show striking, system-
"normal" behavior may be excessively conservative. Using a
atic deficits.
forced-choice staircase method, which measured the exposure
The fact that these deficits showed up in intercept rather than
time necessary to reach a fixed accuracy, Zacks and Zacks found
in slope leads us to argue that DA does not play a role in the
that slope estimates were reduced, compared to the standard,
basic computation of visual salience. If we consider only target-
unlimited exposure RT method. Thus, had we forced our partic-
present trials, search is equally efficient in patients and elderly
ipants to respond within a shorter time window, we might have
controls, and patient behavior varies in the expected fashion with
obtained faster RTs and shallower slopes in the conjunction con-
the salience of the search array. Thus, instead of disrupting visual
dition without loss of accuracy.
input salience per se, PD seems to interfere with the conversion
This may explain the long RTs for the elderly controls. How-
of a salient visual signal into action. This is consistent with the
ever, why were the PD patients' slopes shallower? Paradoxi-
view of DA as a gatekeeper for the transmission of signals in
cally, the patients may have performed somewhat better in this
corticostriatal processing loops
condition because they had difficulty sustaining task-relevant
Our findings raise a new set of questions. First, where does
performance over long periods
the intercept effect arise? A different set of paradigms will
overly conservative thresholds
be required to parse the downstream impairment into VSTM,
require too much time per trial from the patients. Note that the
decision and response components. Second, what are the neural
patients pay no additional cost in discriminability for their impa-
mechanisms by which high "bottom-up" stimulus salience and
tience, possibly because both groups are already operating at the
"top-down" instructional guidance operate to mitigate the diffi-
asymptote of their respective speed-accuracy trade-off functions
culties that medicated PD patients face in this task? Targets with
high stimulus salience may boost DA within the nigrostriatal
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
system to acceptable levels, allowing striatal DA to increase
tions that increase stimulus salience either through bottom-up or
signal-to-noise ratio more effectively. Alternatively, these tar-
gets may possess sufficient salience at the input level such thatthis modulation is less critical for gating responses. Top-down
knowledge may further improve the salience of the signal suchthat modulation by DA is less critical for normal behavior.
This research was supported by grants to J.A. Mangels from
As with any study of medicated PD patients, we must exer-
the National Institute of Mental Health (R21-MH066129), to
cise caution in concluding that this pattern of results is due
J.C. Horvitz from the National Institute of Drug Abuse (R29-
solely to DA dysfunction in the nigrostriatal pathway
DA11653), and to Columbia University from the W.M. Keck
Although in more severe
foundation. We thank Emily Stern, Gillian Diercks, and Emily
patients, medication is less likely to completely restore DA func-
Voigt for their assistance in testing patients and controls. We
tion in the dorsal striatum (putamen and dorsal caudate nucleus)
would also like to thank Shlomo Bentin and three anonymous
or to overmedicate parts of the ventral striatum and mesocorti-
reviewers for constructive criticism of the manuscript. Portions
colimbic system (as may be the case with more mild PD patients,
of this study were presented at the 2003 Cognitive Neuroscience
Society Meeting, New York, NY.
with the moreadvanced patient it is also more difficult to isolate nigrostriatal
DA depletion from dysfunction in the mesocorticolimbic DApathway, as well as in non-dopaminergic systems (
Agid, Y., Ruberg, M., Dubois, B., & Pillon, B. (1987). Anatomoclinical
and biochemical concepts of subcortical dementia. In E. C. Goodman
In order to better understand the direct contribution of
(Ed.), Cognitive neurochemistry (pp. 248–271). Oxford: Oxford Univer-
DA to the pattern of results shown here, future studies should
assess patients in the earlier stage of the disease both "on" and
Alexander, G. E., & Crutcher, M. D. (1990). Functional architecture of basal
"off" l-Dopa medication.
ganglia circuits: Neural substrates of parallel processing. Trends in Neu-
In the present task, top-down knowledge may operate via a
rosciences, 13, 266–271.
Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organiza-
network involving prefrontal regions, similar to that which has
tion of functionally segregated circuits linking basal ganglia and cortex.
been shown to be involved in top-down biasing of task-relevant
Annual Review of Neuroscience, 9, 357–381.
features or objects (
Antonini, A., Schwarz, J., Oertel, W. H., Pogarell, O., & Leenders, K. L.
