Growth factors for rotator cuff repair
for Rotator Cuff Repair
LawrenceV. Gulotta, MD, Scott A. Rodeo, MD
KEYWORDS! Tendon biology ! Cytokines ! BMP ! Growth factors! Gene therapy ! Repair scaffolds
Rotator cuff repair surgeries are one of the most common procedures performed byorthopedic surgeons, with over 250,000 performed annually in the United Statesalone. Despite its prevalence, there is concern regarding the ability of the rotatorcuff to heal back to the insertion site on the humerus following repair. Clinical studieshave shown radiographic failures at the repair site at 2 years in anywhere from 11% to95% of patients, depending on the size and chronicity of the tear, presence of fattyinfiltration, and the age and general health status of the Although patientswith re-tears or failed healing may have pain relief, these studies show that they haveinferior functional results when compared with patients with healed An un-derstanding of the histology and biology that occur during the healing process maylead to therapies that can improve the healing rate and improve the functional resultsof patients following repair.
Our understanding of tendon healing is largely based on animal studies because
there is little histologic information on healing rotator cuff tendons in human beings.
From this animal data, it is known that rotator cuff healing occurs in 3 stages: inflam-mation, repair, and remodeling In the inflammatory stage, inflammatory cellsmigrate into the repair site guided by chemotactic factors followed by an influx ofblood vessels and fibroblasts. In the repair phase, several growth factors are upregu-lated that induce cellular proliferation and matrix deposition. Finally, this tissue un-dergoes remodeling due to extracellular matrix turnover mediated by matrixmetalloproteinases (MMPs).
At the conclusion of the healing process, a normal rotator cuff insertion site is not
regenerated. Normally, the rotator cuff inserts into bone through 4 distinct transitionzones: tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone. Af-ter repair, the tendon heals to bone with an interposed layer of fibrovascular scar tis-sue that persists (A and B).The mechanical properties of this fibrous tissueare weaker than the native insertion site and may render repairs prone to failure.
The Hospital for Special Surgery, Weill Medical College of Cornell University, 535 East 70thStreet, New York, NY 10021, USA* Corresponding author.
E-mail address: (S.A. Rodeo).
Clin Sports Med 28 (2009) 13–23doi:10.1016/j.csm.2008.09.002
0278-5919/08/$ – see front matter ª 2008 Elsevier Inc. All rights reserved.
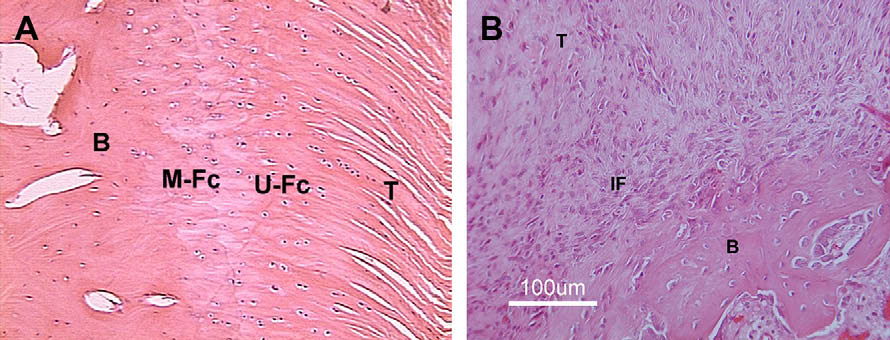
Fig. 1. The stages of rotator cuff healing involve inflammation, repair, and remodeling.
Growth factors are expressed during the repair phase, because they promote cell prolifera-tion and matrix production. This timeline must be kept in mind in growth factor therapies,because the addition of growth factors too early or late in the healing process may decreasetheir effectiveness.
In an effort to limit failures, researchers have focused on ways to minimize the for-
mation of scar tissue at the interface, while at the same time promoting the regener-ation of the fibrocartilaginous insertion zones. Initial studies have focused onimproving the biomechanical strength of the repair through stronger sutures and byrecreating the surface area of the footprint through double-row repairs or their equiv-alent. Even with these techniques, re-tears or failed healing still occur in up to 12% ofpatients.Although improved biomechanics may modestly improve healing, it appearsthat biologic augmentation of the healing process is needed to further reduce failurerates. Biologic therapies that can limit the amount of scar tissue formation at the repairsite, and help regenerate a normal fibrocartilaginous transition zone, may theoreticallyimprove the strength of repairs.
