Vbn.aau.dk
Effect of tetracycline residues in pig manure slurry on tetracycline-resistant bacteria
and resistance gene tet(M) in soil microcosms
Agersø, Yvonne; Wulf, Gitte; Bräuner, Elvira; Halling-Sørensen, Bent; Jensen, Lars
Published in:Environment International
Document VersionEarly version, also known as pre-print
Citation for published version (APA):Agersø, Y., Wulf, G., Bräuner, E., Halling-Sørensen, B., & Jensen, L. (2006). Effect of tetracycline residues inpig manure slurry on tetracycline-resistant bacteria and resistance gene tet(M) in soil microcosms. EnvironmentInternational, 32, 876-882.
General rights
Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners
and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights.
? Users may download and print one copy of any publication from the public portal for the purpose of private study or research.
? You may not further distribute the material or use it for any profit-making activity or commercial gain ? You may freely distribute the URL identifying the publication in the public portal ?
Take down policy
If you believe that this document breaches copyright please contact us at
[email protected] providing details, and we will remove access to
the work immediately and investigate your claim.
Downloaded from vbn.aau.dk on: October 08, 2016
Environment International 32 (2006) 876 – 882
Effect of tetracycline residues in pig manure slurry on tetracycline-resistant
bacteria and resistance gene tet(M) in soil microcosms
Yvonne Agersø a,⁎, Gitte Wulff a, Elvira Vaclavik b, Bent Halling-Sørensen b, Lars Bogø Jensen a
a Danish Institute for Food and Veterinary Research, Bülowsvej 27, 1790 Copenhagen V, Denmark
b Section of Environmental Chemistry, Department of Analytical Chemistry, The Danish University of Pharmaceutical Sciences, Universitetsparken 2,
2200 Copenhagen Ø, Denmark
Received 11 October 2005; accepted 17 May 2006
Available online 30 June 2006
Effects of tetracycline residues from pig manure slurry on the prevalence of tetracycline-resistant bacteria and the tetracycline resistance gene,
tet(M), were studied in soil microcosms. Four types of soil microcosms were established for a period of 152 days, supplemented withcombinations of pig manure slurry and a tetracycline-resistant Enterococcus faecalis, CG110, containing the tetracycline resistance gene tet(M)(on the conjugative transposon, Tn916). The prevalence of both tetracycline-resistant aerobic bacteria and tetracycline-resistant enterococcideclined rapidly until day 45 where no significant differences in the levels of tetracycline-resistant bacteria in any of the four types of microcosmscould be detected. tet(M) could be detected in microcosms supplemented with either pig manure slurry and/or E. faecalis CG110 (tet(M)) for thewhole period (152 days). tet(M) could be detected longer than tetracycline-resistant enterococci could be isolated (limit of detection 100 CFU/gsoil) probably due to viable but not culturable (VBNC) bacteria with tet(M), horizontal gene transfer of tet(M) to indigenous soil bacteria orpresence of "free" DNA. The concentration of chlortetracycline and oxytetracycline were almost stable through out the experimental period, butthe tetracycline concentrations had no effect on prevalence of tetracycline-resistant bacteria. The presented microcosm approach simulated naturalfarmland conditions well and supported results from previous field studies.
2006 Elsevier Ltd. All rights reserved.
Keywords: Soil; Pig manure slurry; Tetracycline; Resistance; tet(M)
with 20,000–30,000 l/ha of slurry per year In studies by manure from
The use of tetracyclines in Danish food animal production
calves treated with oxytetracycline contained detectable
has increased in recent years due to discontinuation of the use of
amounts (820 μg/kg) after 5 months of maturation and
growth promoters in Denmark. Tetracyclines are counting for
oxytetracycline was detected in manured soil, but could not
26% (29,500 kg active compound in 2004) of the total
be detected in water courses (
antimicrobial consumption and are the most common used
found that tetracycline and chlortetra-
antimicrobial agents in the Danish food animal production
cycline could be measured in soil treated with liquid manure at
(Tetracyclines are used for therapeutic
soil depths down to 30 cm. They concluded that tetracycline and
treatment, especially against infections in pigs where the drugs
chlortetracycline persisted and accumulated in soil and may
are administrated orally. Tetracyclines are excreted as active
pose a risk to the environment (
compounds via faeces and urine and can be detected in slurry
investigated the fate of chlortetracycline
in a field study and could detect the compound for the whole
Tetracyclines enter the soil environment primarily via slurry
experimental period of 5 months, while
used as fertiliser; a typical Danish agricultural field is treated
could not detect the presence of tetracycline 1 year aftertreatment with pig manure slurry.
Beside residues of antimicrobial drugs, animal manure
Corresponding author. Tel.: +45 72 34 62 73; fax: +45 72 34 6001.