(1997). Long-term changes of striatal dopamine D2 receptors in patients
tion, given that participants were only shown the target at
with Parkinson's disease: A study with positron emission tomography and[11C]raclopride. Movement Disorders, 21(1), 33–38.
the start of the block, one might expect frontal regions to be
Aosaki, T., Graybiel, A. M., & Kimura, M. (1994). Effect of the nigrostriatal
necessary for the maintenance of target identity in working
dopamine system on acquired neural responses in the striatum of behaving
memory throughout the subsequent block of trials. Therefore,
monkeys. Science, 265(5170), 412–415.
it may at first seem surprising that PD patients demonstrate
Bach, M. (1996). The Freiburg Visual Acuity test—automatic measurement
such a dramatic benefit from top-down information, given ample
of visual acuity. Optometry and Vision Science, 73(1), 49–53.
Bacon, W. F., & Egeth, H. E. (1994). Overriding stimulus-driven attentional
evidence for working memory deficits associated with fronto-
capture. Perception & Psychophysics, 55(5), 485–496.
straital dysfunction in PD (e.g.
Beats, B. C., Sahakian, B. J., & Levy, R. (1996). Cognitive performance
in tests sensitive to frontal lobe dysfunction in the elderly depressed.
ever, impairments are generally greatest for working memory
Psychological Medicine, 26(3), 591–603.
tasks that have high demands on manipulation of information,
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for beck depression
inventory-II. San Antonio, TX: Psychological Corporation.
with simple maintenance being relatively unimpaired in mild-
Berry, E. L., Nicolson, R. I., Foster, J. K., Behrmann, M., & Sagar, H. J.
to-moderate PD, regardless of medication status
(1999). Slowing of reaction time in Parkinson's disease: The involvement
The demands on working memory capacity in this task
of the frontal lobes. Neuropsychologia, 37(7), 787–795.
are relatively low, given that there is only one target per block. In
Botella, J. (1996). Decision competition and response competition: Two main
addition, top-down information can be obtained not only directly
factors in the flanker compatibility effect. In A. F. Kramer, M. G. H.
Coles, & G. D. Logan (Eds.), Converging operations in the study of visual
from instruction (but also indirectly, from
selective attention. Washington, DC: American Psychological Association.
having found the same target several times in a row (
Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10,
In the Target Known conditions
in the present study, explicit and implicit top-down information
Briand, K. A., Hening, W., Poizner, H., & Sereno, A. B. (2001). Automatic
were working in concert, making it difficult to separate out the
orienting of visuospatial attention in Parkinson's disease. Neuropsycholo-gia, 39(11), 1240–1249.
relative contribution of each to the improved performance of the
Broadbent, D. E., & Broadbent, M. H. (1987). From detection to identifi-
PD patients.
cation: Response to multiple targets in rapid serial visual presentation.
In summary, our study demonstrates that the decision and
Perception & Psychophysics, 42(2), 105–113.
response components of visual search in PD are strongly influ-
Cepeda, C., & Levine, M. S. (1998). Dopamine and N-methyl-d-aspartate
enced by the interaction of bottom-up stimulus salience and
receptor interactions in the neostriatum. Developmental Neuroscience,20(1), 1–18.
top-down information. Not only do these results serve to clar-
Chan, A. H., & Courtney, A. J. (1998). Revising and validating the random
ify inconsistencies in the literature on visual search in PD, they
search model for competitive search. Perceptual & Motor Skills, 87(1),
also provide support for implementation of therapeutic interven-
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
Chan, F., Armstrong, I. T., Pari, G., Riopelle, R. J., & Munoz, D. P. (2005).
Greenwood, P. M., & Parasuraman, R. (1999). Scale of attentional focus in
Deficits in saccadic eye-movement control in Parkinson's disease. Neu-
visual search. Perception & Psychophysics, 61(5), 837–859.
Hammar, A. (2003). Automatic and effortful information processing in
Chun, M. M., & Potter, M. C. (1995). A two-stage model for multiple tar-
unipolar major depression. Scandinavian Journal of Psychology, 44(5),
get detection in rapid serial visual presentation. Journal of Experimental
Psychology: Human Perception & Performance, 21(1), 109–127.