Fig. 2. (A) Histologic section of a normal supraspinatus tendon-insertion site in a rabbit,demonstrating the 4 zones of a direct insertion. T, tendon; U-Fc, unmineralized fibrocarti-lage; M-Fc, mineralized fibrocartilage; B, bone. (B) Histologic sections of the tendon-boneattachment site 4 wk after supraspinatus tendon repair in a rat. The resulting attachmentsite is characterized by a fibrovascular scar tissue interface (IF), without formation of anintermediate zone of fibrocartilage between tendon (T) and bone (B). (Reprinted fromRodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg2007;16(5S):191S–7S; with permission.)
Growth Factors for Rotator Cuff Repair
Growth factors play an important role in cell chemotaxis, proliferation, matrix
synthesis, and cell differentiation. Several growth factors are upregulated during therotator cuff healing process. Basic fibroblast growth factor (bFGF), bone morphoge-netic protein 12 (BMP-12), BMP-13, BMP-14, cartilage oligomeric matrix protein(COMP), connective tissue growth factor (CTGF), platelet-derived growth factorbeta (PDGF-B), and transforming growth factor-beta 1 (TGF-b1) have all been shownto be upregulated during the normal healing process of a rat supraspinatus tendon.Because these factors are present during the normal repair process of rotator cuffhealing, the theory is that exogenous addition of these factors can further augmentthe healing process, much as BMP-2 and 7 have done for bone healing.
Several challenges exist in developing an effective biologic therapy to augment
rotator cuff healing. First, the most effective growth factor or combination of growthfactors must be determined. As research progresses, it is clear that a single factortherapy may not be sufficient. Rather, it is probable that several factors may be nec-essary, and the various possible combinations are numerous.
The second challenge is determining the optimum time for growth factor delivery.
Growth factors are upregulated during the healing process in a temporal fashion,with most growth factors being upregulated 1 week following repair in ratmodels.In the first week after injury/repair, the healing process is in the inflamma-tory phase. It is possible that this inflammatory response may override any anabolicagent that is added at this time. Therefore, timing of growth factor application is crit-ical. This is supported by a study by Chan and in which they found thataddition of PDGF into a rat patellar tendon defect at 3 days had no effect on the bio-mechanical strength of the repair, whereas PDGF injection on day 7 improved peakloads-to-failure. Therefore, any growth factor added at the time of surgery needs tobe incorporated into a sustained-release drug delivery vehicle that ensures that thefactor is present during the regenerative phase of healing.
The final challenge involves developing a delivery vehicle for the growth factor.
Many rotator cuff repair surgeries are now performed arthroscopically, so the deliveryvehicle must be amenable to placement through cannulas and the growth factor mustnot be eluted in the fluid-filled arthroscopic environment. These technical consider-ations make gels, pastes, cements, and glues less desirable than scaffolds or patches.
In this review, the most recent research into the ability of growth factors to augment
rotator cuff healing is discussed. Because healing depends on tendon-to-bone healingat the footprint, as well as tendon-to-tendon healing for side-to-side repairs, investiga-tions that examine both processes are discussed. This is followed by a brief review onnovel advances for the delivery of growth factors to the repair site. At the conclusion ofthe review, the reader should have an understanding of the various growth factors thathave been highlighted as being potentially clinically useful, an appreciation for the re-cent research into delivery modalities, and the challenges of growth factor therapy foraugmentation of rotator cuff repair.
STUDIES ON THE APPLICATION OF GROWTH FACTORS TO IMPROVE HEALING
In animal models, growth factors are effective in increasing the cellularity and overalltissue volume at the repair site. These findings usually result in increased failure loadson biomechanical testing; however, these failure loads become less significant whenthey are normalized to the volume or cross-sectional area of the repaired tissue. Thisimplies that growth factors are able to improve the strength of the repair by promotingthe formation of more scar tissue (ie, the structural properties are improved but thematerial properties are not improved). Excessive scar tissue at the healing attachment
site may predispose patients to impingement postoperatively. The ultimate outcomeof the repair depends on both pullout strength and stiffness. Stiffness and creepmay be more important parameters. Ideally, biologic therapies are able to induce tis-sue formation with material properties close to that of normal tissue.