E-mail address: (Y. Agersø).
contains antimicrobial-resistant bacteria and resistance genes
0160-4120/$ - see front matter 2006 Elsevier Ltd. All rights reserved.
Y. Agersø et al. / Environment International 32 (2006) 876–882
that may survive in the soil or transfer resistance genes
The soil was collected from a field on Zealand (owned by The Royal
horizontally to indigenous soil bacteria (
Veterinary and Agricultural University, Denmark) with no history of manureamendment. The soil characteristics were pH-H
2O, 7.5; organic matter, 1.4% (w/
w); clay, 12.2% (w/w); silt, 12.8% (w/w); fine sand, 32.4% (w/w); coarse sand,
study has shown that the presence of pig manure slurry in a field
30.7% (w/w). The water content of the soil was determined to 11% (w/w). The
study created a hot-spot for conjugal transfer caused by
soil was collected 7 days prior to the start of the microcosm experiment, passed
increased availability of nutrition from manure
twice through a 4 mm sieve and stored in a sealed container refrigerated in the
The conjugation and mobilisation rate of
dark to maintain its natural moisture content.
plasmids increased more than 10-fold (
2.3. Set up of microcosms
reported that the greater part ofantimicrobial-resistant bacteria spread with pig manure slurry
An experimental approach as close as possible to the natural Danish field
only survived a limited time, but that the amount of pig manure
conditions, from the time of spread of slurry (May) and 5 months ahead
slurry spread on the soil might have an effect on the occurrence
concerning the light, temperature and amount of slurry was established (see
of tetracycline resistance. The tetracycline resistance gene tet
(M) confer resistance to several tetracyclines including:
Four different kind of microcosms were set up in triplicates consisting of:
type A: soil added 0.9% sterile NaCl, type B: soil added pig manure slurry, type
oxytetracycline, chlortetracycline and minocycline
C: soil added E. faecalis CG110 suspended in 0.9% sterile NaCl, type D: soil
found that the tetracycline
added E. faecalis CG110 suspended in pig manure slurry.
resistance gene, tet(M), was most probably spread with manure
Microcosms of 100 g were prepared in cylindrical transparent plastic
and was present in a higher frequency in manured soil compared
containers (diameter: 8.5 cm, height: 8 cm, surface area: 37 cm2) allowing light
with non-manured soil. Furthermore, tet(M) present on a
influence. A load of 19,000 l slurry/ha was simulated resulting in a load of 7 mlof slurry to each microcosm or sterile 0.9% NaCl for control.
Tn916-like conjugative transposon was found in indigenous
For microcosms, types C and D E. faecalis CG110 were grown in Luria
soil bacteria belonging to the Bacillus cereus group isolated
Bertani (LB) medium supplemented with tetracycline
from manure and manured farmland soil (
8 μg/ml and incubated over night at 37 °C, shaking (120 rpm). 1 ml of overnight
Indigenous soil bacteria isolated from groundwater underlying
culture was added to 100 ml of fresh medium and grown to an OD600 = 0.7. Cells
swine production facilities harboured tet(M) genes, which were
of 120 ml culture was washed twice in 0.9% saline and resuspended in 4.5 ml0.9% saline. Two ml of this suspension was added to 160 ml filtered slurry or
identical to those found in the pig manure lagoons (
saline to a concentration of 1.6 × 108 CFU/ml. 7 ml was added per microcosm to
a final concentration of 1.1 × 107 CFU E. faecalis CG110/g soil.
The aim of this study was to investigate whether
All microcosms contained 100 g of soil and 7 ml of liquid (saline or
tetracycline residues in pig manure slurry selects for
manure). The microcosms were mixed with a sterile wooden spoon, closed
tetracycline-resistant bacteria and tetracycline resistance
with a tight lid and incubated. The next day a needle was used to make a tinywhole in the lid allowing air change. Once a week, the microcosms were aired,
genes in controlled microcosms experiments simulating
weighed and sterile water was added if necessary to obtain stable water content
farmland soil fertilized with pig manure slurry. Furthermore,
of 18% w/w (approx. once a month).
the degradation of the added tetracyclines during the
The microcosms were incubated in a type KBWF incubator (WTB Binder,
experimental period was followed. In the experiment, we
Germany) simulating the weather condition on a Danish farm field from May to
used both oxytetracycline and chlortetracycline as model
September according to day/night temperatures set as 12 h/12 h cycles given asaverage temperatures for the respective months ), see
compounds. The tetracycline residues present in the micro-
Light conditions were set as 12 h/12 h light/dark cycles. The incubator
cosms originated from pig manure slurry due to therapeutic
malfunctioned from day 106 to day 118, resulting in a temperature at approx.
treatment of the pigs. Therefore, the used concentrations of
24 °C in this period.
tetracyclines where similar to the actual concentration oftetracyclines in manured farmland soil.