Hammar, A., Lund, A., & Hugdahl, K. (2003). Selective impairment in
Chun, M. M., & Wolfe, J. M. (1996). Just say no: How are visual searches
effortful information processing in major depression. Journal of the Inter-
terminated when there is no target present? Cognitive Psychology, 30(1),
national Neuropsychological Society, 9(6), 954–959.
Hillstrom, A. P. (2000). Repetition effects in visual search. Perception &
Cohen, A., & Shoup, R. (1997). Perceptual dimensional constraints in
response selection processes. Cognitive Psychology, 32(2), 128–181.
Hochstein, S., & Ahissar, M. (2002). View from the top: Hierarchies and
Cools, R., Stefanova, E., Barker, R. A., Robbins, T. W., & Owen, A. M.
reverse hierarchies in the visual system. Neuron, 36(5), 791–804.
(2002). Dopaminergic modulation of high-level cognition in Parkinson's
Hodgson, T. L., Tiesman, B., Owen, A. M., & Kennard, C. (2002). Abnormal
disease: The role of the prefrontal cortex revealed by PET. Brain, 125(Pt
gaze strategies during problem solving in Parkinson's disease. Neuropsy-
3), 584–594.
chologia, 40(4), 411–422.
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and
Hong, S.-K. (2005). Human stopping strategies in multiple-target search.
stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3,
Industrial Ergonomics, 35, 1–12.
Hornykiewicz, O. (1979). Brain dopamine in Parkinson's disease and other
Cormack, F., Gray, A., Ballard, C., & Tovee, M. J. (2004). A failure of 'pop-
neurological disturbances. In A. S. Horn, J. Korf, & B. H. C. Westerning
out' in visual search tasks in dementia with Lewy Bodies as compared to
(Eds.), The neurobiology of dopamine (pp. 633–654). London: Academic
Alzheimer's and Parkinson's disease. International Journal of Geriatric
Psychiatry, 19(8), 763–772.
Horowitz, T. S., & Wolfe, J. M. (1998). Visual search has no memory. Nature,
Cousineau, D., & Shiffrin, R. M. (2004). Termination of a visual search with
large display size effects. Spatial Vision, 17(4–5), 327–352.
Horowitz, T. S., & Wolfe, J. M. (2003). Memory for rejected distractors in
Crofts, H. S., Dalley, J. W., Collins, P., Van Denderen, J. C., Everitt, B.
visual search? Visual Cognition, 10(3), 257–298.
J., Robbins, T. W., et al. (2001). Differential effects of 6-OHDA lesions
Horvitz, J. C. (2000). Mesolimbocortical and nigrostriatal dopamine responses
of the frontal cortex and caudate nucleus on the ability to acquire an
to salient non-reward events. Neuroscience, 96(4), 651–656.
attentional set. Cerebral Cortex, 11(11), 1015–1026.
Horvitz, J. C. (2002a). Dopamine gating of glutamatergic sensorimotor and
Desimone, R. (1998). Visual attention mediated by biased competition in
incentive motivational input signals to the striatum. Behavioural Brain
extrastriate visual cortex. Philosophical Transactions of the Royal Society
Research, 137(1–2), 65–74.
of London Series B-Biological Sciences, 353(1373), 1245–1255.
Horvitz, J. C. (2002b). Dopamine, Parkinson's disease, and volition. Behav-
Desimone, R., & Duncan, J. (1995). Neural mechanisms of selective visual
ioral and Brain Sciences, 25(5), 586.
attention. Annual Review of Neuroscience, 18, 193–222.
Horvitz, J. C., Stewart, T., & Jacobs, B. L. (1997). Burst activity of ventral
Di Lollo, V., Kawahara, J., Zuvic, S. M., & Visser, T. A. (2001). The
tegmental dopamine neurons is elicited by sensory stimuli in the awake
preattentive emperor has no clothes: A dynamic redressing. Journal of
cat. Brain Research, 759(2), 251–258.
Experimental Psychology: General, 130(3), 479–492.
Hsieh, S., Hwang, W. J., Tsai, J. J., & Tsai, C. Y. (1996). Visuospatial
Dosher, B. A., Han, S., & Lu, Z. L. (2004). Parallel processing in visual
orienting of attention in Parkinson's disease. Perceptual & Motor Skills,
search asymmetry. Journal of Experimental Psychology: Human Percep-
82(3 Pt 2), 1307–1315.
tion & Performance, 30(1), 3–27.
