Vitamin e supplementation and lifespan in model organisms


Contents lists available at
Ageing Research Reviews
Vitamin E supplementation and lifespan in model organisms
I.M.A. Ernst , K. Pallauf , J.K. Bendall , L. Paulsen , S. Nikolai , P. Huebbe , T. Roeder , G. Rimbach
a Institute of Human Nutrition and Food Science, Christian-Albrechts-University Kiel, Hermann-Rodewald-Straße 6-8, D-24118 Kiel, Germany
b Zoological Institute, Zoophysiology II, Christian-Albrechts-University Kiel, Olshausenstraße 40, D-24098 Kiel, Germany
c Department of Cardiovascular Medicine, University of Oxford, John Radcliffe Hospital, OX39DU, UK
We have conducted a comprehensive literature review regarding the effect of vitamin E on lifespan in
Received 23 July 2012
model organisms including single-cell organisms, rotifers, Caenorhabditis elegans, Drosophila melanogaster
Received in revised form 2 October 2012
and laboratory rodents. We searched Pubmed and ISI Web of knowledge for studies up to 2011 using the
Accepted 4 October 2012
terms "tocopherols", "tocotrienols", "lifespan" and "longevity" in the above mentioned model organisms.
Available online 23 October 2012
Twenty-four studies were included in the final analysis. While some studies suggest an increase in life-
span due to vitamin E, other studies did not observe any vitamin E-mediated changes in lifespan in model
organisms. Furthermore there are several studies reporting a decrease in lifespan in response to vitamin E
supplementation. Different outcomes between studies may be partly related to species-specific differ-
ences, differences in vitamin E concentrations and the vitamin E congeners administered. The findings
of our literature review suggest that there is no consistent beneficial effect of vitamin E on lifespan in
model organisms which is consistent with reports in human intervention studies.
2012 Elsevier B.V. All rights reserved.
the lifespan of single cell organisms and rotifers, nematodes, flies,
mice and rats.
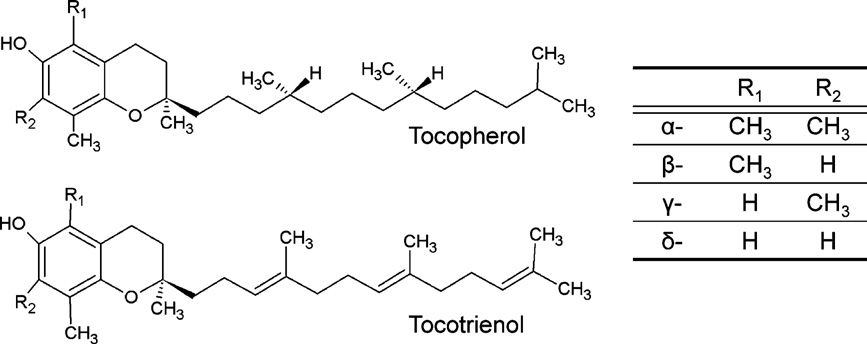
The term vitamin E is used to describe a group of eight lipid
soluble substances with a chromanol ring and a saturated (toco-
pherols) or unsaturated (tocotrienols) carbon side chain (see
2. Vitamin E supplementation in different model
Depending on the methyl groups found at the chromanol groups
these compounds are referred to as ␣-, -, ␥- or ␦-tocopherols and
tocotrienols (As a free radical
2.1. Single-celled organisms and rotifers
scavenger and lipophilic molecule, vitamin E may protect the mem-
branes from oxidative damage by reacting with fatty acid peroxides
The effect of vitamin E on single-cell organisms and rotifers
via electron transfer Additionally, vita-
was examined in five studies
min E may regulate gene expression (
Over the last few decades a possible influence of
Of these studies, four reported
vitamin E on longevity has been studied in animals and humans.
an increase in lifespan with vitamin E and one, on Saccharomyces
However, it remains unclear whether this group of antioxidants
cerevisiae (showed a reduction in lifespan. These
can prolong or, on the contrary, decrease lifespan. In this review, we
findings are summarized in
summarize the studies on vitamin E supplementation and lifespan
in model organisms of increasing biological complexity, thereby
2.1.1. Rotifer Philodina
addressing the question if and to what extent vitamin E increases
The first study to investigate the influence of vitamin E on the
lifespan of rotifers was conducted by
the rotifer Philodina. In this study, dl-␣-tocopherol at
a concentration of 0.05 l/ml (solubilized in Tween 80) was added
∗ Corresponding author. Tel.: +49 431 880 5312; fax: +49 431 880 2628.
to the medium in which the rotifers were grown. The rotifers were
E-mail addresses: (I.M.A. Ernst),
transferred to new medium every 24 h and checked for vitality.
(K. Pallauf), (J.K. Bendall),
Vitamin E treatment significantly increased mean lifespan com-
(L. Paulsen), (S. Nikolai),
pared to both the solvent control and the non-solvent control
(P. Huebbe), (T. Roeder),
(G. Rimbach).
(by 1.9 days (10.2%) and 1.7 days (9.2%) respectively), whilst maxi-
1 These authors contributed equally to this review.
mum lifespan was unaffected. Furthermore, the average number of
1568-1637/$ – see front matter
2012 Elsevier B.V. All rights reserved.
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
Fig. 1. Chemical structures of tocopherols and tocotrienols.
Vitamin E lifespan studies on single-cell organisms and rotifers in chronological order.
−7.5 days (n.s.)
–, data is not available; n.s., not significant.
offspring per rotifer was significantly increased by 10% compared
to both control groups.
Maximum clonal lifespan in days and fissions of P. tetraurelia supplemented with
different amounts of ␣-tocopherol.
2.1.2. Asplanchna brightwelli
Sawada and Enesco examined the effect of vitamin E on
another rotifer, Asplanchna brightwelli. dl-␣-Tocopherol solubi-
lifespan in fissions
lized in Tween-80 was used at concentrations ranging between
5 and 100 g/ml. They demonstrated that 25 g/ml of
tocopherol extended mean lifespan (±S.E.M.) of the rotifers from
Differences are not statistically significant (p > 0.05) (modified from
5.5 days (±0.13) to 6.4 days (±0.17) compared to the control,
whilst lower concentrations had little effect. Interestingly, the
highest concentration used, 100 g/ml, caused a reduction in life-
lifespan, measured in days and fissions. The supplemented sub-
span. Based on these initial results, they used ␣-tocopherol at
groups demonstrated a bulk increase in maximum lifespan by 17.6%
a concentration of 25 g/ml in subsequent experiments aimed
and in mean lifespan by 14.1% compared with controls (supplemen-
at elucidating the exact timing of the effects. They solubilized
tation 58.5 ± 16.6 vs. control 50.5 ± 10.6). Subsequent experiments
25 g/ml ␣-tocopherol in either Tween-80 or ethanol and found
investigated the impact of switching the organisms from control
that the increase in mean lifespan was similar with both solvents.
to vitamin E (25 g/ml) media early in life at days 0, 1, 9, 17 or
By separating the rotifers into pre-reproductive, reproductive and
25. Only those subgroups transferred at day 9 showed a signif-
post-reproductive periods, they found that lifespan was only signif-
icant increase in mean lifespan in days and fissions. Those that
icantly increased during the pre-reproductive period. The number
were transferred to ␣-tocopherol-containing media after 9 days of
of offspring was unaffected in this experiment (
clonal lifespan showed only minor, inconsistent effects on lifespan
This study also evaluated the effects of higher concentrations of
2.1.3. Paramecium tetraurelia
␣-tocopherol (100 and 1000 g/ml) on maximum clonal lifespan.
In 1988, Thomas and Nyberg were the first to investigate the
An increase in the maximum lifespan was observed with increasing
effects of vitamin E on the lifespan of a single-celled organism,
doses of ␣-tocopherol, yet these data were non-significant.
