Hypothalamic proopiomelanocortin neurons are glucose responsive and express katp channels
Hypothalamic Proopiomelanocortin Neurons Are
Glucose Responsive and Express KATP ChannelsNurhadi Ibrahim
Department of Physiology and Pharmacology
Martha A. Bosch
Department of Physiology and Pharmacology
James L. Smart
George Fox University,
[email protected]
Jian Qiu
Department of Physiology and Pharmacology
Marcelo Rubinstein
Instituto de Investigaciones en Ingenierıa Genetica y Biologia Molecular
See next page for additional authors
Follow this and additional works at:
Part of the , and the
Recommended CitationPreviously published in Endocrinology, 2003, 144(4), pp. 1331–1340 http://press.endocrine.org/doi/full/10.1210/en.2002-221033
This Article is brought to you for free and open access by the Department of Biology and Chemistry at Digital Commons @ George Fox University. Ithas been accepted for inclusion in Faculty Publications - Department of Biology and Chemistry by an authorized administrator of Digital Commons @George Fox University. For more information, please contact .
Authors
Nurhadi Ibrahim, Martha A. Bosch, James L. Smart, Jian Qiu, Marcelo Rubinstein, Oline K. RØnnekleiv,
Malcolm J. Low, and Martin J. Kelly
This article is available at Digital Commons @ George Fox University:
Printed in U.S.A.
Copyright 2003 by The Endocrine Society
Hypothalamic Proopiomelanocortin Neurons Are
Glucose Responsive and Express K
NURHADI IBRAHIM, MARTHA A. BOSCH, JAMES L. SMART, JIAN QIU, MARCELO RUBINSTEIN,OLINE K. RØNNEKLEIV, MALCOLM J. LOW, AND MARTIN J. KELLY
Department of Physiology and Pharmacology (N.I., M.A.B., J.Q., O.K.R., M.J.K.), The Vollum Institute (J.L.S., M.J.L.), andDepartment of Behavioral Neuroscience (M.J.L.), Oregon National Primate Research Center (O.K.R.), Oregon Health &Science University, Portland, Oregon 97239-3098; and Instituto de Investigaciones en Ingenierı´a Gene´tica y Biologı´aMolecular (M.R.), Consejo Nacional de Investigaciones Cientı´ficas y Te´cnicas, and Department of Biology, School ofSciences, University of Buenos Aires, Buenos Aires, Argentina
Hypothalamic proopiomelanocortin (POMC) neurons are crit-
ceptor agonist baclofen (40
M) caused an outward current
ical for controlling homeostatic functions in the mammal. We
(21.6 ⴞ
4.0 pA) that reversed at EKⴙ
in these same neurons. The
used a transgenic mouse model in which the POMC neurons
ATP-sensitive potassium channel opener diazoxide also in-
were labeled with enhanced green fluorescent protein to per-
duced an outward Kⴙ
current (maximum of 18.7 ⴞ
2.2 pA) in
form visualized, whole-cell patch recordings from prepuber-
the majority (92%) of POMC neurons with an EC
tal female hypothalamic slices. The mouse POMC-enhanced
response to diazoxide was blocked by the sulfonylurea tolbu-
green fluorescent protein neurons expressed the same endog-
tamide, indicating that the POMC neurons express both Kir6.2
enous conductances (a transient outward Kⴙ
current and a
and sulfonylurea receptor 1 channel subunits, which was ver-
hyperpolarization-activated, cation current) that have been
ified using single cell RT-PCR. This pharmacological and mo-
described for guinea pig POMC neurons. In addition, the se-
lecular profile suggested that POMC neurons might be sen-
lective
-opioid receptor agonist DAMGO induced an outward
sitive to metabolic inhibition, and indeed, we found that their
current (maximum of 12.8 ⴞ
1.2 pA), which reversed at Kⴙ
firing rate varied with changes in glucose concentrations.
equilibrium potential (E
Therefore, it appears that POMC neurons may function as
Kⴙ
), in the majority (85%) of POMC
neurons with an EC
an integrator of metabolic cues and synaptic input for con-
M. This response was blocked by
the opioid receptor antagonist naloxone with an inhibition
trolling homeostasis in the mammal.
constant of 3.1 nM. In addition, the ␥
-aminobutyric acid re-
THEMEDIOBASALHYPOTHALAMUS(MBH)contains is G␣i/o-coupled to either the activation of an inwardly-
the largest concentration of proopiomelanocortin
rectifying K⫹ channel (Kir3.1–3.4, GIRKs), the inhibition of
(POMC) neurons in the central nervous system (1–3). These
adenylyl cyclase, or the inhibition of Ca2⫹ channels (15). The
POMC neurons project to regions throughout the forebrain
GIRK-type subfamily of inwardly rectifying K⫹ channels
(3). Two of the major posttranslational products of MBH
comprises four different channel subtypes, all of which are
POMC neurons, -endorphin (-END) and ␣-MSH, have
expressed in the rat hypothalamus (16). Hypothalamic neu-
been associated with many physiological functions including
rons are inhibited through a -opioid receptor that is coupled
reproduction, metabolic homeostasis, stress responses, and
to GIRKs (7, 12, 17–19).
natural reward (4 – 6). At the cellular level, the opioid peptide
ATP-sensitive potassium (K
-END has been shown to postsynaptically modulate the
ATP) channels are another
excitability of local ␥-aminobutyric acid (GABA) and dopa-
member of the inwardly-rectifying K⫹ channel family (20).