2.4. Harvesting of microcosms
Triplicate samples for each of the four types of soil microcosms were
2. Materials and methods
harvested at days 0, 3, 7, 45, 90 and 152. Samples were transferred to sterileplastic bags and homogenised manually by hand. Ten grams from each bag was
2.1. Bacterial strains
used immediately for recovery of bacteria. A 1.5 ml Eppendorf tube filled with
Enterococcus faecalis, CG110, is a tetracycline-resistant bacteria that
contains tet(M) on the transposon Tn916 present in one copy at the chromosome
Conditions for incubation of microcosms
Days for actual run
(day (light)/night (dark))
2.2. Data on farm and collection of pig manure slurry and soil
Pig manure slurry was collected from a manure slurry tank at a farm on
Zealand, Denmark. The number of animals at the farm was approx. 95 sows, 15
hogs, 750 weaners and 470 slaughter pigs. The consumption of chlortetracycline
and oxytetracycline in the 2-month period, where slurry was collected in thetank, was approx. 145 g and 50 g, respectively. The tank volume when manure
Temperature and light follows a 12:12 h cycle. The temperatures were the
slurry was collected was 380 m3. The predicted maximum concentrations in the
average temperatures in Denmark for the actual month in 2000, given by the
slurry tank of chlortetracycline and oxytetracycline were calculated to 379 μg/
Danish Meteorological Institute.
l and 147 μg/l, respectively. The collected manure was stored at 5 °C over night.
a The incubator malfunctioned from day 106 to day 118, resulting in a
The manure was filtered through a layer of gauze, to remove larger particles.
temperature at approx. 24 °C in this period.
Y. Agersø et al. / Environment International 32 (2006) 876–882
sample was stored at −20 °C for direct detection of the tet(M) gene. The remains
platform-based procession. The detection limit of chlortetracycline and
of the sample (approx. 90 g of soil) was kept at −20 °C in the plastic bag and
oxytetracycline was 0.6 μg/kg and 1.9 μg/kg, respectively (
saved for chemical analysis of chlortetracycline and oxytetracycline.
2.5. Recovery of bacteria from microcosm samples
Bacteria from 10 g of soil sample (wet weight) were recovered after adding
3.1. Recovery of chlortetracycline and oxytetracycline from soil
90 ml of saline and subsequently shaking in a water bath at 125 rpm and 25 °C
for 1 h. The flask was left for 15 min allowing soil particles to settle. Onehundred microlitres of serial 10-fold dilution were plated on selective agar
Each microcosm (100 g) of types B and D was added 7 ml pig
plates: LB for recovery of total aerobic culturable bacteria, LB supplemented
manure slurry containing 2.65 μg chlortetracycline and 1.03 μg
with 8 μg/ml of tetracycline for recovery of aerobic culturable tetracycline-
oxytetracycline, which resulted in a calculated soil maximum
resistant bacteria; Slanetz (Merck, Darmstadt) for recovery of enterococci andSlanetz supplemented with 8 μg/ml of tetracycline for recovery of tetracycline-
concentration of 24.8 μg/kg of chlortetracycline and 9.62 μg/kg of
oxytetracycline. The initial (day 0) measured chlortetracycline and
The LB agar plates with and without tetracycline added were incubated at
oxytetracycline concentration levels in soil treated with pig manure
25 °C for 2 days. Colony forming units (CFU) were counted as described by
slurry were 12.8 ± 1.35 μg/kg and 3.24 ± 1.65 μg/kg. The initial
The Slanetz agar plates with and without tetracycline
recovery in soil of the calculated maximum concentration was,
were incubated overnight at 37 °C and enterococci were counted as violet
therefore, 52% and 34% of chlortetracycline and oxytetracycline
colonies. CFU/g soil was calculated for all microcosms at days 0, 3, 7, 45, 90
originating from the manure slurry, respectively.
and 152 as the average of three samples. The detection limit of bacteria was
No chlortetracycline and oxytetracycline was detected in micro-
100 CFU/g soil.
cosms of types A and C, where no pig manure slurry was added (LOD
The data is presented as the fraction of resistant bacteria, which is the
1 μg/kg). In microcosms with pig manure slurry added (types B and D),
average of the ratio (tetracycline-resistant aerobic counts of bacteria to the totalaerobic counts of bacteria culturable on LB medium) of triplicates (X
chlortetracycline and oxytetracycline could be measured in all samples,
except for one sample, harvested at day 152 from type B microcosms
1total + X2TcR/X2total + X3TcR/X3total) / 3 = (TcR / total) in
(soil with pig manure slurry) where oxytetracycline concentration was
2.6. Direct detection of tet(M) gene
below the level of detection. Results are presented in .