Paramecium tetraurelia. The effect of 25 g/ml dl-␣-tocopherol was
summarizes the results of this experiment.
examined in eight different genotypes, of which only one (that with
Interestingly, however, when comparing controls to organisms
the shortest mean lifespan) showed a significant increase in mean
given 25 g/ml of ␣-tocopherol and organisms given 100 g/ml
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
to those given 1000 g/ml of ␣-tocopherol, the subgroups that
received the higher concentrations of ␣-tocopherol showed a
Caenorhabditis briggsae (
slower increase in death rate despite a higher initial mortality,
and Turbatrix aceti
indicating that an adaptive process may have occurred. The same
Vitamin E, with few exceptions, has been shown to extend the
pattern was observed for fission rates. Furthermore 25 g/ml of
lifespan of nematodes (see One study showed that ␣-
␣-tocopherol increased the survival of non-dividing organisms
tocopherol treatment increased both mean and maximum lifespan
beyond their clonal lifespan from 1.05 days to 1.49 days when com-
(whereas others only demonstrated
pared to controls (
an effect on mean lifespan
Minogue and Thomas provided further evidence of the effects
of higher doses of vitamin E on P. tetraurelia. The authors
or showed no effect at all (
observed that high concentrations of ␣-tocopherol (100, 1000 and
10,000 g/ml) dose-dependently enhanced mean lifespan, despite
2.2.1. C. briggsae
lower concentrations modestly reducing lifespan compared to
Epstein and Gershon in 1972 were the first to investigate the
controls with or without medium. 1000 g/ml of ␣-tocopherol
effects of vitamin E on aging in nematodes, specifically in C. briggsae.
extended mean lifespan by 126.5 fissions (49.5%) and 115 days
␣-Tocopheryl-quinone at 400 g/ml had no effect on the length
(151.3%) compared to controls (mean lifespan in days ± S.E.M.:
of their reproductive period nor on the number of progeny. Con-
supplementation 191 ± 16 vs. control 80 ± 3; mean lifespan in
tinuous supplementation with 400 g/ml ␣-tocopheryl-quinone
fissions ± S.E.M.: supplementation 382 ± 4 vs. control 278.5 ± 11)
increased the median lifespan by 11 days (31%) and the maxi-
whilst 10,000 g of ␣-tocopherol increased lifespan by 83.5 fissions
mum lifespan by 13 days (23%) compared to medium and solvent
(32.7%) and 216.5 days (234.9%) (mean lifespan in days ± S.E.M.:
controls. Administering the ␣-tocopheryl-quinone on days 1, 10,
supplementation 292.5 ± 8.5 vs. control 80 ± 3; mean lifespan in
20 or 30 revealed that only an early initiation of the treatment
fissions ± S.E.M.: supplementation 339 ± 13 vs. control 278.5 ± 11).
prolonged median and maximum lifespan; starting treatment at
Maximum lifespan was not reported. Minogue and Thomas also
days 20 or 30 had no effect. The duration of ␣-tocopheryl-quinone
found that the survival curves of P. tetraurelia supplemented with
treatment correlated with the increase in the nematodes lifespan;
10 and 100 g/ml of ␣-tocopherol were no different to controls,
treating for 6 days after hatching prolonged median lifespan by
displaying little mortality in the early phase of lifespan with a
5 days (16%) and maximum lifespan by 6 days (10%), treating for
sudden and strong reduction in survival later in the lifespan. How-
10 days prolonged median lifespan by 11 days (31%) (supplemen-
ever, organisms treated with 1000 g/ml of ␣-tocopherol had a
tation 46 ± 2 days vs. control 35 ± 2 days) and maximum lifespan by
high mortality rate in the first 4 weeks with only 75% surviving.
14 days (25%) (supplementation 69 ± 4 days vs. control 56 ± 3 days).
Mortality in this group then slowed until survival dropped again
␣-Tocopheryl-quinone treatment was found to delay the accumu-
in the late stages of their lifespan. Those organisms administered
lation of lipofuscin, suggesting that it has antioxidant properties
10,000 g/ml of ␣-tocopherol demonstrated a strong initial decline
in survival such that only ∼50% survived until 5 weeks. Survival
then plateau'd until 15 weeks when the death rate slowly increased
again. Death rates confirmed the survival curves with the high dose
Following on from the work of Epstein and Gershon, Kahn and
treatments showing a consistently elevated death rate indicating a
Enesco found that dl-␣-tocopherol at a concentration of 100 g/ml
cytotoxic effect of ␣-tocopherol (
significantly increased the mean lifespan (±S.D.) of the nema-
tode T. aceti by 15.3 days (33.7%) (supplementation 60.7 ± 30.6 days
2.1.4. S. cerevisiae
vs. control 45.4 ± 29.5 days) and increased the maximum lifespan
More recently, the effect of ␣-
by 8 days (7%) compared to the control. This increase in lifespan
tocopherol on the lifespan of the baker's yeast, S. cerevisiae. The
occurred in the early phase, before day 30, and in the late phase,
organisms of the K6001 strain were grown in the linear phase in
after day 72. In subsequent experiments, nematodes were initially
glucose medium with vitamin E concentrations ranging between
grown in treatment or control medium and then transferred to
20 and 150 M. They found that ␣-tocopherol dose-dependently
treatment or control medium, yielding four supplementation pat-
decreased replicative lifespan (estimated by measuring the opti-
terns (control–treatment, control–control, treatment–control and
cal density of the medium). However, cell viability was unaltered
treatment–treatment). The results demonstrated that early treat-
by these ␣-tocopherol concentrations. To elucidate the molecu-
ment with ␣-tocopherol (i.e. within the first 24 h after hatching)
lar properties of ␣-tocopherol responsible for the observed effects
significantly increased mean lifespan by up to 46.2%. Transferring
they used ␣-tocopherol acetate, which lacks antioxidant activity,
the nematodes 96 h after hatching had no significant effect on life-
2,2,5,7,8-pentamethyl-6-hydroxychroman (PMC) and trolox, two
span. Together, this study indicates that ␣-tocopherol exerts its
synthetic analogs of vitamin E which lack the phytyl tail. Only
positive effects on nematodes in the early phase of their lifespan
PMC decreased the replicative lifespan in the same dose-dependent
manner as ␣-tocopherol. However, PMC also decreased cell viabil-
ity indicating a toxic effect. Thus, Lam et al. concluded that both
2.2.3. C. elegans
the antioxidant capacity and lipid solubility of ␣-tocopherol are
2.2.3.1. Influence of time and dose of ˛-tocopherol supplemen-
responsible for its effects on S. cerevisiae. In contradiction to its
tation. Zuckermann and Geist evaluated whether there is an
antioxidant activity, vitamin E was found to increase ROS pro-
age-dependent effect of ␣-tocopherol on lifespan. Parental C. ele-
duction and thus oxidative stress when administered for 20 h.
gans were grown in an ␣-tocopherol (200 g/ml)-supplemented or
Combining ␣-tocopherol with coenzyme Q10, another antioxidant
solvent control medium and newly born nematodes transferred to
which may potentially restore ␣-tocopherol, was also unsuccessful;
either an ␣-tocopherol or control medium at hatch or day four. The
replicative lifespan was similarly decreased
maximum lifespan and the time point at which 50% of the worms
were still alive were measured. These survival rates showed that
there seems to be no carryover effect from the paternal genera-
tion to the offspring as the progeny from vitamin E supplemented
The most commonly used nematodes to test the responses to
and control parents showed no difference regarding lifespan. How-
tocopherols and tocotrienols are Caenorhabditis elegans (
ever, if the nematodes hatched from egg masses and stayed in
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
Vitamin E lifespan studies on nematodes in chronological order.
Form of vitamin E
Caenorhabditis briggsae
␣-Tocopheryl quinone
Caenorhabditis elegans
Caenorhabditis elegans
Caenorhabditis elegans
␣-Tocopherol acetate
Tocotrienol mix.
Caenorhabditis elegans
−1.8 days (n.s.)
␣-toc + ␥-toc
–, data is not available; n.s., not significant; #, data are given as median.
vitamin E supplemented medium their lifespan increased by 7 days
compared to the solvent control. At concentrations of 200 and
compared to those without vitamin E supplementation (supple-
400 g/ml, dl-␣-tocopherol significantly decreased the number of
mentation 54 days vs. control 47 days). Later supplementation did
offspring per animal by 23–30% and significantly delayed the onset
not significantly affect the 50% survival rate but increased the maxi-
of their reproductive period by 19–28%. The authors concluded
mum lifespan (supplementation 74 days vs. control 66 days). These
that higher doses of vitamin E can lead to growth retardation or
results suggest that ␣-tocopherol exerts its effects in the early
developmental delay
stages of the lifespan of nematodes. Zuckermann and Geist also
demonstrated that high concentrations of ␣-tocopherol (400 g/ml
and 800 g/ml) had detrimental effects on T. aceti, reducing
2.2.3.3. Supplementation with tocopherol and tocotrienols. To com-
their size and the number of offspring compared to controls and
pare the influence of tocotrienols and tocopherols on the lifespan
lower concentrations of ␣-tocopherol (200 g/ml). These results
of C. elegans, Adachi and Ishii used dl-␣-tocopherol acetate and
again suggest that ␣-tocopherol is toxic at higher concentrations
an extract of palm oil (referred to as "tocotrienol mixture") con-
taining 95% of vitamin E composed of 22% ␣-tocopherol, 24%
␣-tocotrienol, 37% ␥-tocotrienol and 12% ␦-tocotrienol. When
given in the adult stage, the tocotrienol mixture at doses of 8
2.2.3.2. Effects of ˛-tocopherol on development and aging. Harring-
and 80 g/ml medium significantly increased the mean lifespan
ton and Harley examined in more detail the distinct effects of
of C. elegans by 1.5 days (8.7%) and 3.2 days (19.8%) (supplemen-
␣-tocopherol on development and aging in C. elegans. The addition
tation 18.7 ± 1.9 and 20.6 ± 1.6 days vs. control 17.2 ± 1.8 days),
of 200 g/ml dl-␣-tocopherol after hatching (levels were depleted
respectively, while maximum lifespan was unaffected. In contrast,
after 3 days) increased maximum lifespan by 5 days (17%) (supple-
80 g/ml of ␣-tocopherol acetate did not significantly alter life-
mentation 34 days vs. control 29 days), median lifespan by 3 days
span (The tocotrienol mixture (at 80 g/ml)
(16.6%) (supplementation 21 ± 1 days vs. control 18 ± 2 days) and
significantly decreased the levels of carbonylated proteins by one
mean lifespan by 4 days (20%) (supplementation 24 ± 1 days vs.