mine neurons (7–9). In addition, GnRH, oxytocin, and
They are heteromultimeric complexes of sulfonylurea re-
vasopressin neurosecretory (8) neurons are inhibited by
ceptors (SUR; the regulatory subunit) and inwardly rec-
-END (10–13). The other putative neurotransmitter of
tifying K⫹ channel (Kir6.1– 6.2) subunits (21, 22). These
POMC neurons, ␣MSH, has been shown to modulate syn-
channel complexes couple membrane excitability to cel-
aptic input to paraventricular neurons that are thought to be
lular metabolism by directly sensing and integrating in-
involved in the regulation of metabolic homeostasis (14).
tracellular changes in the concentration of nucleotides
The opioid peptide -END modulates target neurons
(23). The Kir6.2 plus SUR1 channel complex is activated by
through a G protein-coupled receptor. The -opioid receptor
diazoxide and by metabolic inhibition and is blocked withhigh affinity by sulfonylureas such as glibenclamide and
Abbreviations: aCSF, Artificial cerebrospinal fluid; DAMGO, d-Ala2,
tolbutamide (23). Sulfonylurea binding and electrophys-
N-Me-Phe4, Gly-ol5-enkephalin; DEPC, diethylpyrocarbonate; EGFP,
iological studies have characterized neuronal KATP chan-
enhanced green fluorescent protein; EK⫹, equilibrium potential; -END,
nels in a variety of neurons. For example, Kir6.2 is widely
-endorphin; GABA, ␥-aminobutyric acid; GIRK, G protein-coupled inwardly rectifying K⫹ channel; HBSS, Hanks' balanced salt
distributed in rat brain and is present in neurons express-
solution; IA, a transient outward K⫹ current; Ih, hyperpolarization-
ing tyrosine hydroxylase, neuropeptide Y, and glutamic
activated, cation current; I/V, current/voltage; KATP, ATP-sensitive
acid decarboxylase (24).
potassium; Kir, inwardly rectifying K⫹ channels; MBH, mediobasal
In the rat hypothalamus, the adipocyte hormone leptin
hypothalamus; POMC, proopiomelanocortin; RT, reverse transcriptase;SUR, sulfonylurea receptors; TTX, tetrodotoxin.
hyperpolarizes glucose-responsive, ventromedial nucleus
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Endocrinology, April 2003, 144(4):1331–1340
Ibrahim
et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
neurons via activation of a K⫹ current that is blocked by
imaging to visualize neurons for whole-cell patch clamp recording.
tolbutamide (25). Also, insulin hyperpolarizes glucose-
Microelectrodes (resistances 3– 6 M⍀) were fabricated from borosilicate
responsive neurons via a tolbutamide-sensitive K⫹ current
glass pipettes (1.5 mm OD) and filled with an internal solution (pH 7.30)containing the following constituents, in mm: K-gluconate, 128; NaCl, 10;
(26). More recent studies have shown that these glucose-
MgCl2, 2; EGTA, 11; HEPES, 10; ATP, 1; GTP, 0.25. Standard whole-cell
responsive neurons express Kir6.2 and SUR1 transcripts (27),
voltage clamp procedures were followed using an Axopatch 200A am-
which renders them diazoxide and tolbutamide sensitive.
plifier (2-kHz lowpass filter, Axon Instruments, Union City, CA) as
Previous findings suggest that the G protein-coupled recep-
previously described (32). Signals were digitized with a Digidata 1200,and analyzed using pClamp 7.0 software (Axon Instruments). The liquid
tors such as the dopamine D2, GABAB, and somatostatin SST5
junctional potential of ⫺10 mV was corrected in the data analysis.
receptors are coupled to both GIRKs and Kir6.2 in pancreatic
Current and voltage traces were also recorded on a analog chart recorder
-cells and substantia nigra neurons (28, 29). However, the
(Gould Instruments, Valley View, OH).
relationship between expression of GIRKs and Kir6.2 chan-
Following formation of a greater than 1 G⍀ seal, intracellular access
nel subtypes in hypothalamic neurons is not known. Because
was achieved by suction, followed by perfusion with 1 m tetrodotoxin(TTX, Alomone Labs, Jerusalem, Israel) for at least 4 – 6 min to block
POMC neurons are so critical for regulating homeostasis and
spontaneous firing and action potential-generated synaptic potentials.
motivated behaviors in the mammal (6, 30), we hypothesized
All the responses to agonists and antagonists were measured in voltage
that POMC neurons would respond to activators of GIRKs
60 mV) with the exception of the glucose experiments.
and Kir6.2 channels and serve as integrators of both synaptic
The access resistance was checked before and after each drug treatment,and only those cells that showed less than 10% change in access resis-
and hormonal (metabolic) input. We used a transgenic
tance throughout the recording were included in this study. The access
mouse model in which we could visualize enhanced green
varied from 20 –30 m⍀ (x ⫽ 24.4 ⫾ 0.8 m⍀), which ensured adequate
fluorescent protein (EGFP)-labeled POMC neurons and mea-
voltage clamp of this slow outward K⫹ current and minimal rundown
sure the direct effects of the -opioid agonist DAMGO, the
during pharmacological testing due to rapid dialysis of intracellular
(second messenger) constituents.
ATP channel opener diazoxide and metabolic inhibition.