Osteoinductive Proteins
Secure healing between tendon and bone requires bone ingrowth into the fibrovascu-lar scar tissue and outer tendon. Therefore, factors that induce bone formation maytheoretically improve the strength of the repair. Several studies have used this strategyto improve tendon healing in a bone tunnel in animal models, analogous to an anteriorcruciate ligament (ACL) repair. However, there are few studies on improving rotatorcuff repairs with osteoinductive factors. Rodeo and colleaguesstudied the effectsof an osteoinductive bone protein extract derived from bovine cortical bone (Sulzer Bi-ologics, Wheat Ridge, CO) in a sheep model. This extract contains BMPs 2 through 7,TGF-b1, TGF-b2, TGF-b3, and FGF. The experimental group received 1.0 mg of thebone protein extract on a type-I collagen sponge, which was placed between the infra-spinatus and the bone before repair and animals were sacrificed at 6 weeks and 12weeks. Based on magnetic resonance imaging, repairs that received the bone-proteinextract had a greater volume of bone and soft tissue at the repair site at both timepoints when compared with controls. Histologic examination showed significantlymore fibrocartilage between the tendon and the bone in the experimental group.
The imaging and histology results correlated with greater failure loads at both 6 and12 weeks in the treated group. However, when the failure loads were normalized bytissue volume, there were no differences between groups. This suggests that growthfactor treatment resulted in the formation of poor-quality scar tissue rather than truetissue regeneration.
Bone Morphogenetic Proteins-12 and -13 (BMP-12 and -13)
BMP-12 (also known as growth and differentiation factor 7) and BMP-13 (growth anddifferentiation factor 6) are both expressed at the embryonic development sites thatform tendons and their insertions.These molecules are distinct from the osteoinduc-tive BMPs (BMP-2,-4,-7) and induce formation of tendon and fibrocartilage. Studieshave reported that administration of recombinant human BMP-12 (rhBMP-12) andrhBMP-13 leads to induction of neo-tendon/ligament formation in rats and improvedhealing of tendon laceration.A study conducted in conjunction with WyethResearch, Inc., investigated the effects of rhBMP-12 (Wyeth Research, Cambridge,MA) on rotator cuff tendon-bone healing in a sheep model.In this study, 4 treatmentgroups were evaluated: rhBMP-12 in injectable hyaluronan paste, rhBMP-12 in hyalur-onan sponge, rhBMP-12 in absorbable type-I collagen sponge, and rhBMP-12 type-I/IIIcollagen sponge. These were compared with a control group that underwent detach-ment and repair of the infraspinatus. At 8 weeks, specimens treated with rhBMP-12in collagen sponges were 2.7 times stronger than untreated specimens, whereas thosetreated with rhBMP-12 in hyaluronan sponges were 2.1 times stronger than controls.
Interestingly, specimens treated with rhBMP-12 in hyaluronan paste were similar to un-treated controls, again demonstrating the importance of the delivery vehicle in growthfactor therapy. Histologic evaluation found reestablishment of collagen fiber continuitybetween the bone and the fibrovascular interface scar tissue, with increased glycos-aminoglycan content in the rhBMP-12-treated specimens. These results suggest thatrhBMP-12 may be useful in improving rotator cuff repair healing.
Growth Factors for Rotator Cuff Repair
Platelet-Derived Growth Factor
PDGF-BB has been found to act as a mitogen and chemotactic cytokine that canpotentially enhance ligament and tendon healing. In a rat model of knee medial liga-ment (MCL) healing after transection, it was found that treatment with PDGF alonewhen compared with a combination of growth factors improved the structural proper-ties of the femoral-MCL-tibial complexes.In a rabbit knee medial collateral ligamentrupture model, the application of PDGF-BB delivered in fibrin sealant significantly im-proved the ultimate load, energy absorbed to failure, and ultimate elongation values ofthe femur–MCL–tibia complex when compared with the control In a rat patel-lar-tendon defect model, there was an increased proliferative response when PDGF-BB was supplemented on day 3 after surgery by way of syringe injection, whereassupplementation on day 7 improved peak load and pyridinoline content after admin-istration of the highest dosage of In a rat model of rotator cuff repair, deliveryof cells expressing PDGF-BB with a polyglycolic acid (PGA) scaffold showed restora-tion of normal crimp patterning and collagen bundle alignment compared with suturerepair A study conducted in the authors' laboratory evaluated the ability ofPDGF-BB on a collagen scaffold to improve rotator cuff healing in a rat. Increased cel-lular proliferation and angiogenesis were found in a dose-dependant fashion at 5 days;however, this did not correlate with improved healing at 28 days based on histology orbiomechanical These studies demonstrate that improved healing with PDGFis dependent on the dosage, timing, and delivery vehicle used.