third in 15 day old animals, indicating that the mixture has antiox-
control 27 ± 1 days), resulting in an overall significant increase in
idant properties. The tocotrienol mixture (80 g/ml) was also able
the lifespan of C. elegans by 17% compared to the solvent control.
to abolish the significant reduction in lifespan caused by irradia-
Continuous administration of dl-␣-tocopherol increased lifespan
tion with ultraviolet light when given after the irradiation, whereas
by 22%. Administration of ␣-tocopherol at 100 and 400 g/ml
␣-tocopherol acetate (80 g/ml) offered only minimal protection
slightly, but not significantly, increased the nematodes lifespan
which was non-significant. Furthermore, when treatment was
by 9% and 4%, respectively. They found that when 200 g/ml ␣-
initiated before the irradiation insult, the tocotrienol mixture sig-
tocopherol was administered to the nematodes from day 4 of life
nificantly extended the lifespan by 2.1 days (12.1%) compared to
until death, mean lifespan was significantly increased from 18
untreated, non-irradiated controls This
to 21 days and maximum lifespan increased from 29 to 31 days
is in accordance with a study showing that the lifespan increased
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
in C. elegans that were fed ␥-cyclodextrin tocotrienol complexes
exogenous antioxidants may cause a compensatory depression of
Complexation with ␥-cyclodextrin has been
endogenous defenses. On the other hand, ␣-tocopherol treatment
shown to enhance the absorption of lipid-soluble substances and
did not alter catalase activity at both concentrations. The glu-
may be used as a strategy to improve tocotrienol bioavailability
tathione content of flies supplemented with both concentrations
of ␣-tocopherol was also not statistically different from controls.
Hydrogen peroxide levels were dose-dependently increased by ␣-
2.2.3.4. Wild-type and mev-1 mutant. Another study investigating
tocopherol, reaching statistical significance at 2% ␣-tocopherol.
the effects of vitamin E on longevity in nematodes was conducted
The appearance of fluorescent material (e.g. lipofuscin) which is
by Ishii et al. in 2004. They treated wild-type C. elegans and the
believed to accumulate in the aging organism upon free-radical
mev-1 mutant that has increased superoxide production and is
induced damage was not affected by the ␣-tocopherol treatment
highly susceptible to oxidative damage, with Coenzyme Q10 and ␣-
tocopherol. Mev-1 encodes a succinate dehydrogenase cytochrome
b subunit. The mev-1 mutant has a defective electron transport in
2.3.2. Z. paravittiger
complex II and is therefore characterized by an increased super-
␣-Tocopherol was administered to male and female fruit
oxide production and is highly susceptible to oxidative damage.
flies of the species Z. paravittiger at concentrations ranging
A mutation in the succinate dehydrogenase cytochrome b causes
between 1 and 50 g/ml of medium. On the one hand, median
oxidative stress and aging in nematodes (
and maximum lifespan were significantly increased by ␣-
␣-Tocopherol (400 g/ml) significantly increased the lifespan of
tocopherol supplementation at concentrations of 1 g/ml (median
wild-type nematodes by 11% compared to untreated controls, yet
lifespan mean ± S.D. male/female in days, 37.4 ± 0.1/43.3 ± 0.2;
the lifespan of the mev-1 mutant remained unaltered. However,
maximum lifespan ± S.D., 83.3 ± 1.2/85.0 ± 1.0), 5 g/ml (median
they found that, unlike Coenzyme Q10, vitamin E failed to diminish
lifespan mean ± S.D. male/female in days, 46.9 ± 0.3/48.8 ± 0.5;
levels of superoxide anion production in both wild-type nematodes
maximum lifespan, 88.7 ± 1.2/99.3 ± 1.2) and 10 g/ml (median
and the mev-1 mutants (
lifespan mean ± S.D. male/female in days, 43.3 ± 0.7/46.4 ± 0.4;
maximum lifespan, 85.7 ± 1.5/94.3 ± 1.5) in comparison to the
2.2.3.5. Influence of ˛- and �-tocopherol. Zou et al. studied the
control (median lifespan mean ± S.D. male/female in days,
effects of 20 and 200 g/ml ␣-tocopherol or ␥-tocopherol on
35.1 ± 0.7/41.7 ± 0.1; maximum lifespan, 76.7 ± 1.2/82.0 ± 2.0). On
the sterilized C. elegans mutant, fem-1, in adulthood. Their
the other hand, ␣-tocopherol at concentrations of 25 and 50 g/ml
findings revealed opposing effects of the two tocopherols on life-
decreased median and maximum lifespan by 33.7–54% and
span. 200 g/ml ␣-tocopherol marginally and non-significantly
16.1–35.5%, respectively (median lifespan mean ± S.D. male/female
decreased mean lifespan and did not affect maximum lifespan. Con-
in days, 23.3 ± 0.2/23.8 ± 0.3 and 16.8 ± 0.1/19.2 ± 0.2; maximum
trastingly, ␥-tocopherol significantly extended mean lifespan to
lifespan, 64.3 ± 0.6/65.0 ± 1 and 50.3 ± 1.2/53.7 ± 2.3). Supplemen-
14.7 days (16.7%) at 20 g/ml and to 15.2 days (20.6%) at 200 g/ml
tation of ␣-tocopherol at 5 g/ml decreased TBARS levels in fly
compared to controls with a mean lifespan of 12.6 days. A combined
homogenates at all ages, measured at 7-day intervals during a
administration of ␣- and ␥-tocopherol in equal proportions at 20
period of 43 days starting from day 1. Furthermore, this concen-
and 200 g/ml did not significantly affect the lifespan of C. elegans.
tration of ␣-tocopherol ameliorated the age-dependent decrease
The authors hypothesize that this might be due to ␣-tocopherol
in the activity of the antioxidant enzymes catalase and peroxidase
negating the positive effects of ␥-tocopherol (
2.3.3. D. melanogaster
Miquel et al. fed ∼3000 adult male flies of the species D.
The available data relating to the effects of vitamin E on the
melanogaster a medium containing 0.06%, 0.12% or 0.25% ␣-
lifespan of flies originates from five studies, of which four were con-
tocopherol acetate. All three concentrations resulted in a significant
ducted in Drosophila melanogaster
increase in the flies' lifespan with the highest dose having the
largest effect, increasing the mean and maximum lifespan by 8–15%
lifespan in the fruit fly Zaprionus paravit-
and by 12% compared to controls, respectively (
tiger and examined the effects on the Mexican
Driver and Georgeou supplemented male flies with vitamin E
fruit fly, Anastrepha ludens. In addition, the effects of ␣-tocopherol
solubilized in ethyl acetate at concentrations of 3, 20, 100 and
on the housefly Musca domestica were tested in a study by
200 g/ml. The lifespan of control flies was 79 days (±6) whilst that
main results of these studies are summarized in
of flies administered 3, 20, and 200 g/ml vitamin E was 86 (±6),
92 (±6) and 76 days (±2), respectively. As such, only the 20 g/ml
dose of vitamin E significantly elevated lifespan compared to con-
2.3.1. M. domestica
trols. When given in conjunction with paraquat, a chemical that
Sohal et al. fed flies sucrose containing 0.5% and 2% ␣-
acts as a redox cycler and generates a superoxide anion radical
tocopherol. The lower dose of ␣-tocopherol did not alter mean
when metabolized in mitochondria, vitamin E at a concentration
lifespan, whereas the higher dose significantly decreased lifespan
of 200 g/ml was able to extend lifespan and abolish the detri-
by 5.4 days (26.1%) (supplementation 15.3 ± 4.2 days vs. control
mental effects of paraquat. When flies were administered vitamin
20.7 ± 5.9 days). The metabolic rate, measured as oxygen consump-
E at a concentration of 250 l/ml for the first 6 weeks of life, their
tion per time per weight of flies, was not affected by the treatment
lifespan significantly decreased by 12.6 days (13.6%; supplemen-
at either concentration. However, SOD activity was significantly
tation 11.6 ± 0.31 vs. control 13.4 ± 0.3). Furthermore, the authors
decreased in flies given 2% ␣-tocopherol at 6, 9 and 12 days of age
found that vitamin E supplementation altered the circadian rhythm
by between 20.3% and 24.9% compared to controls. Interestingly,
of flies, causing a significantly different pattern and length of their
the SOD activity of ␣-tocopherol-treated flies remained almost
diurnal activity cycle when the lighting conditions were changed
constant with age, while SOD activity in control flies increased
from a 12 h dark, 12 h light cycle to continuous light (
from days 3 to 9 then slightly decreased on day 12. This indi-
cates an age-related variation in SOD activity that is abolished
Zou et al. investigated potential sex-specific differences in
by ␣-tocopherol. The authors suggest that the administration of
responses to ␣- and/or ␥-tocopherol at concentrations of 20, 100 or
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
Vitamin E lifespan studies on flies in chronological order.