For the cell attached recordings, the patch pipettes were filled with
the external solution (aCSF), and a loose seal (100 m⍀) was formed on
Materials and Methods
the identified POMC neurons to measure spontaneous activity in current
POMC-EGFP transgenic mice
clamp. After a stable baseline was established after several minutes ofrecording, the glucose concentration was rapidly decreased from 10 –5
Transgenic mice were generated on an inbred C57BL/6 genetic back-
mm. The firing activity was measured over a minute period after sta-
ground as previously described (31). All animals for these studies were
bilization and compared with the firing rate measured 1 min before the
maintained under controlled temperature (25 C) and photoperiod con-
change in the glucose concentration. Only cells that showed a full re-
ditions (14-h light, 10-h dark; lights on between 0530 and 1930) with food
covery were used to calculate the change in firing frequency with altered
and water
ad libitum. The animal procedure protocols were done in
accordance with the NIH Guide for the Care and Use of LaboratoryAnimals and were approved by our local animal care and use committee.
Drug application
Based on the immunocytochemical staining of fixed tissue sectionsthrough the arcuate nucleus, greater than 99% of the EGFP-tagged
Following generation of a control current-voltage plot in the presence
neurons contained -END, and there were over 3000 POMC neurons
of TTX, drugs were perfused until a steady-state outward current was
counted in each hypothalamus (31). Therefore, we were confident that
obtained. Diazoxide (7-chloro-3-methyl-2H-1,2,4-benzo-thiadiazin 1,1-
we could target POMC neurons based on the presence of EGFP expres-
dioxide) and tolbutamide (Sigma, St. Louis, MO) were dissolved in
sion in a hypothalamic slice preparation.
dimethylsulfoxide 99.5% to a stock concentration of 300 mm and 100 mm,respectively. Perfusion of aCSF containing 0.1– 0.3% dimethylsulfoxide
(vehicle controls) had no effect on the cells. Naloxone (Sigma) andDAMGO (d-Ala2,
N-Me-Phe4, Gly-ol5-enkephalin; Peninsula Laborato-
Female POMC-EGFP transgenic mice (14 –21 d) were selectively bred
ries, Inc., Belmont, CA) were dissolved in Milli-Q H2O to a stock con-
in-house, and maintained under the conditions described above. On the
centration of 1 mm. Baclofen (Sigma) was dissolved in 0.1 n HCl to a
day of experiment, the mice were anesthetized with halothane, decap-
concentration of 40 mm. Aliquots of the stock solutions were stored
itated, the brain rapidly removed from the skull and a block containing
appropriately until needed. Final drug concentrations were made up in
the hypothalamus immediately dissected. (The trunk blood was col-
10 ml volumes and perfused at 1.5 ml/min. On the average, it took 2–5
lected, and serum estrogen levels determined by chromatography and
min to reach a steady-state outward current with DAMGO, baclofen, or
subsequent RIA by Oregon National Primate Research Center. Serum
diazoxide. The drug-induced change in conductance was determined by
estrogen levels in these immature female mice were 5.9 ⫾ 0.8 pg/ml,
subtracting the pre- from the postdrug current/voltage (I/V) slopes.
which were significantly below castrate levels of adult females.) The
Composite dose-response curves were generated from the following
hypothalamic block was submerged in cold (4 C) oxygenated (95% O
logistic equation fitted by computer (Origin 4.1, Microcal) to the data:
⫽ 100 䡠 ([agonist]n/([agonist]n ⫹ EC n)), where ⌬I
2) artificial cerebrospinal fluid (aCSF) with low Ca2⫹ containing
the following constituents, in mm: NaCl, 124; KCl, 5; NaHCO
imum outward current for a given agonist, EC
50 represents the agonist
potency, and
n is the Hill slope.
2PO4, 2.6; dextrose, 10; HEPES, 10; MgSO4, 2; CaCl2, 1. Coronal
slices (300 m) through the caudal-rostral extent of the arcuate nucleus
The pharmacodynamics sometimes were reevaluated after the drug
were cut with a vibratome during which time (20 min) the slices were
wash-out in the presence of antagonists. Estimates of the Ki for antag-
bathed in aCSF with low Ca2⫹ at 4 C. The arcuate slices were then
onists were derived from the logistic equation fitted by computer
transferred to an auxiliary chamber where they were kept at room
(SigmaPlot 2000, Jandel Scientific) to the data: ⌬I
100 䡠 ([agonist]n/
temperature (25 C) in aCSF with normal Ca2⫹ (2 mm) until recording
([agonist]n ⫹ (EC n 䡠
(1 ⫹ ([antagonist]n/Ki ))))).
(recovery ⬃1.5 h), at which time a single slice was transferred to therecording chamber. Once in the recording chamber, the slices were kept
Acutely dispersed neurons
viable by continually perfusing with warm (35 C), oxygenated normalaCSF at 1.5 ml/min.
For these experiments, we prepared hypothalamic slices from adult
For imaging and recording, slices were viewed with a Zeiss Axioskop
Topeka guinea pigs using the same procedures as for the preparation of
outfitted for fluorescence (fluorescein isothiocyanate filter) and infrared
mouse hypothalamic slices (see above). The 300-m coronal hypotha-
differential interference contrast videomicroscopy. After visualizing flu-
lamic slices were cut on a vibratome from caudal to rostral and placed
orescent POMC-EGFP neurons with the 5⫻ objective, a 40⫻ water im-
in an auxiliary chamber containing oxygenated, normal aCSF. The slices
mersion objective was used for infrared differential interference contrast
were allowed to recover for 1–2 h in the chamber before dispersion. The
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Ibrahim
et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
Endocrinology, April 2003, 144(4):1331–1340
arcuate nucleus of the hypothalamus was microdissected and incubated
The PCR product from single cells for each primer pair was sequenced
in 2–3 ml of Hanks' balanced salt solution [HBSS (in mm): CaCl2 ,1.26;
in our core facilities.