There are currently several commercially available systems to create a ‘‘platelet-rich
plasma'' or ‘‘platelet gel'' from autologous blood. These systems involve spinning au-tologous blood in a centrifuge to form a dense, suturable fibrin matrix that can be eas-ily placed directly at the tendon repair site. One technical problem with these systemsis that many use human or bovine thrombin to form the platelet-rich plasma. Excessthrombin causes premature platelet activation and degranulation, causing immediaterelease of the platelet-derived cytokines. Newer systems have omitted the use ofthrombin to prevent this phenomenon during processing. Currently, there are no clin-ical studies on the efficacy of this treatment though theoretically it holds promise.
Transforming Growth Factor-b
During wound healing, TGF-b is released from degranulating platelets and secreted byall the major cell types participating in the healing process, including lymphocytes,macrophages, endothelial cells, smooth muscle cells, epithelial cells, and fibroblasts.Scar tissue formation has been closely associated with the presence of the 3 TGF-b iso-forms (TBF-b1, 2, and 3). Although adult wounds heal with an abundance of scar tissue,which is correlated with increased expression of TGF- b1, fetal wounds heal withoutscar and without expression of TBF-b1. Therefore, inhibition of TGF-b1 or exogenousapplication of TGF-b3 may reduce scar tissue formation in the interface. TBF-b3is expressed during fetal tendon development. In a study on a rat rotator cuff repairmodel, Kim and colleaguesfound that exogenous application of TGF-b3 resultedin improved mechanical properties when compared to specimens treated with TGF-b1.
Conversely, application of TGF-b1 coupled with suppression of TGF-b2 and -3 led tomechanically inferior tissue despite increased cross-sectional area. This suggeststhat although TGF-b1 results in the exuberant production of scar tissue at the repairsite, this tissue is mechanically weaker than normal tissue. The ultimate goal in devel-oping strategies to improve rotator cuff healing is to limit the amount of scar formation,while maximizing the strength of the repair.
Vascular Endothelial Growth Factor
It is well established that the rotator cuff tendon is hypovascular in the area adjacent tothe distal insertion site.Therefore, it seems reasonable that increased vascularitycould improve rotator cuff healing. VEGF is known to have a potent angiogenic effectand is expressed in high concentration in healing flexor tendons 7 to 10 days followingrepair, with a return to normal by 14 No studies have directly evaluated the roleof these molecules in rotator cuff repair. However, Zhang and injectedVEGF into repaired Achilles tendons in a rat model and found improved tensilestrength early in the course of healing. In contrast, a recent study on the ability ofVEGF on graft healing in a sheep ACL reconstruction model showed no benefit inVEGF therapy over In this study, the grafts were soaked in VEGF in theexperimental group, whereas the control group grafts were soaked in phosphate buff-ered saline. Although there was increased vascularity in the VEGF-treated group, thestiffness of the femur–graft–tibia complex in the VEGF-treated group was significantlylower than in controls. Although only a single concentration of VEGF solution wasused, and the animals were evaluated at only 1 time point (12 weeks), thesepreliminary data suggest that excessive vascularity may have detrimental effects onthe healing ACL graft. It is unclear if these findings can be extrapolated to rotatorcuff healing.
Basic Fibroblast Growth Factor
bFGF causes fibroblasts to produce collagenase and stimulates proliferation of cap-illary endothelial cells, both of which are necessary for angiogenesis. It also helps toinitiate the formation of granulation tissue. In vitro work has shown bFGF results incell proliferation and collagen production in cultured flexor-tendon
Recent in vivo work also appears encouraging, though there are no studies to date
that have evaluated bFGF in a rotator cuff repair model. Chan and colleaguesin-jected bFGF in various doses into rat patella tendons 3 days after a window defectwas created. At 7 days, there was a dose-dependent increase in the number of pro-liferating cells and the level of expression of type-III collagen. However, these resultswere not seen at 14 days, nor were there any differences in the ultimate stress and thepyridinoline content of the healing tendons. Tang and used a digitalflexor-tendon repair model in chickens to evaluate the efficacy of injecting bFGF inan adeno-associated viral vector into the lacerated tendon ends before repair. Theyfound that tendons treated with this vector had increased ultimate loads-to-failurewhen compared with those treated with a sham vector, or no vector at all, at 2, 4,and 8 weeks. Exogenous bFGF loaded onto a monofilament nylon suture has alsobeen shown to result in more cellularity and increased failure loads at 3 weeks in an-other flexor-tendon repair model.