−11.8/17.9 d. #
−12.3/17 d.
−18.3/22.5 d. #
−26.3/28.3 d.
0.005, 0.05, 0.5,
–, data is not available; n.s., not significant; d, days; # data are given as median.
200 g/ml in canton-S wild-type flies of the species D. melanogaster.
At 100 g/ml, ␣-tocopherol modestly, yet significantly, increased
the mean lifespan of female flies by 1.7% but had no affect on males.
As vertebrates and mammals, rodents are far more complex
In contrast, the same dose of ␥-tocopherol as well as the two highest
organisms than the others included so far in this review. A number
doses of ␣- and ␥-tocopherol significantly reduced the mean life-
of studies have been conducted in rodents to elucidate the effects
span of female flies by 1.3 days (9.9%), 1.6 days (6.9%) and 2.9 days
of vitamin E on lifespan; one in rats and seven
(12.5%), respectively. The authors also reported that 100 g/ml of
␣- or ␥-tocopherol did not significantly affect the lifespan of flies of
the species A. ludens, with mean lifespan even slightly decreasing
The main results of these
eight studies are discussed below and summarized in
Bahadorani et al. examined the effects of ␣-tocopherol in male
SOD 1-deficient and wild-type D. melanogaster both under normal
and elevated oxygen partial pressures. Food intake was found to be
Lipman et al. investigated whether initiating antioxidant
unaltered by treatment compared to controls. ␣-Tocopherol was
supplementation during the late stage of a rodent's life is bene-
tested under normal oxygen conditions at concentrations of 0.005,
ficial in terms of longevity. Eighteen month old mice were given
0.05, 0.5, 5.0 and 25.0 IU/ml and was not found to significantly alter
acetate at a concentration of 500 g/g or a control
dl-␣-tocopherol
longevity. In addition to the lifespan extension in SOD 1-deficient
diet containing 30 g/g of vitamin E. They found that the vitamin
flies, ␣-tocopherol significantly increased the antioxidant capac-
E group showed an increase in bodyweight of 0.07 mg/kg day−1
ity of these animals, measured as trolox equivalents, by about 32%
(based on the average food intake of the mice). However, whilst
compared to the control (
serum levels of ␣-tocopherol were significantly higher in supple-
Taken together, the studies examining the effects of ␣-
mented mice, this did not lead to an improvement in longevity
tocopherol in flies are inconsistent and do not show a uniform effect
of vitamin E in these insects. Two studies reported a decrease in the
Selman et al. induced oxidative stress in C57BL/6 mice by
lifespan of flies supplemented with vitamin E
decreasing room temperature (7 ◦C). Four month old male and
whilst Zou et al. found no significant effect of
female mice were supplemented with ␣-tocopherol at either
vitamin E on the lifespan of A. ludens. Similarly,
22 g/g or 550 g/g. Supplementation had no effect on the rate
our own experiments showed no significant effects on
compared to controls but significantly increased both the median
wild-type Drosophila. Both Driver and Georgeou as well as Kakkar
and maximum lifespan in both sexes of mice. Median lifespan
et al. observed differing effects on lifespan according to the dose of
of ␣-tocopherol-supplemented mice was increased from 682 to
785 days (15.1%) compared to the control. Maximum lifespan,
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
Vitamin E lifespan studies on rodents in chronological order.
Rat (Wistar, male)
–, data is not available; n.s., not significant; #, data are given as median.
determined as the age of the oldest 10% in each cohort, was signif-
a higher tendency than the LAF1 strain. The mice were supple-
icantly increased from 834 to 945 days (5.7%) in the ␣-tocopherol
mented with 2500 g/g dl-␣-tocopherol starting at 5 weeks of
group compared to the control. The content of ␣-tocopherol in the
age. Maximum longevity in ␣-tocopherol-supplemented mice of
liver was significantly increased in supplemented animals but, in
both strains was not significantly altered compared to control ani-
accordance with the findings from below),
mals. However, there was a significant increase in mean lifespan
the levels of TBARS and the amount of oxidative damage to DNA
of mice administered dl-␣-tocopherol compared to the control,
were not affected by the higher amount of ␣-tocopherol. Addition-
mainly caused by a decreased death rate prior to 24 month of age.
ally, decreasing the temperature in this experimental setting could
␣-Tocopherol treatment also decreased the number of fatal tumors
also have induced incidents other than oxidative stress.
by 33.3% compared to the control. Furthermore, the predisposition
Macroarray analysis revealed that the expression of several
of the CH3H/He strain to develop more tumors was abolished in the
hepatic genes was elevated in ␣-tocopherol-treated animals,
supplemented subgroup. Furthermore, the content of lipofuscin in
including seven genes encoding cytochrome p450 enzymes and
the murine heart muscles was decreased by ␣-tocopherol by 28%
genes encoding enzymes associated with phase II detoxification.
in CH3H/He mice and by 36% in LAF1 mice compared to the respec-
However, Selman et al. found no significant increase in the expres-
tive controls, indicating an antioxidant effect of dl-␣-tocopherol
sion of genes encoding enzymes involved in cellular DNA repair or
in these mice at the high concentration of 2500 g/g (
antioxidant defense, such as SOD and catalase
Ledvina and Hodanova conducted experiments in female mice
Morley and Trainer evaluated the effects of supplementing with
of the C3 strain. ␣-Tocopherol acetate solubilized in 1 ml of ether
vitamin E straight after conception in Balb/c mice. Although serum
and 1 ml of ethanol was added to the diet at a concentration of
concentrations of vitamin E increased dose dependently with 20,
4.4 mg/g. The diet also contained 13.6 mg/g of sunflower oil. A sec-
400 and 4000 g/g of vitamin E supplementation, the median life-
ond experimental subgroup was fed on a diet containing sunflower
span values did not significantly change with different vitamin E
oil with an approximately equal iodine number of 127–136, at a
concentrations or differ from controls (804, 830 and 802 days for
concentration of 12.0 mg/g and solubilized in the same manner as
female offspring supplemented with 20, 400 and 4000 g of vita-
the ␣-tocopherol diet. Animals commenced these diets at 46 days
min E per g of food, respectively) (
of age. Food intake and body weight were significantly altered by
Another study involved continuously supplementing adult CD-
the treatments. Up to day 130, the mice ingested about 15.4 mg of
1/UCadiz mice with 5000 g/g dl-␣-tocopherol from 28 weeks of
␣-tocopherol acetate, dropping to 13.6 mg from day 131 onwards
age. The mice were monitored for their lifespan, neuromuscu-
due to decreased food consumption. Mean and maximum lifespan
lar and cognitive performance (tightrope and maze tests). The
were higher in the ␣-tocopherol supplemented animals compared
control diet contained 29 g/g of dl-␣-tocopherol. Median and
to control subgroups, yet this did not reach significance. Mean and
maximum lifespan of supplemented male mice increased signif-
maximum lifespan for the ␣-tocopherol supplemented subgroup,
icantly from 61 ± 4 to 85 ± 4 weeks (39.3%) and from 116 ± 4 to
the sunflower oil subgroup and the control were 704.2 ± 209 and
136 ± 4 weeks (17.2%), respectively. Median and maximum lifespan
1200 days, 620.4 ± 217.2 and 1078 days as well as 690.4 ± 168.1 and
of female mice also increased from 78 ± 4 to 88 ± 5 weeks (12.8%)
933 days, respectively (
and 148 ± 4 to 155 ± 4 weeks (6.9%), respectively, but these changes
A comparative approach on the effects of vitamin E on the life-
were not significant. Furthermore, ␣-tocopherol-supplemented
span of two strains of mice, the CH3H/He and LAF1 strains, was
male mice demonstrated significantly enhanced neuromuscular
taken by Blackett and Hall. These two strains differ in their pre-
and cognitive performance at 50 and 76 weeks of age. The levels
disposition for tumor development; the CH3H/He strain having
of protein carbonylation products and TBARS in the liver and brain
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
were significantly decreased in ␣-tocopherol-supplemented mice
highlights that vitamin E has inconsistent effects on longevity in
at 76 weeks of age. ␣-Tocopherol also improved the age-associated
single-celled organisms, rotifers, nematodes, flies and rodents.