MgSO4,1; KCl, 5.37; KH2PO4, 0.44; NaCl,136.89; Na2HPO4, 0.34; d-
glucose, 5.55; HEPES,15 in diethylpyrocarbonate (DEPC)-treated water,
pH 7.3, 300 mOsm] containing 1 mg/ml protease XIV (Sigma) for ap-
Passive membrane properties and endogenous conductances
proximately 15 min at 37 C. The tissue was then washed four times inone volume low calcium aCSF and two times in HBSS. The cells were
Initially, whole cell recordings were made from 103
isolated by trituration with flame-polished pasteur pipettes, dispersed
POMC-EGFP neurons from prepubertal female mice
on a 35-mm Petri dish and continuously perfused with HBSS at a rate
(C57BL/6J background) using visualized, whole-cell patch
of 1.5 ml/min. Cells were visualized using an inverted microscope, andindividual neurons were patched and harvested into the patch pipette
recording. We were confident that all of the cells that we
by applying negative pressure. The content of the pipette was expelled
targeted were POMC neurons based on a previous study in
into a siliconized microcentrifuge tube containing 5 l of the following
which 99% of the neurons expressing EGFP were identified
solution: 0.5 l of 10⫻ buffer (100 mm Tris-HCl, 500 mm KCl, 1% Triton
as -END neurons (31). For the electrophysiology analysis,
X-100; Promega Corp., Madison, WI), 15 U RNasin (Promega Corp.), 0.5
l 100 mm dithiothreitol, and DEPC-treated water.
only POMC cells with gigaohm or better seals were includedin this study. The mean resting membrane potential was
Tissue total RNA purification
⫺55.4 ⫾ 2.2 mV at a 0 pA holding current, and the mean inputresistance was 1.1 ⫾ 0.1 G⍀. Moreover, the majority (68%) of
Hypothalamic tissue was homogenized and total RNA extracted us-
female mouse POMC neurons exhibited the same endoge-
ing the RNeasy kit (QIAGEN, Valencia, CA) according to the manu-facturer's protocol. Total RNA was treated with deoxyribonuclease I,
nous conductances that we have described in female guinea
which was then inactivated and removed using DNA-free reagents as
pig POMC neurons under low steroid (ovariectomized) con-
described by the manufacturer (Ambion, Inc., Austin, TX). The RNA was
ditions, which included expression of a hyperpolarization-
diluted and used as a positive (⫹ reverse transcriptase [⫹RT]) or neg-
activated, cation current (I
ative (⫺RT) control for the PCRs.
h) and a transient outward K⫹
current (IA; Fig. 1). Hence, the passive membrane propertiesof mouse POMC neurons labeled with EGFP are similar to
RT-PCR of single cells and tissue RNA
what we have reported for guinea pig and rat POMC neurons
The harvested cell solution and 25 ng of hypothalamic total RNA in
identified
post hoc by immunocytochemistry (17, 18). There-
1 l were denatured for 5 min at 65 C then cooled on ice for 5 min.
fore, we do not think that the EGFP expression in POMC
Single-stranded cDNA was synthesized from cellular RNA by adding 50
neurons altered the physiological properties of these
U murine leukemia virus RT (Applied Biosystems, Foster City, CA), 1.5
l 10⫻ buffer, 2 mm MgCl
2, 0.2 m deoxynucleotide triphosphate, 15 U
RNasin, 10 mm dithiothreitol, 100 ng random hexamers, and DEPC-treated water to a final volume of 20 l. Cells and tissue RNA used as
Coupling of
-opioid receptor to GIRK: effects of DAMGO
negative controls, were processed as described above but without RT.
Based on our previous findings in guinea pig POMC neu-
The reaction mixtures were incubated at 42 C for 60 min, denatured at99 C for 5 min, and cooled on ice for 5 min.
rons that indicated the -opioid receptor mediates autoin-
PCR was performed using 2–3 l of cDNA template from each RT
hibition of these opioid neurons, we evaluated the coupling
reaction in a 30-l PCR volume containing: 3 l 10⫻ buffer, 2.4 l MgCl2
of the -opioid receptor to GIRK in mouse POMC-EGFP
(2 mm final concentration for POMC, Kir6.1, and SUR1) or 4.8 l MgCl2
neurons. In the presence of TTX (1 m), DAMGO (30 –1000
(4 mm final concentration for Kir6.2), 0.2 mm deoxynucleotide triphos-phate, 0.2 m forward and reverse primers, 2 U Taq DNA polymerase
nm) induced an outward current in 54 out of 63 (85%) of
(Promega Corp.), and 0.22 g TaqStart Antibody (CLONTECH Labo-
mouse POMC neurons. DAMGO (1 m) caused a maximum
ratories, Inc., Palo Alto, CA). Taq DNA polymerase and TaqStart An-
outward current of 12.8 ⫾ 1.2 pA (n ⫽ 22) that reversed near
tibody were combined and incubated at room temperature for 5 min, the
⫽ ⫺81.0 ⫾ 2.5 mV
vs. E
remainder of the reaction contents were added to the tube and incubated
K⫹ ⫽ ⫺84.5 mV) and
increased the whole-cell slope conductance by 1.3 ⫾ 0.2 nS
at 94 C for 2 min. Then, each reaction went through 60 cycles of am-plification according to the following protocols: 94 C, 45 sec (denatur-
(Fig. 2). Moreover, the nonselective opioid receptor antago-
ation); 60 C, 45 sec (annealing); 72 C, 1 min 10 sec (elongation), with a
nist naloxone attenuated the response to DAMGO (Fig. 2). A
final 72 C extension for 5 min (POMC, Kir6.1, and SUR1) or 94 C, 45 sec
concentration-response relationship was generated from 54
(denaturation); 68 C, 1 min (annealing and elongation combined), with
neurons, and the majority of POMC neurons were tested
a final 72 C extension for 5 min (Kir6.2). Ten microliters of the PCRproducts were visualized with ethidium bromide on a 1.5% agarose gel.