Humphrey, D. G., & Kramer, A. F. (1997). Age differences in visual search
Downes, J. J., Roberts, A. C., Sahakian, B. J., Evenden, J. L., Morris, R.
for feature, conjunction, and triple-conjunction targets. Psychology and
G., & Robbins, T. W. (1989). Impaired extra-dimensional shift perfor-
Aging, 12(4), 704–717.
mance in medicated and unmedicated Parkinson's disease: Evidence for
Itti, L., & Koch, C. (2001). Computational modelling of visual attention.
a specific attentional dysfunction. Neuropsychologia, 27(11–12), 1329–
Nature Reviews Neuroscience, 2(3), 194–203.
Jahanshahi, M., & Frith, C. D. (1998). Willed action and its impairments.
Duncan, J., & Humphreys, G. (1992). Beyond the search surface: Visual
Cognitive Neuropsychology, 15, 483–533.
search and attentional engagement. Journal of Experimental Psychology:
Julesz, B. (1986). Texton gradients: The texton theory revisited. Biological
Human Perception & Performance, 18(2), 578–588.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). "Mini-mental state".
Kastner, S., & Ungerleider, L. G. (2001). The neural basis of biased compe-
A practical method for grading the cognitive state of patients for the
tition in human visual cortex. Neuropsychologia, 39(12), 1263–1276.
clinician. Journal of Psychiatric Research, 12(3), 189–198.
Kingstone, A., Klein, R., Morein-Zamir, S., Hunt, A., Fisk, J., & Maxner,
Frischer, M. (1989). Voluntary vs autonomous control of repetitive finger
C. (2002). Orienting attention in aging and Parkinson's disease: Distin-
tapping in a patient with Parkinson's disease. Neuropsychologia, 27,
guishing modes of control. J Clin Exp Neuropsychol, 24(7), 951–967.
Kiyatkin, E. A., & Rebec, G. V. (1996). Dopaminergic modulation of
Gauntlett-Gilbert, J., Roberts, R. C., & Brown, V. J. (1999). Mechanisms
glutamate-induced excitations of neurons in the neostriatum and nucleus
underlying attentional set-shifting in Parkinson's disease. Neuropsycholo-
accumbens of awake, unrestrained rats. Journal of Neurophysiology,
gia, 37(5), 605–616.
Georgiou, N., Bradshaw, J. L., Iansek, R., Phillips, J. G., Mattingley, J. B.,
Klein, R. (1988). Inhibitory tagging system facilitates visual search. Nature,
& Bradshaw, J. A. (1994). Reduction in external cues and movement
sequencing in Parkinson's disease. Journal of Neurology, Neurosurgery
Koch, C., & Ullman, S. (1985). Shifts in selective visual attention: Towards
and Psychiatry, 57, 368–370.
the underlying neural circuitry. Human Neurobiology, 4, 219–227.
Glickstein, M., & Stein, J. (1991). Paradoxical movement in Parkinson's
Kulisevsky, J. (2000). Role of dopamine in learning and memory: Implica-
disease. Trends in Neurosciences, 14(11), 480–482.
tions for the treatment of cognitive dysfunction in patients with Parkin-
Gordon, A. M., & Reilmann, R. (1999). Getting a grasp on research:
son's disease. Drugs & Aging, 16(5), 365–379.
Does treatment taint testing of parkinsonian patients? Brain, 122(Pt 8),
Lamy, D., & Egeth, H. E. (2003). Attentional capture in singleton-detection
and feature-search modes. Journal of Experimental Psychology: Human
Gotham, A. M., Brown, R. G., & Marsden, C. D. (1988). ‘Frontal' cognitive
Perception & Performance, 29(5), 1003–1020.
function in patients with Parkinson's disease ‘on' and ‘off' levodopa.
Lange, K. W., Robbins, T. W., Marsden, C. D., James, M., Owen, A. M., &
Brain, 111(Pt 2), 299–321.
Paul, G. M. (1992). l-Dopa withdrawal in Parkinson's disease selectively
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
impairs cognitive performance in tests sensitive to frontal lobe dysfunc-
Rosenholtz, R. (1999). A simple saliency model predicts a number of motion
tion. Psychopharmacology (Berlin), 107(2–3), 394–404.
popout phenomena. Vision Research, 39(19), 3157–3163.
Lewis, S. J., Dove, A., Robbins, T. W., Barker, R. A., & Owen, A. M.