reduction in activity of several protective enzymes, including nitric
In accordance with the studies in model organisms described
oxide synthase and SOD, in aging mice
herein, studies in humans have yielded similarly inconsistent
Hsieh and Lin supplemented MRL/lpr mice that suffer from an
results regarding the beneficial effects of vitamin E, with ran-
autoimmune disease and are susceptible to die within the first
domized clinical trials reporting positive, negative and no effect
6 month of age with all-rac-␣-tocopherol acetate at concentrations
depending on the outcome measured
of 250, 375, and 500 g/g. Control food contained 50 g/g all-rac-␣-
tocopherol acetate. ␣-Tocopherol supplementation had no effect on
Because of its properties as a lipid-soluble
feeding behavior and body weight of mice. They found that mice fed
antioxidant and membrane protector, vitamin E was assumed
250 g/g of all-rac-␣-tocopherol acetate lived significantly longer
to ameliorate or prevent arteriosclerosis and thereby promote
than mice supplemented with 500 g/g all-rac-␣-tocopherol
healthy aging. However, many well-designed randomized placebo-
acetate (213 ± 76 days vs. 157 ± 49 days, respectively), and also
controlled studies with sufficient power to detect clinical events,
longer than control animals (177 ± 45 days) and those fed 375 g/g
such as HOPE, GISSI and PPP, have demonstrated a null effect of
all-rac-␣-tocopherol acetate (162 ± 57 days). The higher doses of
vitamin E in the treatment or prevention of coronary heart dis-
all-rac-␣-tocopherol acetate led to significantly increased lev-
els of the vitamin E in the liver, kidney and plasma but did
However, two secondary prevention studies, CHAOS
not reduce the levels of TBARS in these tissues (
and SPACE, suggest that vitamin E supplementation may be benefi-
cial to coronary artery disease in certain sub-populations, although
these studies were small with relatively short follow up (
In the Women's Health Study on
vitamin E prevention of cardiovascular disease it was shown that
Porta et al. studied the effects of
dl-␣-tocopherol on the lifespan
in spite of slightly decreasing cardiovascular mortality, vitamin E
of male rats that were fed a diet enriched with 15% of either coconut
supplementation could not lower total mortality
oil (rich in saturated fatty acids), safflower oil (rich in unsaturated
A large meta-analysis by comes to the con-
fatty acids) or both oils combined from weaning.
clusion that vitamin E supplementation at high doses may elevate
was added at a concentration of either 20 g/g or 2000 g/g but,
the all-cause mortality. However, whilst the authors state that the
due to the different amounts of vitamin E in the oils, the final con-
high-dose trials were often carried out in patients with chronic
centration was increased by 0.7 g/g in the coconut oil diet, 5.2 g/g
diseases thereby making the analysis of the mortality data more
in the safflower oil diet and by 3.4 g/g in the oil combination
complicated, a more recent meta-analysis concludes that vitamin E
diet. Rats fed on the coconut oil-containing diet had significantly
supplementation including high doses does not affect overall mor-
reduced body weights soon after initiation of the diets. The mean
tality The inconsistent results from large and
and maximum lifespan of rats were not significantly affected by
smaller scale human studies investigating the potential benefits of
the different amounts of unsaturated fatty acids in the diets or by
vitamin E supplementation clearly warrant the studies in model
dl-␣-tocopherol supplementation. The 50% survival time (the day
organisms in which confounding factors can be much more easily
at which only 50% of a subgroup was still alive) was significantly
controlled. Yet this review highlights that the discrepant results
increased in the group receiving 2000 g/g of
dl-␣-tocopherol and
prevail in studies using model organisms.
safflower oil compared to all other experimental subgroups and the
These inconsistencies are likely to be attributable to a number
control (median lifespan in days ± S.E.M. 594.7 ± 35.6 vs. control
of factors. For example, there may be species–species differences
600.1 ± 30.6; mean lifespan ± S.E.M. 739.5 ± 34.1 vs. 724.7 ± 29.3;
and the complexity of the model organism may be an important
maximum lifespan ± S.E.M. 1013 ± 50.0 vs. 1037 ± 63.1). This bene-
determinant of the function of vitamin E. However, a number of
ficial effect was related to a delay in the onset, as well as a reduction
distinct studies in the same species, including rodents and humans,
in the incidence, of malignant neoplasms. The 50% survival time
have reported contradictory results. The dose of vitamin E used in
was also increased in rats fed on the combined oil diet supple-
the studies differs greatly and many studies have shown a ben-
mented with 20 g/g
dl-␣-tocopherol compared to the subgroups
eficial effect of vitamin E at lower concentrations with higher
supplemented with the same concentration of vitamin E but in dif-
doses exhibiting adverse effects on lifespan (
ferent oils This indicates that the amount of
unsaturated fatty acids in the oils may influence longevity.
Taken together, these studies in rodents show inconsistent
Unfortunately, when using non-mammalian model orga-
effects of vitamin E on longevity. Whilst three studies demonstrated
nisms it is difficult to categorize the amounts used according to
that vitamin E supplementation improves lifespan
high and low doses because of a lack of daily dose recommendations
making it difficult to deduce a dose-dependent effect of vitamin E
failed to show significant lifespan effects but did reveal a signifi-
on lifespan in flies and worms. Nevertheless, very high vitamin E
cant increase in the time at which 50% animals were still alive (
concentrations have been used in many human studies which may
Another study found divergent effects with different
account for the lack of beneficial effect observed
doses of vitamin E with a low concentration increasing lifespan
and a higher concentration reducing it Three
other studies found no significant effect of vitamin E administra-
3.1. Vitamin E as an antioxidant
tion on longevity (
Deducing a possibly life-prolonging effect of vitamin E because
of its antioxidant properties seems to have been a too rapid conclu-
sion. Recently we have shown that in rodents, both vitamin E in the
liver and biomarkers of oxidative stress increased with age, argu-
The potential benefits of vitamin E on health and lifespan have
ing against the hypothesis that a higher vitamin E status in older
been intensely investigated and this review article focuses on
organisms could attenuate oxidative damage (
the studies carried out in model organisms. This appraisal clearly
Additionally, although oxidative stress accompanies the aging
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
process it is not necessarily its underlying cause and promoting the
cells which could explain the different findings in vitamin E tri-
organism's stress resistance rather than simply lowering ROS levels
als on cardiovascular disease. On the one hand,
seems to be the better strategy to slow down aging (
describe that ␥-tocopherol, in contrast to ␣-tocopherol, decreased
Schulz et al. demonstrated that increased mitochondrial
the synthesis of pro-inflammatory prostaglandin E2 by inhibi-
ROS formation, as a consequence of reduced glucose availability,
ting the cyclooxigenase 2 (COX-2). On the other hand, Bernikovs
induces catalase activity and enhances oxidative stress resistance
et al. found ␣-tocopherol as inhibiting inflammation by regulat-
in C. elegans. Their results provide evidence for the mitochondrial
ing endothelial cell signals during leukocyte recruitment. At a
hormesis (or mitohormesis) theory of aging, which leads to an
10% ␥-tocopherol/␣-tocopherol ratio which is similar to the con-
increase in overall life expectancy. Indeed, they showed that treat-
centrations physiologically found in humans, ␥-tocopherol could
ment of nematodes with various antioxidants and vitamins had
abolish this anti-inflammatory effect (
the unexpected effect of decreasing lifespan
Dietary vitamin E sources contain mostly ␣- and ␥-tocopherols
Consistent with a counteracting role in ROS-induced hormesis,
with ␥-tocopherol being more present in US-American diets than
the antioxidant vitamin E prevented the health-promoting effects
in European diets In the body, tocopherol
of exercise in young men (It is well known
is transported from the liver via very low density lipoproteins
that exercise, in spite of promoting healthy aging and health in
(VLDLs). Incorporation into the lipoproteins happens via the ␣-
general, leads to increased ROS formation
tocopherol transfer protein (␣-TTP) which has a higher affinity to
In line with the "mitohormesis" concept (
␣-tocopherol albeit transferring around 10% ␥-tocopherol
exercise-induced mitochondrial ROS led to induction of endoge-
Therefore ␣-tocopherol supple-
nous ROS defense. However, with vitamin E supplementation this
mentation is likely to decrease ␥-tocopherol bioavailability and
up-regulation of the anti-oxidative resistance was blocked (
the extent up to which the ␣-/␥-tocopherol rates change also
Due to its antioxidant properties, vitamin E may abolish
depends on the type of vitamin E that is consumed with the
this increase in stress resistance (such as the induction of endoge-
nous antioxidant enzymes), accounting for the reduced lifespan
As a side effect of vitamin E supplementation, increased bleed-
observed in many of the studies in model organisms described
ing has been reported. Farley et al. gave a possible explanation for
this phenomenon by showing that menaquinone (vitamin K) lev-
Although in mice the antioxidant properties of vitamin E were
els are decreased by high vitamin E tissue concentrations
shown to be independent of transcriptional events (
In the context of lifespan extension it is especially
vitamin E seems to induce Nrf2 Nrf2 in mammals
interesting to note that vitamin K can extend lifespan in C. ele-
(or its homologue SKN-1 in C. elegans) is a well known longevity-
gans (A putatively negative effect on longevity
promoting transcription factor with its target
with vitamin E supplementation could therefore be due in part
genes encoding for antioxidant proteins and molecules that are
to vitamin E ousting the lifespan-prolonging vitamin K from the
involved in xenobiotic metabolism (Vitamin E
could induce Nrf2 directly through increased Nrf2 expression and
Unfortunately, in most studies the vitamin E levels in the model
translocation to the nucleus as suggested by (or
organisms were not studied and so the amount of the supple-
more indirectly by protecting the cell from Nrf2 inhibitors. This
mented vitamin E which was bioavailable cannot be given. This
mechanism was elucidated in another study with an asthma model
is especially relevant in the case of tocopherol acetate supplemen-
in which allergens caused inflammation and suppressed Nrf2 in
tation because the acetate needs to be hydrolyzed in vivo to exert
alveolar macrophages from asthmatics. Administering tocopherol
anti-oxidative effects. Therefore, an analysis of vitamin E tissue
to these patients could attenuate the allergen-induced Nrf2 inhibi-
levels should be conducted in future studies.