The maximum concentrations of chlortetracycline were measured at
The tet(M) gene was detected directly by PCR as previously described
day 45 in both microcosms where pig manure slurry was added (B and
(DNA extraction of soil samples was performed by use of
D), highest in type D microcosms (with slurry and E. faecalis, CG110
UltraClean Soil DNA Isolation kit #12800-100 (MObio Laboratories, USA). An
added) (20.2 μg/kg). Chlortetracycline in type D microcosm showed a
amount of 250 mg of soil was used. The DNA was extracted as described by the
14% decrease in the recovery of chlortetracycline from day 45 to day
manufactures, by use of a beet-beater (Model 3-15-1301, Howard Industries,
152. The concentration levels in soil of oxytetracycline remained fairly
Illinois, USA) at full speed for 3 min. DNA extractions were stored at −20 °C.
constant during the whole period of 152 days.
For PCR and dot-blot, a pure culture of B. cereus group, R89, containing thegene tet(M) was used as positive control ). For negative
3.2. Levels of tetracycline-resistant bacteria
controls, sterile DNA free soil (farmland soil radiated with 25 kGrey) and sterile0.9% saline was used.
For each sample 2 μl of undiluted and 10 times diluted DNA was used as
Observed levels of tetracycline resistance in the four types of
template for a tet(M) specific PCR using primers tetM-1 (5′-GTTAAA-
microcosms are shown in CFU counts of both tetracycline-
TAGTGTTCTTGGAG-3′) and tetM-up (5′-CTGGCAAACAGGTTC-3′). A
resistant aerobic bacteria and total aerobic bacteria are presented in
volume of 20 μl PCR product was boiled for 10 min and added to a Hybond-N +
membrane using the S&S Minifold I Dot-Blot System (Schleicher and Schuell,Dassel, Germany) following the guidelines given by the manufactures aspreviously described (). The dot-blot was hybridised anddeveloped as previously described (A sample was positiveif either the undiluted or the diluted sample was positive. Detection limit orapprox. concentration of tet(M) for 10 times diluted DNA was as follows: If bothPCR and dot-blot were negative (< 103–104 tet(M) copies/gram of soil), onlydot-blot was positive (103–104 tet(M) copies/g soil), PCR was weakly positiveand dot-blot was positive (104–105 tet(M) copies/g soil), and PCR and dot-blotwere strongly positive (> 104–105 tet(M) copies/g soil)
2.7. Measurement of chlortetracycline and oxytetracycline concentra-tions by use of HPLC-MS-MS
Subsamples (10 g of soil) from each of the microcosms were extracted, pre-
concentrated and analysed by use of HPLC-MS-MS as previously described(The samples were analysed by combining the HPLCsystem (Agilent 1100 series, Agilent Technologies, Palo Alto, CA, USA), withan MS-MS system (Sciex API 3000 triple quadropole detector, AppliedBiosystems Foster City, CA, USA) as described by .
The HPLC system was equipped with a degasser, a cooled autosampler
Fig. 1. Concentration levels (μg/kg soil) of chlortetracycline (Ctc) and
(4 °C) and a cooled column oven (13 °C). The MS-MS system was equipped
oxytetracycline (Otc) over time in microcosms with pig manure slurry
with an ESI source (Turbo Ionspray). Collection and treatment of data was
amendment. Type B: soil with pig manure slurry; type D: soil with pig manure
performed using Analyst software (Applied Biosystems) in Windows NT
slurry and CG110.
Y. Agersø et al. / Environment International 32 (2006) 876–882
CFU counts of enterococci were determined in all four types ofmicrocosms at day 90 and day 152. In microcosms where E. faecalis(CG110) was added (types A and B), enterococci were in additiondetermined at day 45.
No tetracycline-resistant enterococci were detected in type A soil
microcosms. In types B, C and D, tetracycline-resistant enterococciwere measured at day 0 to 591 ± 64 CFU/g soil, 1.72 × 106 ±8.01 × 105 CFU/g soil and 2.64 × 106 ± 7.31 × 105 CFU/g soil, respec-tively. At day 3, the number of culturable tetracycline-resistantenterococci had decreased in types C and D microcosms to1.37 × 106 ± 7.07 × 103 CFU/g soil and 1.71 × 106 ± 9.73 × 105 CFU/gsoil, respectively. At day 45 and day 152 (for types C and D), notetracycline-resistant enterococci were detected in any of the micro-cosms (LOD = 102 CFU/g soil). Neither was any enterococci detectedin microcosms where total counts of enterococci were determined(LOD = 102 CFU/g soil).