Rosenholtz, R. (2001). Search asymmetries? What search asymmetries? Per-
(2003). Cognitive impairments in early Parkinson's disease are accompa-
ception & Psychophysics, 63(3), 476–489.
nied by reductions in activity in frontostriatal neural circuitry. Journal of
Rothblat, D. S., & Schneider, J. S. (1993). Response of caudate neurons
to stimulation of intrinsic and peripheral afferents in normal, symp-
Lewis, S. J. G., Slabosz, A., Robbins, T. W., Barker, R. A., & Owen, A.
tomatic, and recovered MPTP-treated cats. Journal of Neuroscience,
M. (2005). Dopaminergic basis for deficits in working memory but not
attentional set-shifting in Parkinson's disease. Neuropsychologia, 43, 823–
Scatton, B., Javoy-Agid, F., Rouquier, L., Dubois, B., & Agid, Y. (1983).
Reduction of cortical dopamine, noradrenaline, serotonin and their
Li, Z. (2002). A saliency map in primary visual cortex. Trends in Cognitive
metabolites in Parkinson's disease. Brain Research, 275, 321–328.
Sciences, 6(1), 9–16.
Schettino, L. F., Rajaraman, V., Jack, D., Adamovich, S. V., Sage, J., &
Lieb, K., Brucker, S., Bach, M., Els, T., Lucking, C. H., & Greenlee, M.
Poizner, H. (2004). Deficits in the evolution of hand preshaping in Parkin-
W. (1999). Impairment in preattentive visual processing in patients with
son's disease. Neuropsychologia, 42, 82–94.
Parkinson's disease. Brain, 122(Pt 2), 303–313.
Schneider, J. S. (1991). Responses of striatal neurons to peripheral sensory
Ljungberg, T., Apicella, P., & Schultz, W. (1992). Responses of monkey
stimulation in symptomatic MPTP-exposed cats. Brain Research, 544(2),
dopamine neurons during learning of behavioral reactions. Journal of
Schultz, W. (1998). Predictive reward signal of dopamine neurons. Journal
Lubow, R. E., Dressler, R., & Kaplan, O. (1999). The effects of target and
of Neurophysiology, 80(1), 1–27.
distractor familiarity on visual search in de novo Parkinson's disease
Sharpe, M. (1990). Distractibility in early Parkinson's disease. Cortex, 26,
patients: Latent inhibition and novel pop-out. Neuropsychology, 13(3),
Stern, E. R., Horvitz, J. C., Cˆot´e, L. J., & Mangels, J. A. (2005). Maintenance
Macmillan, N. A., & Creelman, C. D. (2004). Detection theory: A user's
of response readiness in patients with Parkinson's disease: Evidence from
guide (2nd ed.). unknown: Lawrence Erlbaum.
a simple reaction time task. Neuropsychology, 19(1), 54–65.
Maddox, W., Filoteo, J. V., Delis, D., & Salmon, D. (1996). Visual selec-
Stern, Y., Sano, M., Paulson, J., & Mayeux, R. (1982). Modified mini-mental
tive attention deficits in patients with Parkinson's disease: A quantitative
state examination: Validity and reliability. Neurology, 27(Suppl. 1), 179.
model-based approach. Neuropsychology, 10(2), 197–218.
Strecker, R. E., & Jacobs, B. L. (1985). Substantia nigra dopaminergic unit
Maljkovic, V., & Nakayama, K. (1994). Priming of pop-out. I. Role of fea-
activity in behaving cats: Effect of arousal on spontaneous discharge and
tures. Memory & Cognition, 22(6), 657–672.
sensory evoked activity. Brain Research, 361(1–2), 339–350.
McCarley, J. S., Wang, R. F., Kramer, A., Irwin, D. E., & Peterson, M. S.
Swainson, R., Rogers, R. D., Sahakian, B. J., Summers, B. A., Polkey, C.
(2003). How much memory does oculomotor search have? Psychological
E., & Robbins, T. W. (2000). Probabilistic learning and reversal deficits
Science, 14(5), 422–426.
in patients with Parkinson's disease or frontal or temporal lobe lesions:
McElree, B., & Carrasco, M. (1999). The temporal dynamics of visual search:
Possible adverse effects of dopaminergic medication. Neuropsychologia,
Evidence for parallel processing in feature and conjunction searches.