with a single concentration of DAMGO. In addition, a small
The primers used were as follows: guinea pig POMC; 344-bp product
number of POMC neurons were tested with two concentra-
(accession no. S78260), forward primer (bases 40 – 60) 5⬘-CTGGCCTT-
tions of DAMGO, a lower concentration followed by a higher
GCTGCTTCAGAT-3⬘; reverse primer (bases 383–363) 5⬘-ATGGAG-
concentration of agonist to establish a maximum (outward
TAGGAGCGCTTGTC-3⬘. Guinea pig Kir6.2; 398-bp product (accession
current) response within a given cell. A logistics equation fit
no. AF183920), forward primer (bases 1608 –1627) 5⬘-GCCCGCTTTGT-GTCCAAGAA-3⬘; reverse primer (bases 2005–1985) 5⬘-CCCAGCAT-
to the data points yielded an EC50 of 102.8 nm (Fig. 3).
GATGGCGTTGAT-3⬘. Guinea pig SUR1; 238-bp product (accession no.
In an additional 22 POMC neurons, the DAMGO-induced
AF183921), forward primer (bases 1325–1345) 5⬘-GCCACGGCTTC-
outward current was antagonized by the opioid receptor
CATCGACAT-3⬘; reverse primer (bases 1562–1542) 5⬘-CGCTGGCAG-
antagonist naloxone. A single concentration of DAMGO
GTCACTTGTCT-3⬘. Guinea pig Kir6.1; 220-bp product (accession no.
AF183918), forward primer (bases 379 –399) 5⬘-GGACATCTACGCTTA-
(300 –3000 nm) was applied to each neuron in the presence of
CATGG-3⬘; reverse primer (bases 598 –578) 5⬘-GACAGCGTTGATGAT-
20 nm naloxone. The antagonism by naloxone produced a
CAGAC-3⬘. Guinea pig glyceraldehyde-3 phosphate dehydrogenase
rightward shift in the agonist dose-response curve with an
(GAPDH); 212-bp product (accession no. CPU51572), forward primer
(bases 123–143)5⬘CATCCACTGGTGCTGCCAAG-3⬘; reverse primer
i of 3.1 nm (Fig. 3). This Ki for naloxone inhibition
of -opioid response is similar to what we and others have
(bases 334 –314) 5⬘-GTCCTCGGTGTAGCCCAAGA-3⬘. Primers weresynthesized by Invitrogen (Carlsbad, CA), and the optimum PCR con-
published for naloxone antagonism of -opioid receptor-
ditions for each primer pair was established in preliminary experiments.
mediated responses in the central nervous system (33, 34).
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Endocrinology, April 2003, 144(4):1331–1340
Ibrahim
et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
FIG. 1. Female mouse POMC-EGFP neurons express I and I currents. A, Transient outward currents (I ,
arrow) generated in a whole-cell
recording of a mouse EGFP-POMC neuron elicited by holding the cell at ⫺60 mV and giving a series of 1-sec prepulses ranging from ⫺50 mVto ⫺140 mV (in 10 mV increments) and stepping back to ⫺60 mV. Resting V ⫽ ⫺54 mV. B, I generated in mouse POMC-EGFP neuron. This
current has the appearance of a sag (
dotted line) following the instantaneous current observed at the onset of the hyperpolarizing voltagecommand. The cell was held at ⫺60 mV and given a series of hyperpolarizing pulses from ⫺65 to ⫺130 mV (5-mV increments for 600 msec).
Resting V
⫽ ⫺60 mV. The
inset graph is the composite I/V curve (leak subtracted) showing the differences between the instantaneous and
steady-state inward current for POMC neurons with the symbols on the current traces showing where the instantaneous (f) and the steady-state(E) currents were measured.
FIG. 2. Female mouse POMC neurons respond to -opioid receptor agonist DAMGO. A, In the presence of 1 M TTX, bath application of DAMGOinduced a 15-pA outward current. This effect was blocked by opioid receptor antagonist naloxone (20 nM). The break in the recording traceindicates where I/V data were obtained. V
⫽ ⫺60 mV (resting V ⫽ ⫺62 mV). The
dotted line in this figure and subsequent figures serves
as a point of reference only. B, The pre-DAMGO, post-DAMGO, and post-DAMGO ⫹ naloxone current-voltage relationships from another POMCneuron. The reversal potential for the outward current was close to the predicted equilibrium potential for potassium (E
⫽ ⫺90 mV).