Fig. 2. Observed levels of tetracycline resistance in the four types ofmicrocosms. The data is presented as the fraction of resistant bacteria, which
3.4. Direct detection of tetracycline resistance gene tet(M)
is the ratio of tetracycline-resistant, aerobic counts (colony forming units (CFU)/g soil) of bacteria to the total aerobic counts of bacteria culturable on LB
All four types of microcosms were examined for the presence of the
medium at 25 °C. Type A: soil; type B: soil with pig manure slurry; type C: soil
tetracycline resistance gene tet(M) by extraction of total community
with CG110; type D: soil with pig manure slurry and CG110.
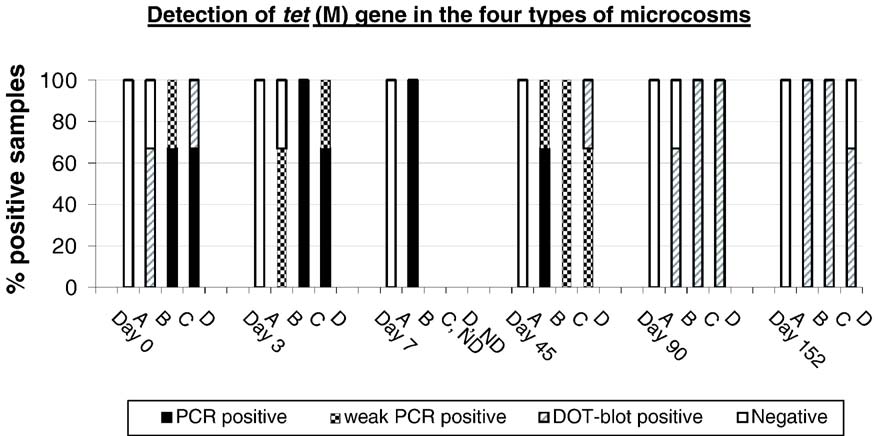
DNA from the samples followed by tet(M) specific PCR and dot-blotassays. Results are presented in In all samples except for one, tet
A statistical analysis using the Student's t-test showed that types A
(M) was only detected after dilution of the DNA 10 times. Samples
and B (soil without and with pig manure slurry) were significantly
were taken in triplicates and were measured twice.
different (p value < 0.05) at day 0 and day 7, but at days 45, 90 and 152
tet(M) could not be detected in any type A soil microcosms, but was
no significant differences were observed between these two type of
found in the microcosms types B, C and D where either manure or E.
microcosms: type A microcosms (soil) day 0 to days 7, 45, 90 and 152,
faecalis, CG110 were added to the soil. In microcosms supplemented
day 7 to day 45, day 90 to day 152; and type B (soil with pig manure
with E. faecalis CG110 (types C and D), the highest level of tet(M)
slurry) day 90 to day 152 were compared, but no significantly
genes were detected at day 0 and day 3. At least two out of three
differences were observed. Neither were significant differences
samples were positive already after the PCR step (> 104–105 tet(M)
observed between types C and D (soil + E. faecalis without and with
copies/g soil). At day 45, the level had decreased 10-fold being weakly
pig manure slurry) at days 0, 3, 45, 90 and 152, respectively.
positive after the PCR step (104–105 tet(M) copies/g soil). At days 90and 152, the samples were only positive when dot-blot was used (103–
3.3. Survival of tetracycline-resistant E. faecalis, CG110
104 tet(M) copies/g soil) and one sample at day 152 was negative(< 103–104 tet(M) copies/g soil). The microcosms with pig manure
Tetracycline-resistant enterococci were determined in all four types
slurry added (B) was positive using dot-blot (103–104 tet(M) copies/g
of microcosms (A, B, C and D) at day 0 and day 45. In microcosms
soil) for two out of three samples at day 0. At day 3 and day 7, an
where E. faecalis (CG110) was added (C and D), tetracycline-resistant
increase was observed and, at day 45, the level of tet(M) was still
enterococci were in addition determined at day 3 and day 152. Total
higher than in types C and D microcosms, but at days 90 and 152 no
Table 2Prevalence of aerobic and tetracycline-resistant (TcR) aerobic bacteria in the four types of microcosms over time
Type A: soilTcR bacteria
(6.2 ± 2.3) × 103
(4.1 ± 0.63) × 103
(1.8 ± 1.0) × 103
(1.7 ± 0.37) × 103
(2.9 ± 1.2) × 103
Total no. of bacteria
(8.1 ± 1.2) × 105
(7.7 ± 2.5) × 105
(2.1 ± 0.24) × 105
(1.6 ± 0.28) × 105
(2.0 ± 1.2) × 105
Type B: soil with slurryTcR bacteria
(8.8 ± 2.1) × 104
(1.2 ± 0.49) × 105
(1.7 ± 2.0) × 104
(2.7 ± 2.6) × 103
(6.6 ± 2.9) × 103
Total no. of bacteria
(1.1 ± 0.38) × 106
(6.9 ± 5.0) × 106
(7.5 ± 3.9) × 105
(3.9 ± 1.4) × 105
(5.2 ± 3.3) × 105
Type C: soil + E. faecalis (CG110)TcR bacteria
(1.7 ± 0.83) × 106
(1.6 ± 0.19) × 106
(6.1 ± 3.1) × 103
(2.0 ± 0.86) × 103
(1.9 ± 0.57) × 103
Total no. of bacteria
(1.2 ± 1.5) × 107
(3.1 ± 3.0) × 107
(2.4 ± 0.98) × 105
(3.2 ± 0.89) × 105
(1.6 ± 0.81) × 105
Type D: soil + slurry and E. faecalis (CG110)TcR bacteria
(3.2 ± 1.8) × 106
(1.8 ± 1.1) × 106
(3.5 ± 2.6) × 104
(2.0 ± 0.46) × 103
(1.6 ± 0.92) × 103
Total no. of bacteria
(1.1 ± 0.68) × 107
(7.1 ± 3.8) × 106
(1.3 ± 0.72) × 106
(2.6 ± 0.78) × 105
(5.8 ± 2.0) × 105
Given as colony forming units (CFU)/g soil on LB media with and without 8 μg/ml of tetracycline, respectively.