Journal of Experimental Psychology: Human Perception & Performance,
Torstenson, R., Hartvig, P., Langstrom, B., Westerberg, G., & Tedroff, J.
(1997). Differential effects of levodopa and dopaminergic function in early
Musen, G., & Treisman, A. (1990). Implicit and explicit memory for visual
and advanced Parkinson's disease. Annals of Neurology, 41(3), 334–340.
patterns. Journal of Experimental Psychology: Learning, Memory, and
Treisman, A. (1996). The binding problem. Current Opinion in Neurobiology,
Cognition, 16(1), 127–137.
Niebur, E., & Koch, C. (1998). Computational architectures for attention.
Treisman, A., & Gelade, G. (1980). A feature-integration theory of attention.
In R. Parasuraman (Ed.), The attentive brain (pp. 163–186). Cambridge,
Cognitive Psychology, 12, 97–136.
MA, USA: The Mit Press.
Treisman, A., & Sato, S. (1990). Conjunction search revisited. Journal
Nieoullon, A. (2002). Dopamine and the regulation of cognition and attention.
of Experimental Psychology: Human Perception & Performance, 16(3),
Progress in Neurobiology, 67, 53–83.
O'Donnell, P. (2003). Dopamine gating of forebrain neural ensembles. Eur
Troscianko, T., & Calvert, J. (1993). Impaired parallel visual search mech-
J Neurosci, 17(3), 429–435.
anisms in Parkinson's disease: Implications for the role of dopamine in
O'Donnell, P., Greene, J., Pabello, N., Lewis, B. L., & Grace, A. A. (1999).
visual attention. Clinical Vision Science, 8(3), 281–287.
Modulation of cell firing in the nucleus accumbens. Annals of the New
Vogel, E. K., & Luck, S. J. (2002). Delayed working memory consolida-
York Academy of Sciences, 877, 157–175.
tion during the attentional blink. Psychonomic Bulletin & Review, 9(4),
Pessoa, L., Kastner, S., & Ungerleider, L. G. (2003). Neuroimaging studies
of attention: From modulation of sensory processing to top-down control.
Vogel, E. K., Woodman, G. F., & Luck, S. J. (2001). Storage of features, con-
Journal of Neuroscience, 23, 3990–3998.
junctions and objects in visual working memory. Journal of Experimental
Plude, D. J., & Doussard-Roosevelt, J. A. (1989). Aging, selective attention,
Psychology: Human Perception & Performance, 27(1), 92–114.
and feature integration. Psychology and Aging, 4(1), 98–105.
Weinstein, A., Troscianko, T., & Calvert, J. (1997). Impaired visual search
Ravizza, S. M., & Ciranni, M. A. (2002). Contributions of the prefrontal cor-
mechanisms in Parkinson's disease (PD): A psychophysical and event-
tex and basal ganglia to set shifting. Journal of Cognitive Neuroscience,
related potentials study. Journal of Psychophysiology, 11(1), 33–47.
Wheeler, M. E., & Treisman, A. M. (2002). Binding in short-term visual
Raymond, J. E., Shapiro, K. L., & Arnell, K. M. (1992). Temporary suppres-
memory. Journal of Experimental Psychology: General, 131(1), 48–64.
sion of visual processing in an RSVP task: An attentional blink? Journal
Wolfe, J. M. (1994). Guided search 2.0: A revised model of visual search.
of Experimental Psychology: Human Perception & Performance, 18(3),
Psychonomic Bulletin & Review, 1(2), 202–238.
Wolfe, J. M. (1996). Extending guided search: Why guided search needs a
Redgrave, P., Prescott, T. J., & Gurney, K. (1999). Is the short-latency
preattentive "item map". In A. F. Kramer & M. G. H. Coles (Eds.), Con-
dopamine response too short to signal reward error? Trends in Neuro-
verging operations in the study of visual selective attention. Washington,
sciences, 22(4), 146–151.
DC: American Psychological Association.
Rogers, R. D., Sahakian, B. J., Hodges, J. R., Polkey, C. E., Kennard, C., &
Wolfe, J. M. (1998a). Visual search. In H. Pashler (Ed.), Attention (pp.
Robbins, T. W. (1998). Dissociating executive mechanisms of task control
13–73). Hove, England UK: Psychology Press/Erlbaum (UK).
following frontal lobe damage and Parkinson's disease. Brain, 121(Pt 5),
Wolfe, J. M. (1998b). What can 1 million trials tell us about visual search?