Insulin-Like Growth Factor-1
IGF-1 has been shown to have anabolic effects on healing tendons by stimulating pro-tein synthesis, increasing cell proliferation, collagen synthesis, and decreasing swell-ing. In vitro studies have shown that the addition of IGF-1 to tenocytes in cultureinduces matrix synthesis, but did not affect matrix Kurtz and applied exogenous IGF-1 to repaired rat Achilles tendons and found that it stimulatedthe synthesis of DNA, collagen, and proteoglycans and that this resulted in reducedtime to functional recovery. Dines et al. studied the ability of rat tendon fibroblaststransduced with a retroviral vector containing IGF-1.These cells were then seededonto a bioabsorbable polymer scaffold that was made of nonwoven, PGA. The
Growth Factors for Rotator Cuff Repair
scaffold was then tested in a rat rotator cuff model. At 6 weeks, specimens treatedwith the IGF-1 seeded scaffold exhibited better histology scores and a higher ultimateload-to-failure than those with the scaffold alone. This study introduced a novel man-ner by which to deliver growth factors to the healing repair site, and its results are en-couraging and warrant further investigation in larger animal models.
DEVELOPMENTAL BIOLOGYAS A PARADIGM FOR TENDON REGENERATION
Work in developmental biology laboratories have identified several molecules that arethought to play a role in tendon and tendon-bone development during embryogenesis.
The theory is that an understanding of the mechanisms by which tendons and tendon-bone interfaces are formed in the fetus will one day lead to therapies that can induceregeneration of normal tissue as opposed to the fibrosis seen in adults. Scleraxis isa transcription factor that is upregulated in tissues that develop into tendons, leadingresearchers to postulate that it plays a role in driving tenocyte differentiation.Shuka-nami and linked scleraxis expression with another tenocyte marker,a transmembrane glycoprotein named tenomodulin. The role these proteins play inthe formation of tendons is still unclear, but they have been shown to result in tenocyteproliferation. There have been no in vivo studies investigating their ability to improverotator cuff healing.
Initial formation of tendons occurs independently with respect to muscle, but later
development depends on signals from the muscle to drive tendon development andmaturation. FGF-4 is secreted from the muscle of developing chick embryos. Its pres-ence results in upregulation of scleraxis and another tendon marker, tenascin.Thisimplies that FGF-4 may be responsible for proliferation of tenocytes and maturation oftendons during development. Another protein necessary for tendon development ismyostatin (GDF-8). Mendias and showed that the tendons of myostatinknockout mice were smaller, more brittle, had less cellularity, and had a decrease inthe expression of type-I collagen. Conversely, treatment of tendon fibroblasts withmyostatin activated tenocyte proliferation pathways and increased the productionof type-I collagen. Although the field of tendon development and its translational ap-plication to the augmentation of rotator cuff repairs is in its infancy, the possible ther-apies this research can lead to is exciting.
GROWTH FACTOR DELIVERY METHODS
Perhaps the most challenging aspect of growth factor therapy for the augmentation ofrotator cuff repairs is determining a way to deliver the factor to the healing site. As dis-cussed, once the proper combination of growth factors has been determined, as wellas their optimal time for delivery, they then need to be delivered to the tendon-bonehealing site in a fluid-filled arthroscopic environment without interfering with healing.
Gene therapy approaches and tissue regenerative scaffolds are currently beinginvestigated.