Y. Agersø et al. / Environment International 32 (2006) 876–882
Fig. 3. Detection of the tetracycline resistance gene tet(M) in the four types of microcosms over time. Type A: soil; B: soil with pig slurry; C: soil with E. faecalis(CG110); and D: soil with pig slurry and E. faecalis (CG110). Abbreviations: ND, not detected; negative, both PCR and dot-blot were negative (< 103–104 tet(M)copies/g soil); dot-blot positive, only dot-blot was positive (103–104 tet(M) copies/g soil); weak PCR positive, weakly positive by PCR and positive by dot-blot (104–105 tet(M) copies/g soil); PCR positive, strongly positive by PCR and dot-blot (> 104–105 tet(M) copies/g soil).
notable differences between types B, C and D microcosms were
One explanation for the slow degradation of tetracyclines
could be due to strong binding of tetracyclines to soil particleshereby enabling tetracyclines to persist for a considerable time
in the soil environment. Results obtained here might suggest agradual build up of concentrations of tetracyclines in soil matrix
Microcosms are experimental approaches where several
by continuous fertilising with manure.
variable parameters can be controlled, but where the experi-
Pig manure slurry contains high amounts of antimicrobial-
mental set up closely mimics natural field conditions. They are
resistant bacteria ). Addition of pig
routinely used to support for example observations from other
manure slurry to the microcosms increased the levels of
studies performed on farmland soils. The present microcosm
tetracycline-resistant bacteria significantly at day 0 and day 7,
experiments were set up to simulate the conditions on Danish
but after day 45 no significant differences were observed when
farmland from May to September. The study showed that
microcosms types A and B were compared. This confirms that
increased prevalence of aerobic tetracycline-resistant bacteria
addition of pig manure slurry to farmland soil only has a
from pig manure slurry could be detected up to 45 days in
temporary effect on the prevalence of resistant bacteria probably
microcosms with pig manure slurry added compared with non-
due to limited survival of bacteria present in slurry on farm
manured soil. This is in accordance with observations from
fields. Field studies described by
previous field studies ().
identified significant differences in levels of tetracycline
The consumption of chlortetracycline and oxytetracycline
resistance up to 45 days after spread of pig manure slurry on
for therapeutic treatment of infections in the period where pig
manure slurry was collected was approx. 145 g and 50 g,
The microcosms CFU of tetracycline-resistant aerobic
respectively. A considerably amount of the chlortetracycline
bacteria and aerobic bacteria in type B microcosms were
and oxytetracycline could be recovered from the soil, 52% and
highest at day 7, indicating bacterial growth as a result of adding
30%, respectively. Previous publications confirmed this obser-
nutrition present in the pig slurry to the soil.
vation of detectable amounts of tetracyclines in pig and calf
showed that manure also had an effect on horizontal
gene transfer on farmland, so the increase in tetracycline-
concentrations in soil were stable over the whole period of
resistant bacteria could be a result of growth or horizontal gene
152 days except for the concentration of chlortetracycline
transfer of tetracycline-resistant genes to the indigenous soil
measured in type D microcosms, where a minor decrease (14%)
bacterial flora.