GABA receptor agonist baclofen activates GIRK
Activation of Kir6.2 by the K
channel opener diazoxide
We have shown that guinea pig POMC neurons are in-
Based on the RT-PCR detection of both Kir6.2 and SUR1
hibited by both DAMGO and the GABAB receptor agonist
transcripts in the mediobasal hypothalamus of the mouse
baclofen via activation of GIRKs (35). Therefore, we tested
(27), we hypothesized that mouse POMC neurons express
mouse POMC neurons to see if they would show a similar
Kir6.2 and SUR1 and therefore would be sensitive to the KATP
response to the GABAB receptor agonist baclofen. Indeed, all
channel opener diazoxide and the sulfonylurea drug tolbu-
of the mouse POMC cells that were sensitive to DAMGO
tamide. Following perfusion with TTX (1 m), diazoxide
responded to the GABAB receptor agonist baclofen (40 m)
(3–1000 m) induced an outward current in 37 out of 40 (92%)
with an outward current (21.6 ⫾ 4.0 pA) that reversed near
mouse POMC neurons. Diazoxide (300 m) caused a max-
80.7 ⫾ 7.2 mV, n ⫽ 8) and with an increase
imum outward current of 18.7 ⫾ 2.2 pA (n ⫽ 9) that reversed
in slope conductance of 1.8 ⫾ 0.5 nS (Fig. 4). Therefore, it
82.5 ⫾ 1.8 mV) and increased the slope
appears that the GABAB receptor is similarly coupled to
conductance by 1.5 ⫾ 0.1 nS (Fig. 5). Moreover, the SUR1
activation of GIRK as the -opioid receptor in mouse POMC
selective drug tolbutamide antagonized the actions of di-
neurons. Further elucidation of the GABAB-mediated re-
azoxide at equimolar concentrations (n ⫽ 12, Fig. 4). A
sponse was not undertaken because we have extensively
concentration-response relationship for diazoxide was
characterized this response in guinea pig and rat hypotha-
generated, using a single concentration of diazoxide to test
lamic neurons (18, 35–38).
each POMC neuron. A logistics equation fit to the data
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Ibrahim
et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
Endocrinology, April 2003, 144(4):1331–1340
yielded an EC50 of 61.3 m (Fig. 6). We did not see any
produced by DAMGO (Fig. 7A). In fact, 9 of 14 cells showed
evidence for desensitization of the diazoxide response even
an additive response that was not significantly different from
at concentrations of the drug that gave a maximum response
the theoretical maximum based on both channels (GIRK and
(300 –1000 m).
Kir6.2) being activated (27.7 ⫾ 2.8 pA
vs. 31.5 ⫾ 2.2 pA). Theadditive response did not depend on the order of drug ap-
Activation of Kir6.2 and GIRK in mouse POMC neurons
plication. The other five cells of this subpopulation showed
Based on the response of POMC neurons to DAMGO and
an additive response to perfusion of DAMGO followed by
diazoxide, we asked whether the same cells could respond
diazoxide (n ⫽ 3) or diazoxide followed by DAMGO (n ⫽ 2)
to both agonists and therefore exhibit an additive response
that was less than the theoretical maximum additive re-
(
i.e. a greater maximum outward current). Therefore, we
sponse (13.8 ⫾ 0.7 pA
vs. 31.5 ⫾ 1.2 pA). POMC neurons that
recorded from an additional 27 POMC neurons from pre-
responded to both drugs were not localized to any particular
pubertal female mice. Based on the response to both agonists,
region (rostral
vs. caudal) of the mediobasal hypothalamus.
there were three distinct subpopulations of POMC neurons.
In another subpopulation of POMC neurons, DAMGO
In over 50% of the cells (n ⫽ 14), there was an additive effect
induced a large outward current (24.4 ⫾ 2.0 pA, n ⫽ 8)
of both drugs such that the maximum outward current gen-
without any further effect of diazoxide (Fig. 7B). A third
erated by diazoxide added to a maximum outward current
subpopulation showed a robust diazoxide-induced outwardcurrent (18.2 ⫾ 1.1 pA, n ⫽ 5) without any further effect ofDAMGO (Fig. 7C). Again, the order of drug perfusion did notmake any difference in terms of the responses, and the re-sponses did not appear to be region specific. In addition, wedid not see a specific effect of tolbutamide to block theDAMGO-activated GIRK (n ⫽ 5) or the opioid receptor an-tagonist naloxone to block the diazoxide response (n ⫽ 2) inPOMC neurons. Therefore, there appeared to be three dis-tinct subpopulations of mouse POMC neurons. One popu-lation responded to both -opioid receptor activation byDAMGO, coupling to GIRK, and KATP channel activation bydiazoxide. Another population responded to -opioid acti-vation only; and finally, a smaller population of POMC neu-rons responded to the KATP channel opener diazoxide only.
Although there is evidence for activation of GIRK and
KATP channels by G protein-coupled receptors (28, 29), we
FIG. 3. Concentration-response curves for DAMGO and antagonism
did not see any evidence of a direct activation of KATP chan-
by naloxone. DAMGO (30 –1000 nM) induced outward K⫹ currents in
nels by DAMGO. However, over a longer time period there
female POMC neurons in a dose-dependent manner.
Squares repre-
could be a change in activity of KATP channels due to
sent the means, and the
bars SEMs for each concentration of DAMGO
G␣i/o inhibition of adenylyl cyclase activity.
normalized to the maximum outward current (12.8 ⫾ 2.2 pA, n ⫽ 54).
The number of neurons tested at each concentration is given in
pa-rentheses. Based on a logistics equation fit to the data points (see
Expression of Kir6.2 and SUR1 transcripts in
Materials and Methods), the EC
for DAMGO was 102.8 n
oxone (20 nM) antagonized the response to DAMGO and shifted the
POMC neurons
M with an estimated Ki
M (n ⫽ 22). The
circles
The electrophysiological data on the potency of diazoxide
represent the means, and the
bars SEMs for each concentration ofDAMGO in the presence of naloxone normalized to the maximum
to induce an outward K⫹ current in POMC cells suggested
that POMC cells express Kir6.2 subunits. Also, the sensitivity
FIG. 4. Female mouse POMC neurons respond to the GABA agonist baclofen. A, In the presence of TTX (1
M), perfusion of baclofen (40 M)
induced a 20-pA outward current. This cell also responded to DAMGO (see Fig. 2). The break in the recording indicates where current-voltagedata were obtained. V
⫽ ⫺60 mV (resting V ⫽ ⫺54 mV). B, I/V relationships for another POMC neuron that responded to baclofen. The
baclofen-induced outward current that reversed near E
⫽ ⫺78 mV).