tion (It is also possible that vitamin E activates
other transcription or longevity factors. The deacetylase sirt1 was
3.3. Adverse effects of vitamin E by induction of drug metabolism
shown to have a beneficial effect on aging Sirt1
is a regulator of FoxO transcription factors
␣-Tocopherol with ␥-tocopherol and ␣-tocopherol with vita-
are the mammalian homologues of daf-16, a well known longevity
min K interactions do not seem to be the only type of interactions
gene in C. elegans (While there are still very few
for vitamin E. Similar to many prescription drugs, all forms of vita-
available data on the effect of vitamin E on sirtuins or FoxOs, a vita-
min E are metabolized by Phase I cytochrome P 450 (CYP) enzymes
min E analog, ␣-tocopheryl succinate was shown to activate FoxO1
in the liver and seem to be conjugated
In the light of a possible effect of vitamin E on
by Phase II enzymes before Phase III excretion
lifespan and the emerging evidence of its influence on gene expres-
In mice, ␣-tocopherol was shown to induce Phase I CYP
sion further studies on the interplay of vitamin E and
expression (Contrarily, another report states that
its cellular targets are needed to understand how this vitamin acts
there is no up-regulation of CYP enzymes in vivo and no increased
in the organism.
mRNA expression of known CYP drug metabolizers in vitro after
administration of ␣-tocopherol In a fur-
3.2. Opposing functions of the tocopherol isoforms and
ther study, varying results were found with regard to vitamin E
interaction with vitamin K
induced CYP up- or down-regulation (How-
ever, it seems that Phase II conjugation enzymes
A further possible reason for the different outcomes observed
and Phase III hepatic transporters ABCB1b and ABCG2
in the studies in this review may be due to the opposing reg-
are induced upon vitamin E supplementation. Considering
ulatory functions of the different vitamin E isoforms. Indeed, as
the finding that vitamin E is eliminated from the organism like
described above, Zou et al. reported that whilst low dose ␣-
xenobiotics, it seems possible that it decreases the plasma levels
tocopherol had a positive effect on the lifespan of flies of the species
of co-administered drugs by inducing their enzymatic degradation
D. melanogaster, the same dose of ␥-tocopherol had the opposing
(This concern is highly relevant considering
effect by reducing lifespan In fact, other stud-
the fact that in many studies the participating individuals suffer
ies have also reported contradictory effects of the two isoforms of
from diseases and are accordingly on medication (
tocopherols in vitamin E. Some of these studies showed that toco-
pherols can act as pro- or anti-inflammatory agents in endothelial
Under such circumstances vitamin E could interfere
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
with the treatment of these diseases and thereby negatively affect
Epstein, J., Gershon, D., 1972. Studies on ageing in nematodes IV. The effect of
the lifespan of the patients.
anti-oxidants on cellular damage and life span. Mechanisms of Ageing and
Development 1, 257–264.
Farley, S.M., Leonard, S.W., Labut, E.M., Raines, H.F., Card, D.J., Harrington,
D.J., Mustacich, D.J., Traber, M.G., 2012. Vitamin E decreases extra-hepatic
menaquinone-4 concentrations in rats fed menadione or phylloquinone. Molec-
ular Nutrition & Food Research 56, 912–922.
Taken together, these studies in model organisms described
Feng, Z., Liu, Z., Li, X., Jia, H., Sun, L., Tian, C., Jia, L., Liu, J., 2010. Alpha-tocopherol is an
herein together with published studies in humans have failed to
effective phase II enzyme inducer: protective effects on acrolein-induced oxida-
provide compelling evidence for either a beneficial or a detrimental
tive stress and mitochondrial dysfunction in human retinal pigment epithelial
cells. The Journal of Nutritional Biochemistry 21, 1222–1231.
effect on vitamin E on lifespan. However, the interactions of vitamin
Harrington, L.A., Harley, C.B., 1988. Effect of vitamin E on lifespan and reproduction
E with other vitamins and xenobiotics, the possible counteraction
in Caenorhabditis elegans. Mechanisms of Ageing and Development 43, 71–78.
against beneficial, ROS-induced hormesis by the antioxidant and
Hsieh, C.C., Lin, B.F., 2005. Opposite effects of low and high dose supplementation
of vitamin E on survival of MRL/lpr mice. Nutrition 21, 940–948.
the question whether administration of vitamin E decreases oxida-
Hundhausen, C., Bosch-Saadatmandi, C., Augustin, K., Blank, R., Wolffram, S., Rim-
tive damage in healthy humans are arguments against high dose
bach, G., 2005. Effect of vitamin E and polyphenols on ochratoxin A-induced
vitamin E supplementation until the scarce knowledge about the
cytotoxicity in liver (HepG2) cells. Journal of Plant Physiology 162, 818–822.
Hunt, P.R., Son, T.G., Wilson, M.A., Yu, Q.S., Wood, W.H., Zhang, Y., Becker, K.G., Greig,
mechanisms of the action of vitamin E in general and of its distinct
N.H., Mattson, M.P., Camandola, S., Wolkow, C.A., 2011. Extension of lifespan in
isoforms has been further elucidated. Moreover, considering the
C. elegans by naphthoquinones that act through stress hormesis mechanisms.
similarly contradicting data in model organisms and human trials,
PloS One 6, e21922.
Ikeda, S., Uchida, T., Ichikawa, T., Watanabe, T., Uekaji, Y., Nakata, D., Terao, K.,
it is tempting to speculate that studies in model organisms could
Yano, T., 2010. Complexation of tocotrienol with gamma-cyclodextrin enhances
serve as anticipation platforms for human trials with vitamin E.
intestinal absorption of tocotrienol in rats. Bioscience, Biotechnology, and Bio-
chemistry 74, 1452–1457.
Ishii, N., Fujii, M., Hartman, P.S., Tsuda, M., Yasuda, K., Senoo-Matsuda, N., Yanase,
S., Ayusawa, D., Suzuki, K., 1998. A mutation in succinate dehydrogenase
cytochrome b causes oxidative stress and ageing in nematodes. Nature 394,
We are grateful to the DFG cluster of excellence "Inflammation
Ishii, N., Senoo-Matsuda, N., Miyake, K., Yasuda, K., Ishii, T., Hartman, P.S., Furukawa,
at Interfaces" for financial support and we would like to thank Dr.