was observed. This suggests that these compounds persist or
The continuous presence of tetracyclines throughout the
degrade very slowly in soil. Farmland studies also found
experiment did not have any effect on the persistence of
tetracyclines to persist in a period of five months
tetracycline-resistant bacteria. Whether chlortetracycline or
), but had disappeared after one year
oxytetracycline were present in bioavailable concentrations
(). These experiments could not determine
was not investigated in this study, but the presence of
whether the final vanishing of tetracycline were more due to
tetracyclines had previously been shown to increase horizontal
washing out than to degradation of the compounds
gene transfer of tetracycline resistance genes such as tet(M) and
tet(Q), even when present in very low concentrations
here could indicate that washing out could be the reason for
). Horizontal gene
disappearing of tetracyclines rather than degradation in soils.
transfer was not detected in this study but a previous field study
Y. Agersø et al. / Environment International 32 (2006) 876–882
indicated transfer of tet(M) residing on Tn916-like transposons
host, here the E. faecalis, CG110. This indicates that, even
to the indigenous soil bacterial flora ).
though intestinal bacteria can only be isolated in a limited
Increased prevalence of tet(M) was detected in microcosms
period, resistance genes originating from these bacteria can be
added manure (type B) indicating growth or transfer of tet(M) as
identified for an extended period suggesting a resistance gene
a result of increased nutrition and/or low concentrations of
reservoir that are not detected by conventional microbiological
tests. Clearly, more studies on the fate of resistance genes in the
The survival of the tetracycline-resistant E. faecalis, CG110
environment are needed.
(tet(M)), determined by CFU counts was not affected by theaddition of pig manure slurry to the microcosms, since no
significant differences in CFU of tetracycline-resistant entero-cocci were observed in microcosms with and without pig
This study was funded by grants from the Danish
manure slurry (types C and D). A decrease in presence of tet(M)
Agricultural and Veterinary Research Council (no. 23-00-
over time was observed in both types C and D microcosms,
0279 and no. 23-02-0169) and from the Danish Directorate for
indicating that the presence of pig manure slurry had little or no
Food, Fishery and Agricultural Business (project no. 3401-65-
effect on the persistence of tet(M) from E. faecalis CG110. Even
03-45). Furthermore, we would like to acknowledge Hanne
though the numbers of enterococci detected in types C and D
Nørgaard Nielsen and Anette Nielsen for excellent technical
microcosms at day 0 were considerably higher (around
assistance. Part of this work was presented at the 2nd
106 CFU/g soil) than in type B microcosms (6 × 102 CFU/g
International ASM-FEMs Conference on Enterococci, 2005.
soil), culturable enterococci could in the microcosms only bedetected until day 45, indicating that E. faecalis (CG110) had a
limited survival in the soil, and that an increased amount ofenterococci added did not increase the survival time of the
Agersø Y, Jensen LB, Givskov M, Roberts MC. The identification of a
enterococci. Other studies have shown survival of enterococci
tetracycline resistance gene tet(M), on a Tn916-like transposon, in the
in soil microcosms with bovine manure for at least 19 weeks
Bacillus cereus group. FEMS Microbiol Lett 2002;214:251–6.
Agersø Y, Sengeløv G, Jensen LB. Rapid method for direct detection of specific
() and enterococci are frequently present
genes from farm soil, used for detection of tet(M) genes in Danish farmland.
in soil environments (
Environ Int 2004;30:117–22.
The tet(M) gene was not detected in type A microcosms (soil
Anonymous. DANMAP 2004. Use of antimicrobial agents and occurrence of
not treated with manure or CG110) indicating absence or low
antimicrobial resistance in bacteria from food animals, foods and humans in
prevalence of tet(M) in the indigenous bacterial soil population.
Denmark. ISSN 1600–2032; 2005.
Chee-Sanford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI.
In microcosms types B, C and D, where E. faecalis CG110 or
Occurrence and diversity of tetracycline resistance genes in lagoons and
manure was added, tet(M) could be detected during the
groundwater underlying two swine production facilities. Appl Environ
experimental period (152 days). Enterococci could not be
cultured after 45 days by direct plating, while presence of tet(M)
De Liguoro M, Cibin V, Capolongo F, Halling-Sørensen B, Montesissa C. Use
could be detected through out the experiment. Whether the
of oxytetracycline and tylosin in intensive calf farming: evaluation oftransfer to manure and soil. Chemosphere 2003;52:203–12.
presence of tet(M) is caused by the presence of viable but not
Franke AE, Clewell DB. Evidence for a chromosome-borne resistance
culturable bacteria (VBNC), horizontal gene transfer of tet(M)
transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal"
or DNA from dead cells could not be determined by this
transfer in the absence of a conjugative plasmid. J Bacteriol 1981;145:
experimental setup, but demands further investigations.