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Endocrinology, April 2003, 144(4):1331–1340
Ibrahim
et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
FIG. 5. Female mouse POMC neurons respond to the K
channel opener diazoxide. A, In the presence of TTX, a POMC neuron responded
channel opener diazoxide (300
M) with 20 pA outward current. This effect was reversed by the sulfonylurea tolbutamide (300 M).
The break in the recording trace indicates where I/V data were obtained. V
⫽ ⫺60 mV (resting V ⫽ ⫺50 mV). B, The predizoxide,
postdiazoxide, and postdiazoxide ⫹ tolbutamide current-voltage relationships from the POMC neuron in panel A. The reversal potential forthe outward current was close to the predicted equilibrium potential for potassium (E
⫽ ⫺85 mV).
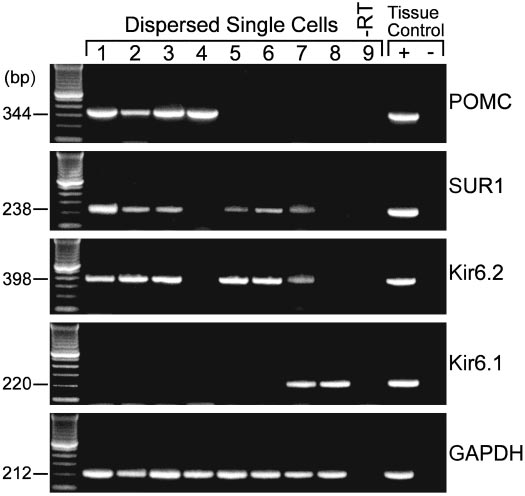
population. Indeed, in our analysis of 20 arcuate neurons thatwere dispersed, patched and then harvested for RT-PCR,four POMC neurons were identified, three of which ex-pressed Kir6.2 and SUR1 (Fig. 8). Although adjacent neuronsalso expressed Kir6.1, Kir6.2 and SUR1 transcripts, Kir6.2plus SUR1 appear to be the predominant transcripts ex-pressed in arcuate neurons (Fig. 8), which agrees with ourpharmacological profile for the KATP channel in theseneurons.
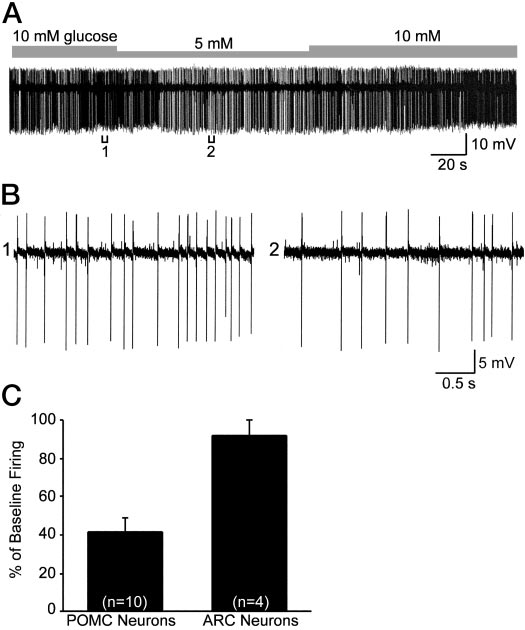
POMC neurons are glucose responsive
The fact that POMC neurons express Kir6.2 and SUR1
transcripts and are sensitive to diazoxide and tolbutamidesuggested that they would be directly modulated by meta-bolic signals. However, the expression of Kir6.2 plus SUR1transcripts is necessary but not sufficient for sensing changesin glucose (41). Therefore, we measured the direct response
FIG. 6. Concentration-response curve for the K
diazoxide. Diazoxide (30 –1000 M) induced outward K⫹ currents in
to changes in glucose concentrations. For these experiments,
female POMC neurons in a dose-dependent manner.
Squares repre-
we did cell attached recordings from mouse POMC neurons
sent the means, and the
bars SEMs for each concentration of di-
to monitor the firing rate and reduced the extracellular con-
azoxide normalized to the maximum outward current (18.7 ⫾ 2.2 pA,
centrations of glucose within physiological limits (42). Re-
n ⫽ 37). The number of cells for each concentration is given in pa-renthesis. Based on a logistics equation fit to the data points (see
duction in extracellular concentrations of glucose from 10
Materials and Methods), the EC
for diazoxide was 61.3
mm to 5 mm significantly decreased the baseline firing rateto 41.5 ⫾ 7.7% in 10 of 12 POMC cells, whereas it did not
of this response to tolbutamide antagonism suggested that
affect the firing rate (91.8 ⫾ 8.2%, n ⫽ 4) of adjacent, un-
SUR1 is the sulfonylurea receptor subunit within the K
identified arcuate neurons (Fig. 9). The spontaneous firing
channel. To define the molecular composition of the K
rate returned to control levels in all of the POMC neurons;
channel directly, we measured the expression of transcripts
however, the time course of recovery from metabolic inhi-
using single cell RT-PCR. These experiments were carried
bition varied among POMC neurons. Therefore, although
out in dispersed guinea pig POMC neurons in which we have
the changes in the firing rate of POMC neurons may be due
measured an equivalent pharmacological response to dia-
in part to altered synaptic input, the direct expression of
zoxide and tolbutamide and have specific primers for Kir6.1,
KATP channels and the sensitivity to glucose suggests that
Kir6.2, SUR1, and POMC. The PCR product from single cells
POMC neurons are glucose-responsive neurons (
i.e. they in-
for each primer was sequenced and found to be specific. In
crease their firing rate in response to increases in glucose
addition, the specificity of the single cell PCR products had
been verified in preliminary experiments using real-timePCR (39). Based on previous electrophysiological recordings
from the caudal mediobasal hypothalamus (17, 36, 40), we
To our knowledge, this is the first study showing that
predicted that POMC neurons make up about 20% of the
POMC neurons express a complement of inwardly rectifying
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Ibrahim et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
Endocrinology, April 2003, 144(4):1331–1340
FIG. 8. Kir6.2 and SUR1 transcripts are detected in POMC neurons.