S., 2004. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative
Mario Hasler for his valuable comments.
stress. Mechanisms of Ageing and Development 125, 41–46.
Jiang, Q., Elson-Schwab, I., Courtemanche, C., Ames, B.N., 2000. Gamma-tocopherol
and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygen-
ase activity in macrophages and epithelial cells. Proceedings of the National
Academy of Sciences of the United States of America 97, 11494–11499.
Abner, E.L., Schmitt, F.A., Mendiondo, M.S., Marcum, J.L., Kryscio, R.J., 2011. Vitamin
Kahn, M., Enesco, H.E., 1981. Effect of alpha-tocopherol on the lifespan of Turbatrix
E and all-cause mortality: a meta-analysis. Current Aging Science 4, 158–170.
aceti. Age 4, 109–115.
Adachi, H., Ishii, N., 2000. Effects of tocotrienols on life span and protein carbonyla-
Kakkar, R., Bains, J.S., Sharma, S.P., 1996. Effect of vitamin E on life span, malon-
tion in Caenorhabditis elegans. The Journals of Gerontology Series A: Biological
dialdehyde content and antioxidant enzymes in aging Zaprionus paravittiger.
Sciences and Medical Sciences 55, B280–B285.
Gerontology 42, 312–321.
Azzi, A., 2007. Molecular mechanism of alpha-tocopherol action. Free Radical Biology
Kamal-Eldin, A., Appelqvist, L.A., 1996. The chemistry and antioxidant properties of
& Medicine 43, 16–21.
tocopherols and tocotrienols. Lipids 31, 671–701.
Bahadorani, S., Bahadorani, P., Phillips, J.P., Hilliker, A.J., 2008. The effects of vitamin
Kashima, N., Fujikura, Y., Komura, T., Fujiwara, S., Sakamoto, M., Terao, K., Nishikawa,
supplementation on Drosophila life span under normoxia and under oxidative
Y., 2012. Development of a method for oral administration of hydrophobic sub-
stress. Journals of Gerontology Series A: Biological Sciences and Medical Sciences
stances to Caenorhabditis elegans: pro-longevity effects of oral supplementation
63, 35–42.
with lipid-soluble antioxidants. Biogerontology 13, 337–344.
Bayram, B., Nikolai, S., Huebbe, P., Ozcelik, B., Grimm, S., Grune, T., Frank, J.,
Kluth, D., Landes, N., Pfluger, P., Muller-Schmehl, K., Weiss, K., Bumke-Vogt, C., Ris-
Rimbach, G., 2012. Biomarkers of oxidative stress, antioxidant defence and
tow, M., Brigelius-Flohe, R., 2005. Modulation of Cyp3a11 mRNA expression by
inflammation are altered in the senescence-accelerated mouse prone 8. Age,
alpha-tocopherol but not gamma-tocotrienol in mice. Free Radical Biology &
(Epub ahead of print).
Medicine 38, 507–514.
Berdnikovs, S., Abdala-Valencia, H., McCary, C., Somand, M., Cole, R., Garcia, A., Bryce,
Kritharides, L., Stocker, R., 2002. The use of antioxidant supplements in coronary
P., Cook-Mills, J.M., 2009. Isoforms of vitamin E have opposing immunoregula-
heart disease. Atherosclerosis 164, 211–219.
tory functions during inflammation by regulating leukocyte recruitment. Journal
Lam, Y.T., Stocker, R., Dawes, I.W., 2010. The lipophilic antioxidants alpha-tocopherol
of Immunology 182, 4395–4405.
and coenzyme Q10 reduce the replicative lifespan of Saccharomyces cerevisiae.
Blackett, A.D., Hall, D.A., 1981. Vitamin E – its significance in mouse ageing. Age and
Free Radical Biology & Medicine 49, 237–244.
Ageing 10, 191–195.
Ledvina, M., Hodanova, M., 1980. The effect of simultaneous administration of
Boaz, M., Smetana, S., Weinstein, T., Matas, Z., Gafter, U., Iaina, A., Knecht, A., Weiss-
tocopherol and sunflower oil on the lifespan of female mice. Experimental
garten, Y., Brunner, D., Fainaru, M., Green, M.S., 2000. Secondary prevention
Gerontology 15, 67–71.
with antioxidants of cardiovascular disease in endstage renal disease (SPACE):
Lee, I.M., Cook, N.R., Gaziano, J.M., Gordon, D., Ridker, P.M., Manson, J.E., Hen-
randomised placebo-controlled trial. Lancet 356, 1213–1218.
nekens, C.H., Buring, J.E., 2005. Vitamin E in the primary prevention of
Brigelius-Flohe, R., 2007. Adverse effects of vitamin E by induction of drug
cardiovascular disease and cancer: the Women's Health Study: a randomized
metabolism. Genes & Nutrition 2, 249–256.
controlled trial. JAMA: The Journal of the American Medical Association 294,
Brunet, A., Sweeney, L.B., Sturgill, J.F., Chua, K.F., Greer, P.L., Lin, Y., Tran, H., Ross, S.E.,
Mostoslavsky, R., Cohen, H.Y., Hu, L.S., Cheng, H.L., Jedrychowski, M.P., Gygi, S.P.,
Li, G., Lee, M.J., Liu, A.B., Yang, Z., Lin, Y., Shih, W.J., Yang, C.S., 2012. The antioxidant
Sinclair, D.A., Alt, F.W., Greenberg, M.E., 2004. Stress-dependent regulation of
and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice.
FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015.
Free Radical Biology & Medicine 52, 1151–1158.
Clarke, M.W., Burnett, J.R., Croft, K.D., 2008. Vitamin E in human health and disease.
Lin, K., Dorman, J.B., Rodan, A., Kenyon, C., 1997. DAF-16: an HNF-3/forkhead fam-
Critical Reviews in Clinical Laboratory Sciences 45, 417–450.
ily member that can function to double the life-span of Caenorhabditis elegans.
Copple, I.M., Goldring, C.E., Kitteringham, N.R., Park, B.K., 2008. The Nrf2–Keap1
Science 278, 1319–1322.
defence pathway: role in protection against drug-induced toxicity. Toxicology
Lipman, R.D., Bronson, R.T., Wu, D., Smith, D.E., Prior, R., Cao, G., Han, S.N., Martin, K.R.,
246, 24–33.
Meydani, S.N., Meydani, M., 1998. Disease incidence and longevity are unaltered
Corbi, G., Conti, V., Scapagnini, G., Filippelli, A., Ferrara, N., 2012. Role of sirtuins,
by dietary antioxidant supplementation initiated during middle age in C57BL/6
calorie restriction and physical activity in aging. Frontiers in Bioscience (Elite
mice. Mechanisms of Ageing and Development 103, 269–284.
Edition) 4, 768–778.
Miller 3rd, E.R., Pastor-Barriuso, R., Dalal, D., Riemersma, R.A., Appel, L.J., Guallar,
Dietrich, M., Traber, M.G., Jacques, P.F., Cross, C.E., Hu, Y., Block, G., 2006. Does
E., 2005. Meta-analysis: high-dosage vitamin E supplementation may increase
gamma-tocopherol play a role in the primary prevention of heart disease and
all-cause mortality. Annals of Internal Medicine 142, 37–46.
cancer? A review. Journal of the American College of Nutrition 25, 292–299.
Mindlen, F., Nordaby, R., Ruiz, M., Zavala, A., Guzman, L., Martinez, F., Diaz, R.R.,
Driver, C., Georgeou, A., 2003. Variable effects of vitamin E on Drosophila longevity.
Mackey, C., Marino, M., Romero, G., Zapata, G., Cuneo, C., Kawamura, T., Coelho,
Biogerontology 4, 91–95.
O., Massayochi, O., Braga, J., Labrunie, A., Bodanese, L., Manenti, E., Vitola,
Dworski, R., Han, W., Blackwell, T.S., Hoskins, A., Freeman, M.L., 2011. Vitamin E
D., Nicolau, J., Kennedy, J.W., et al., 1996. The HOPE (Heart Outcomes Pre-
prevents NRF2 suppression by allergens in asthmatic alveolar macrophages in
vention Evaluation) Study: the design of a large, simple randomized trial
vivo. Free Radical Biology & Medicine 51, 516–521.
of an angiotensin converting enzyme inhibitor (ramipril) and vitamin E in
Enesco, H.E., Verdone-Smith, C., 1980. Alpha-tocopherol increases lifespan in the
patients at high risk of cardiovascular events. Canadian Journal of Cardiology 12,
rotifer Philodina. Experimental Gerontology 15, 335–338.