Götz A, Smalla K. Manure enhances plasmid mobilization and survival of
Pseudomonas putida introduced into field soil. Appl Environ Microbiol
4.1. Overall conclusions
Guardabassi L, Dalsgaard A. Occurrence, structure, and mobility of Tn1546-like
Chlortetracycline and oxytetracycline in the present con-
elements in environmental isolates of vancomycin-resistant enterococci.
centrations did not seem to select for tetracycline-resistant
Appl Environ Microbiol 2004;70:984–90.
bacteria, but are degraded slowly in soil and may accumulate
Haack BJ, Andrews RE. Isolation of Tn916-like conjugal elements from swine
lot effluent. Can J Microbiol 2000;46:542–9.
over time if manure containing tetracyclines is regularly
Halling-Sørensen B, Jacobsen AM, Jensen J, Sengeløv G, Vaclavik EIF.
amended to the soil. Results of this experiment, when
Dissipation and effects of chlortetracycline and tylosin in two agricultural
compared to previous soil experiments may suggest that
soils—a field-scale study in southern Denmark. Environ Toxicol Chem
probably the main reason for disappearance of tetracyclines
from farmland soil could be washing out. Studies on the
Hamscher G, Sczesny S, Hoper H, Nau H. Determination of persistent
tetracycline residues in soil fertilized with liquid manure by high-
bioavailability of tetracyclines in soil are needed to determine
performance liquid chromatography with electrospray ionization tandem
the selective pressure of these compounds on survival of
mass spectrometry. Anal Chem 2002;74:1509–18.
resistant bacteria.
Jacobsen AM, Halling-Sørensen B, Ingerslev F, Hansen SH. Simultaneous
A large load of enterococci did not extend the survival time
extraction of tetracycline, macrolide and sulfonamide antibiotics from
of the enterococci and only a temporary increase in the levels of
agricultural soils using pressurised liquid extraction, followed by solid-phase extraction and liquid chromatography-tandem mass spectrometry.
tetracycline-resistant bacteria after addition of manure was
J Chromatogr A 2004;1038:157–70.
observed. However, the tet(M) gene could be detected for at
Lau MM, Ingham SC. Survival of faecal indicator bacteria in bovine manure
least five months and may persist in soil longer than the original
incorporated into soil. Lett Appl Microbiol 2001;33:131–6.
Y. Agersø et al. / Environment International 32 (2006) 876–882
Nielsen P, Gyrd-Hansen N. Bioavailability of oxytetracycline, tetracycline and
Sengeløv G, Agersø Y, Halling-Sørensen B, Baloda SB, Andersen JS, Jensen
chlortetracycline after oral administration to fed and fasted pigs. J Vet
LB. Bacterial antibiotic resistance levels in Danish farmland as a result of
Pharmacol Ther 1996;19:305–11.
treatment with pig manure slurry. Environ Int 2003;28:587–95.
Oliva B, Chopra I. Tet determinants provide poor protection against some
Shoemaker NB, Vlamakis H, Hayes K, Salyers AA. Evidence for extensive
tetracyclines: further evidence for division of tetracyclines into two classes.
resistance gene transfer among Bacteroides spp. and among Bacteroides and
Antimicrob Agents Chemother 1992;36:876–8.
other genera in the human colon. Appl Environ Microbiol 2001;67:561–8.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning a laboratory manual. A
Su YA, He P, Clewell DB. Characterization of the tet(M) determinant of Tn916:
laboratory manual. 2nd edn. Cold Spring Habor, NY: Cold Spring Habor
evidence for regulation by transcription attenuation. Antimicrob Agents
Laboratory Press; 1989.
Source: http://vbn.aau.dk/files/63143097/www.dmi.dk
CURRICULUM VITAE BSc PhD DSc (Med) DSc (hc) FRSE FFPH FRCPS (Glas) FRCP (Edin) FMedSci Knight's Cross of Order of Merit of Republic of Poland Fellow, National Academy of Scotland Fellow, Academy of Medical Science (United Kingdom) Honorary Member, Hungarian Academy of Science C: Users fourneraj Documents PB 2013-06 PB CV.doc CURRICULUM VITAE Name: Peter
Crawl, Walk Run Approach to Building an IT Service Catalog Tips and techniques to build out your service catalog - in smart, easily digestible pieces. TABle of ConTenTS TABLE OF CONTENTS . 2 ExEcuTivE Summary . 3SErvicE PorTfolio vS. SErvicE caTalog . 5WhaT DiSTinguiShES ThE BEnEfiTS of a SErvicE caTalog . 6a ruSh To juDgEmEnT . 7a Painful craWl . 8collaBoraTivE craWling . 9STEPPing in ThE Wrong DirEcTion . 11Walking Tall . 12running on EmPTy . 13running To DaylighT . 14concluSion . 15BioS anD rESourcE linkS . 16aBouT uS . 17