Gene therapy was first developed to treat inherited genetic defects by replacing a de-fective copy of the gene with a normal one. In orthopedics, however, attention hasbeen turned to this technique as a biologic sustained release, local growth factor de-livery vehicle. There are 2 main strategies for growth factor delivery with gene therapy,‘‘ex vivo'' and ‘‘in vivo'' (The ex vivo technique involves transferring the genethat codes for the growth factor of interest into carrier cells in vitro. These cells thenoverexpress the growth factor for which the gene codes for and releases it into the
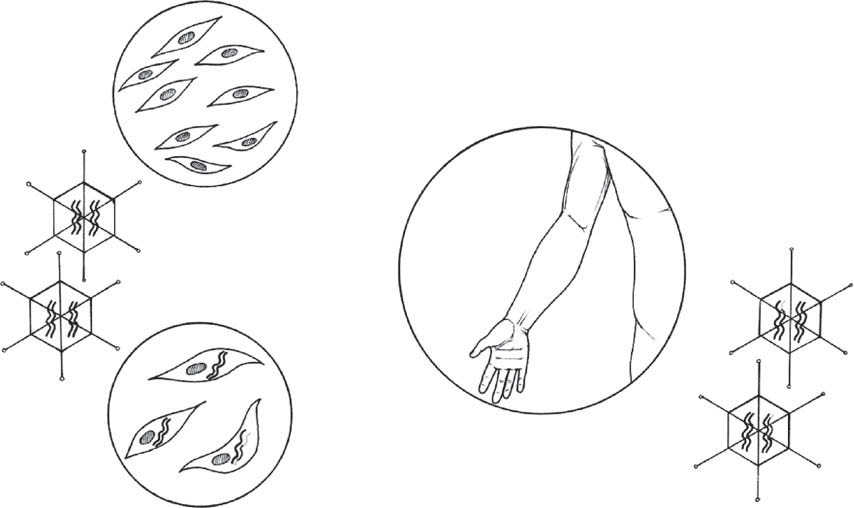
Direct injection of
Fig. 3. The 2 basic gene therapy strategies: in vivo and ex vivo. The in vivo strategy involvesadministering the vector containing the gene of interest directly to the patient. The ex vivomethod involves harvesting cells, transducing them with the vector containing the gene invitro, then re-administering the cells into the repair site. (Reprinted from Musgrave DS, FuFH, Huard J. Gene therapy and tissue engineering in orthopaedic surgery. J Am Acad OrthopSurg 2002;10:6–15; with permission.)
local environment. The transduced stem cells are then added to the repair site wherethey release the growth factor for an extended period of time. The second, less com-mon, method involves delivering the gene of interest directly into the local cells of thehealing tissue. This involves exposing the host tissue to the vector containing the geneof interest. This technique is less attractive because the vector is usually a virus, andthere is a risk of contaminating the surrounding tissue and the surgeon.
Tissue Engineering Scaffolds and Coated Sutures
Scaffolds are 3-dimensional structures that promote regeneration of the surroundingtissue. The scaffold by itself may guide new tissue formation by its 3-dimensional ar-chitecture. It may also be seeded with either cells, growth factors, or both. Scaffoldscan be made of naturally derived polymers, such as collagen and hyaluronic acid, syn-thetic polymers, such as polyL-lactic acid (PLLA), PGA, polyDL-lactic-co-glycolic acid(PLGA), polyvinyl alcohol (PVA), or injectable polymers that cross-link in situ, such asalginate and polyethylene oxide (PEO). In the simplest design, the various scaffoldscan be soaked in a solution containing the growth factor such that the factor wicksonto it. This scaffold can then be added to the repair site where the growth factor iseluted into the local environment. This technique has been outlined by Dines and col-leagues,in which a Vicryl (polyglactin 910, Ethicon, Somerville, NJ) was coated withrhGDF-5. They showed that a consistent amount of growth factor is released from thesutures after passage through soft tissue.
More complex strategies have also been outlined in which hydrogels are embedded
with microspheres that contain growth factors. The microspheres then are able to re-lease the factor at a controlled rate. This technique offers the option to load the scaf-fold with more than 1 growth factor. Furthermore, the microspheres can be engineeredto release different growth factors at different rates, so that the factors can be released
Growth Factors for Rotator Cuff Repair
in a temporal fashion. Although research in this field has progressed substantially,these technologies are still far from being clinically useful.