Psychological Science, 9(1), 33–39.
T.S. Horowitz et al. / Neuropsychologia 44 (2006) 1962–1977
Wolfe, J. M., Butcher, S. J., Lee, C., & Hyle, M. (2003). Changing your
Wolfe, J. M., Horowitz, T. S., Kenner, N., Hyle, M., & Vasan, N.
mind: On the contributions of top-down and bottom-up guidance in visual
(2004). How fast can you change your mind? The speed of top-
search for feature singletons. Journal of Experimental Psychology: Human
down guidance in visual search. Vision Research, 44(12), 1411–
Perception & Performance, 29(2), 483–502.
Wolfe, J. M., Cave, K. R., & Franzel, S. L. (1989). Guided search: An
Zacks, J. L., & Zacks, R. T. (1993). Visual search times assessed with-
alternative to the feature integration model for visual search. Journal
out reaction times: A new method and an application to aging. Journal
of Experimental Psychology: Human Perception & Performance, 15(3),
of Experimental Psychology: Human Perception & Performance, 19(4),
Wolfe, J. M., & Horowitz, T. S. (2004). What attributes guide the deployment
Zink, C. F., Pagnoni, G., Martin, M. E., Dhamala, M., & Berns, G. S. (2003).
of visual attention and how do they do it? Nature Reviews Neuroscience,
Human striatal response to salient nonrewarding stimuli. Journal of Neu-
roscience, 23(2), 8092–8097.
Source: http://search.bwh.harvard.edu/new/pubs/Horowitz%20et%20al,%20(2006)%20Parkinson%20Search.pdf
Level 2 HAPS Building John Hunter Hospital +61 2 4921 4095 [email protected] Myasthenia Gravis Background: Myasthenia gravis (MG) is an acquired autoimmune disorder characterised clinically by weakness of skeletal muscles and fatigability on exertion. The first reported clinical description was in 1672. Pathophysiology: The antibodies in MG are directed toward the acetylcholine receptor (AChR) at the neuromuscular junction (NMJ) of skeletal muscles. The hallmark of MG is muscle weakness that increases during periods of activity and improves after periods of rest. Certain muscles, such as those that control eye and eyelid movement, facial expression, chewing, talking, and swallowing are often, involved in the disorder. The muscles that control breathing and neck and limb movements may also be affected. What causes myasthenia gravis? MG is caused by a defect in the transmission of nerve impulses to muscles. It occurs when normal communication between the nerve and muscle is interrupted at the neuromuscular junction - the place where nerve cells connect with the muscles they control. Normally when impulses travel down the nerve, the nerve endings release a neurotransmitter substance called acetylcholine. Acetylcholine travels through the neuromuscular junction and binds to acetylcholine receptors which are activated and generate a muscle contraction. In MG, antibodies block, alter, or destroy the receptors for acetylcholine at the neuromuscular junction which prevents the muscle contraction from occurring. These antibodies are produced by the body's own immune system. Thus, MG is an autoimmune disease because the immune system - which normally protects the body from foreign organisms - mistakenly attacks itself. Frequency: In the United States MG is uncommon. Estimated annual incidence is 2 per 1,000,000. Mortality/Morbidity: Recent advances in treatment and care of critically ill patients have resulted in marked decrease in the mortality rate. The rate is now 3-4%, with principal risk factors being age older than 40 years, short history of severe disease, and thymoma. Previously, the mortality rate was as high as 30-40%. Sex: The female-to-male ratio is said classically to be 6:4, but as the population has aged, the incidence is now equal in males and females. Age: MG presents at any age. Female incidence peaks around 30 years of age, whereas male incidence peaks at age 60-70. Average age of onset is 28 years in females and 42 years in males. Transient neonatal MG occurs in infants of myasthenic mothers. History: MG is characterized by fluctuating weakness increased by exertion. Weakness increases during the day and improves with rest. Presentation and progression vary.
Family Literacy TIPS Words, Words Everywhere! A three-year-old playing Words, Words Everywhere P.1 with a toy remarks, "For some reason the fan is not Math is for Everyone P.1 working." A four-year-old Home: Where Language P.2 expressing his frustration at a Learning Begins! friend's decision to discontinue play