I.M.A. Ernst et al. / Ageing Research Reviews 12 (2013) 365–375
Minogue, P.J., Thomas, J.N., 2004. An alpha-tocopherol dose response study in
Sontag, T.J., Parker, R.S., 2002. Cytochrome P450 omega-hydroxylase pathway of
Paramecium tetraurelia. Mechanisms of Ageing and Development 125, 21–30.
tocopherol catabolism. Novel mechanism of regulation of vitamin E status. The
Miquel, J., Binnard, R., Howard, W.H., 1973. Effects of dl-alpha-tocopherol in life
Journal of Biological Chemistry 277, 25290–25296.
span of Drosophila melanogaster. Gerontologist 13, pp. 37–37.
Stephens, N.G., Parsons, A., Schofield, P.M., Kelly, F., Cheeseman, K., Mitchinson, M.J.,
Morley, A.A., Trainer, K.J., 2001. Lack of an effect of vitamin E on lifespan of mice.
Brown, M.J., 1996. Randomised controlled trial of vitamin E in patients with
Biogerontology 2, 109–112.
coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 347,
Mustacich, D.J., Bruno, R.S., Traber, M.G., 2007. Vitamin E. In: Litwack, G. (Ed.), Vita-
min E: Vitamins and Hormones Advances in Research and Applications. , pp.
Thomas, J., Nyberg, D., 1988. Vitamin E supplementation and intense selection
increase clonal life span in Paramecium tetraurelia. Experimental Gerontology
Navarro, A., Gomez, C., Sanchez-Pino, M.J., Gonzalez, H., Bandez, M.J., Boveris, A.D.,
23, 501–512.
Boveris, A., 2005. Vitamin E at high doses improves survival, neurological per-
Traber, M.G., Atkinson, J., 2007. Vitamin E, antioxidant and nothing more. Free Rad-
formance, and brain mitochondrial function in aging male mice. American
ical Biology & Medicine 43, 4–15.
Journal of Physiology: Regulatory Integrative and Comparative Physiology 289,
Traber, M.G., Kayden, H.J., 1989. Preferential incorporation of alpha-tocopherol vs
gamma-tocopherol in human lipoproteins. The American Journal of Clinical
Porta, E.A., Joun, N.S., Nitta, R.T., 1980. Effects of the type of dietary fat at two levels of
Nutrition 49, 517–526.
vitamin E in Wistar male rats during development and aging. 1. Lifespan, serum
Traber, M.G., Labut, E.M., Leonard, S.W., Lebold, K.M., 2011. Alpha-tocopherol injec-
biochemical parameters and pathological changes. Mechanisms of Ageing and
tions in rats up-regulate hepatic ABC transporters, but not cytochrome P450
Development 13, 1–39.
enzymes. Free Radical Biology & Medicine 51, 2031–2040.
Powers, S.K., Jackson, M.J., 2008. Exercise-induced oxidative stress: cellular mech-
Tullet, J.M., Hertweck, M., An, J.H., Baker, J., Hwang, J.Y., Liu, S., Oliveira, R.P., Baumeis-
anisms and impact on muscle force production. Physiological Reviews 88,
ter, R., Blackwell, T.K., 2008. Direct inhibition of the longevity-promoting factor
SKN-1 by insulin-like signaling in C. elegans. Cell 132, 1025–1038.
Rimbach, G., Minihane, A.M., Majewicz, J., Fischer, A., Pallauf, J., Virgli, F., Weinberg,
Valagussa, F., Franzosi, M.G., Geraci, E., Mininni, N., Nicolosi, G.L., Santini, M., Tavazzi,
P.D., 2002. Regulation of cell signalling by vitamin E. The Proceedings of the
L., Vecchio, C., Marchioli, R., Bomba, E., Chieffo, C., Maggioni, A.P., Schweiger, C.,
Nutrition Society 61, 415–425.
Tognoni, G., Barzi, F., Flamminio, A.V., Marfisi, R.M., Olivieri, M., Pera, C., Polidoro,
Rimbach, G., Moehring, J., Huebbe, P., Lodge, J.K., 2010. Gene-regulatory activity of
A., Santoro, E., Zama, R., Pagliaro, L., Correale, E., Del Favero, A., Loi, U., Marubini,
alpha-tocopherol. Molecules 15, 1746–1761.
E., Campolo, L., Casari, A., Di Minno, G., Donati, M.B., Galli, M., Gattone, M., Garat-
Ristow, M., Zarse, K., Oberbach, A., Kloting, N., Birringer, M., Kiehntopf, M., Stumvoll,
tini, S., Mancini, M., Marino, P., Santoro, G.M., Scardulla, C., Specchia, G., Cericola,
M., Kahn, C.R., Bluher, M., 2009. Antioxidants prevent health-promoting effects
A., Di Gregorio, D., Di Mascio, R., Levantesi, G., Mantini, L., Mastrogiuseppe, G.,
of physical exercise in humans. Proceedings of the National Academy of Sciences
Tucci, C., Mocarelli, P., Baldinelli, R., Ceriotti, F., Colonna, A., Cortese, C., For-
of the United States of America 106, 8665–8670.
tunato, G., Franzini, C., Gonano, F., Graziani, M.S., Investigators, G.I.-P., 1999.
Sawada, M., Enesco, H.E., 1984. Vitamin E extends lifespan in the short-lived rotifer
Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E
Asplanchna brightwelli. Experimental Gerontology 19, 179–183.
after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354,
Schneider, S.A., Schrader, C., Wagner, A.E., Boesch-Saadatmandi, C., Liebig, J., Rim-
bach, G., Roeder, T., 2011. Stress resistance and longevity are not directly linked
Valis, K., Prochazka, L., Boura, E., Chladova, J., Obsil, T., Rohlena, J., Truksa, J., Dong,
to levels of enzymatic antioxidants in the ponerine ant Harpegnathos saltator.
L.F., Ralph, S.J., Neuzil, J., 2011. Hippo/Mst1 stimulates transcription of the
PloS One 6, e14601.
proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Research
Schulz, T.J., Zarse, K., Voigt, A., Urban, N., Birringer, M., Ristow, M., 2007.
71, 946–954.
Glucose restriction extends Caenorhabditis elegans life span by inducing mito-
Wagner, K.H., Kamal-Eldin, A., Elmadfa, I., 2004. Gamma-tocopherol – an underes-
chondrial respiration and increasing oxidative stress. Cell Metabolism 6,
timated vitamin? Annals of Nutrition & Metabolism 48, 169–188.
Wolf, G., 2006. How an increased intake of alpha-tocopherol can suppress the
Selman, C., McLaren, J.S., Mayer, C., Duncan, J.S., Collins, A.R., Duthie, G.G., Redman,
bioavailability of gamma-tocopherol. Nutrition Reviews 64, 295–299.
P., Speakman, J.R., 2008. Lifelong alpha-tocopherol supplementation increases
Zou, S., Sinclair, J., Wilson, M.A., Carey, J.R., Liedo, P., Oropeza, A., Kalra, A., de Cabo,
the median life span of C57BL/6 mice in the cold but has only minor effects on
R., Ingram, D.K., Longo, D.L., Wolkow, C.A., 2007. Comparative approaches to
oxidative damage. Rejuvenation Research 11, 83–95.
facilitate the discovery of prolongevity interventions: effects of tocopherols on
Sohal, R.S., Allen, R.G., Farmer, K.J., Newton, R.K., Toy, P.L., 1985. Effects of exogenous
lifespan of three invertebrate species. Mechanisms of Ageing and Development
antioxidants on the levels of endogenous antioxidants, lipid-soluble fluorescent
128, 222–226.
material and lifespan in the housefly, Musca domestica. Mechanisms of Ageing
Zuckerman, B.M., Geist, M.A., 1983. Effects of vitamin E on the nematode Caenorhab-
and Development 31, 329–336.
ditis elegans. Age 6, 1–4.
Source: http://age.bjmu.edu.cn/old/progress/pbl/25.pdf
POWERbreathe Guide for Indoor Rowers Fletcher Sport Science In collaboration with Professor Alison McConnell – Centre for Sports Medicine and Human Performance, Brunel University 2. The importance of the breathing muscles to rowing 3. How do you train the inspiratory muscles 4. Using POWERbreathe as part of the warm up
Small Intestinal Bacterial Overgrowth Irritable Bowel Syndrome and "Gas": Bloating, Distention, and Flatulence Adapted and expanded from the book and including the latest science-based research on diagnosis and treatment Draft 2 (February 27 , 2014) This educational offering is provided to inform patients and facilitate a shared and participatory patient-doctor