The 4 fibrocartilaginous transition zones of the rotator cuff insertion site are not recre-ated following surgical repair. Instead, a layer of scar tissue is formed between the ten-don and the bone, which renders repairs prone to failure. Growth factors are a group ofcytokines that induce mitosis, extracellular matrix production, neovascularization, cellmaturation, and differentiation. Research has focused on their ability to augment rota-tor cuff repairs. Studies have shown that several factors are capable of increasing thestrength of repairs in animal models. However, this appears to be accomplishedthrough the production of more scar tissue, as opposed to regeneration of native tis-sue. It is becoming clear that multiple factors may be needed to regenerate the nativetendon-bone insertion site. The optimal timing and vehicle for growth factor deliverhave remained elusive. Gene therapy and tissue scaffolds provide promising optionsfor the future, but the engineering still needs to be optimized for clinical use. Growthfactor therapy for rotator cuff repairs remains a promising therapeutic for the future;however, much work needs to be done to optimize its effectiveness.
1. Fealy S, Adler RS, Drakos MC, et al. Patterns of vascular and anatomical re-
sponse after rotator cuff repair. Am J Sports Med 2006;34(1):120–7.
2. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of com-
pletely arthroscopically repaired large and massive rotator cuff tears. J Bone JointSurg Am 2004;86A(2):219–24.
3. Harryman DT 2nd, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correla-
tion of functional results with integrity of the cuff. J Bone Joint Surg Am 1991;73(7):982–9.
4. Lafosse L, Brozska R, Toussaint B, et al. The outcome and structural integrity of
arthroscopic rotator cuff repair with use of the double-row suture anchor tech-nique. J Bone Joint Surg Am 2007;89(7):1533–41.
5. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator
cuff. J Bone Joint Surg Am 2000;82(4):505–15.
6. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness
tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am2005;87(6):1229–40.
7. Carpenter JE, Thomopoulos S, Flanagan CL, et al. Rotator cuff defect healing:
a biomechanical and histologic analysis in an animal model. J Shoulder ElbowSurg 1998;7(6):599–605.
8. Cohen DB, Kawamura S, Ehteshami JR, et al. Indomethacin and celecoxib impair
rotator cuff tendon-to-bone healing. Am J Sports Med 2006;34(3):362–9.
9. Galatz LM, Sandell LJ, Rothermich SY, et al. Characteristics of the rat supraspi-
natus tendon during tendon-to-bone healing after acute injury. J Orthop Res2006;24(3):541–50.
10. Wurgler-Hauri CC, Dourte LM, Baradet TC, et al. Temporal expression of 8 growth
factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder ElbowSurg 2007;16(Suppl 5):S198–203.
11. Kobayashi M, Itoi E, Minagawa H, et al. Expression of growth factors in the early
phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg 2006;15(3):371–7.
12. Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors
and matrix molecules in healing tendon lesions. J Orthop Res 2005;23(1):84–92.
13. Chan BP, Fu SC, Qin L, et al. Supplementation-time dependence of growth
factors in promoting tendon healing. Clin Orthop Relat Res 2006;448:240–7.
14. Rodeo SA, Potter HG, Kawamura S, et al. Biologic augmentation of rotator cuff
tendon-healing with use of a mixture of osteoinductive growth factors. J BoneJoint Surg Am 2007;89(11):2485–97.
15. Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament
in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-betagene family. J Clin Invest 1997;100(2):321–30.
16. Lou J, Tu Y, Burns M, et al. BMP-12 gene transfer augmentation of lacerated ten-
don repair. J Orthop Res 2001;19(6):1199–202.
17. Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta
Orthop Scand 1999;70(1):51–4.
18. Seeherman HJ, Archambault JM, Rodeo SA, et al. rhBMP-12 accelerates healing
of rotator cuff repairs in a sheep model. J Bone Joint Surg Am 2008;90:2206–19.
19. Letson AK, Dahners LE. The effect of combinations of growth factors on ligament
healing. Clin Orthop Relat Res 1994;308:207–12.
20. Hildebrand KA, Woo SL, Smith DW, et al. The effects of platelet-derived growth
factor-BB on healing of the rabbit medial collateral ligament. An in vivo study.
Am J Sports Med 1998;26(4):549–54.
21. Uggen JC, Dines J, Uggen CW, et al. Tendon gene therapy modulates the
local repair environment in the shoulder. J Am Osteopath Assoc 2005;105(1):20–1.
22. Kovacevic D, Gulotta L, Nickols J, et al. PDGF induces cell proliferation and an-
giogenesis in a rat rotator cuff repair model of tendon-bone healing. Presented atthe Annual Meeting of the American Orthopaedic Society for Sports Medicine,Orlando (FL), July 10–13, 2008.
23. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament
healing. Sports Med 2003;33(5):381–94.
24. Kim HM, Galatz L, Das R, et al. The role of TGF-Beta during tendon to bone heal-
ing. Trans of the Orthopaedic Research Society 2006;31:1060.
25. Zhang F, Liu H, Stile F, et al. Effect of vascular endothelial growth factor on rat
achilles tendon healing. Plast Reconstr Surg 2003;112(6):1613–9.
26. Yoshikawa T, Tohyama H, Katsura T, et al. Effects of local administration of vascu-
lar endothelial growth factor on mechanical characteristics of the semitendinosustendon graft after anterior cruciate ligament reconstruction in sheep. Am J SportsMed 2006;34(12):1918–25.
27. Takahasih S, Nakajima M, Kobayashi M, et al. Effect of recombinant basic fibro-
blast growth factor (bFGF) on fibroblast-like cells from human rotator cuff tendon.
Tohoku J Exp Med 2002;198(4):207–14.
28. Thomopoulos S, Harwood FL, Silva MJ, et al. Effect of several growth factors on
canine flexor tendon fibroblast proliferation and collagen synthesis in vitro.
J Hand Surg [Am] 2005;30(3):441–7.
29. Chan BP, Fu S, Qin L, et al. Effects of basic fibroblast growth factor (bFGF) on
early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand2000;71(5):513–8.
30. Tang JB, Cao Y, Zhu B, et al. Adeno-associated virus-2-mediated bFGF gene
transfer to digital flexor tendons significantly increases healing strength. An invivo study. J Bone Joint Surg Am 2008;90(5):1078–89.
Growth Factors for Rotator Cuff Repair
31. Hamada Y, Katoh S, Hibino N, et al. Effects of monofilament nylon coated with
basic fibroblast growth factor on endogenous intrasynovial flexor tendon healing.
J Hand Surg [Am] 2006;31(4):530–40.
32. Kurtz CA, Loebig TG, Anderson DD, et al. Insulin-like growth factor I accelerates
functional recovery from achilles tendon injury in a rat model. Am J Sports Med1999;27(3):363–9.
33. Dines JS, Grande DA, Dines DM. Tissue engineering and rotator cuff tendon
healing. J Shoulder Elbow Surg 2007;16(5 Suppl):S204–7.
34. Pryce BA, Brent AE, Murchison ND, et al. Generation of transgenic tendon re-
porters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene.
Dev Dyn 2007;236(6):1677–82.
35. Shukunami C, Takimoto A, Oro M, et al. Scleraxis positively regulates the expres-
sion of tenomodulin, a differentiation marker of tenocytes. Dev Biol 2006;298(1):234–47.
36. Edom-Vovard F, Schuler B, Bonnin MA, et al. Fgf4 positively regulates scleraxis
and tenascin expression in chick limb tendons. Dev Biol 2002;247(2):351–66.
37. Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are
small, brittle, and hypocellular. Proc Natl Acad Sci U S A 2008;105(1):388–93.
38. Musgrave DS, Fu FH, Huard J. Gene therapy and tissue engineering in orthopae-
dic surgery. J Am Acad Orthop Surg 2002;10(1):6–15.
39. Dines JS, Weber L, Razzano P, et al. The effect of growth differentiation factor-
5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg 2007;16(Suppl 5):S215–21.
Source: http://adrianoleonardi.com.br/wp-content/uploads/2013/03/GrowthFactor-RC-Repais-ClinSportsMed2009.pdf
New Release Dana Pickel Dana Pickel GYMINI® Sunny Day GYMINI® Belle journée GYMINI® Zonnige Dag GYMINI® Día de Sol GYMINI® Dia Ensolarado Guía de instrucciones PLEASE KEEP THIS INSTRUCTION GUIDE AS IT CONTAINS IMPORTANT INFORMATION. READ CAREFULLY.
EQUINE VETERINARY JOURNAL Equine vet. J. (2011) •• (••) ••-••doi: 10.1111/j.2042-3306.2010.00313.x Comparative efficacy of inhaled albuterol between twohand-held delivery devices in horses with recurrentairway obstruction F. R. BERTIN, K. M. IVESTER and L. L. COUËTIL* Department of Veterinary Clinical Sciences, School of Veterinary Medicine, Purdue University, Indiana, USA.