RT-PCR analysis of Kir6.2, Kir6.1, and SUR1 transcripts in singlecells harvested from dispersed arcuate neurons from the guinea pig.
The expected size of the PCR products is indicated, and the single-cellPCR products were verified with sequencing. GAPDH transcriptswere analyzed in the same cells as an internal control for the RTreaction. The following controls were also included: HBSS from therecording chamber; a water blank (B) and basal hypothalamic (BH)tissue RNA all of which were reversed transcribed in the presence ofRT (⫹RT). In addition, a single cell and tissue RNA were included thatwere processed without RT (⫺RT). PCR was performed for 60 cycles.
rons is not surprising in view of the fact that these commandneurons of the hypothalamus are involved in almost every
FIG. 7. Three populations of POMC neurons based on their response
aspect of hypothalamic control of homeostasis. Indeed,
to DAMGO and diazoxide. A, The effects of DAMGO and diazoxide
POMC neurons have been associated with many physiolog-
were additive in the majority of female mouse POMC neurons. Di-
ical functions including control of the ovulatory cycle and
azoxide (300 M) produced an additional 22-pA outward current when
reproductive behavior, metabolic homeostasis, fluid balance,
coperfused with DAMGO (1 M), which generated a maximal outwardcurrent of 18 pA in the presence of 1 M TTX. The breaks in the
stress responses, and motivated behaviors. One of the me-
recording trace indicates points where current/voltage data were ob-
diators of many of these functions is -END, which is a
⫽ ⫺60 mV (resting V ⫽ ⫺54 mV). B, A subpopulation
posttranslational product of POMC.
of female POMC neurons responded to DAMGO only. Coperfusion of
Acting through G␣i/o-coupled -opioid receptors, -END
diazoxide (300 M) did not augment to maximal outward current
can inhibit its target neurons through activation of GIRK,
produced by 1 M DAMGO (25 pA) in the presence of 1 M TTX. Thebreaks in the recording trace indicates points where current/voltage
inhibition of adenylyl cyclase or inhibition of Ca2⫹ channels
data were obtained. V
⫽ ⫺60 mV (resting V ⫽ ⫺51 mV). C, A
(15). At the cellular level, -opioid receptor agonists have
subpopulation of female POMC neurons responded to diazoxide only.
been shown to modulate the excitability of dopamine neu-
Diazoxide (300 M) induced maximal outward current of 15 pA in the
rons (7), GnRH neurons (10, 12), oxytocin and vasopressin
presence of 1 M TTX following the application of 1 M DAMGO, whichhad no effect in this POMC neuron. The break in the recording trace
neurons (13) and finally local GABA neurons (37). In addi-
indicates when current/voltage data were obtained. V
tion, we have demonstrated that mediobasal hypothalamic
⫽ ⫺57 mV).
POMC neurons are similarly self-inhibited through a -opioid autoreceptor that is coupled to GIRK activation (17).
potassium channels that allows them to be sensitive to both
In fact, the -opioid receptor agonist DAMGO is more potent
neurotransmitter (opioids and GABA) input and metabolic
(EC50 60 nm) but equally efficacious in hyperpolarizing fe-
cues. Furthermore, we have found that POMC neurons ex-
male guinea pig POMC neurons (40) as mouse POMC neu-
press Kir6.2 and SUR1 transcripts and are glucose respon-
rons. However, the present findings indicate that DAMGO
sive. In addition, we have identified two other distinct pop-
is more potent to hyperpolarize POMC neurons (102 nm)
ulations of POMC neurons that were responsive either to
vs. other arcuate neurons (315 nm) in the C57BL/6J strain of
-opioid receptor activation or to KATP channel openers only.
The fact that there are three subpopulations of POMC neu-
The GABAB receptor is also coupled to GIRK channels in
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Endocrinology, April 2003, 144(4):1331–1340
Ibrahim et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
Ref. 45). Based on the sensitivity to tolbutamide and singlecell RT-PCR data, hypothalamic POMC neurons also appearto express SUR1. Liss and colleagues (46, 47) have shown thatGABAergic neurons in the substantia nigra pars recticularisshow a similar KATP channel subunit profile, with Kir6.2 andSUR1 coexpression detected in the majority of the neurons.
In all cases, Kir6.2 plus SUR1-KATP channels are activated bydiazoxide and by metabolic inhibition, and are blocked withhigh affinity by sulfonylureas such as tolbutamide. In addi-tion, we have found that another SUR1-selective antagonist,glipizide, potently blocks the diazoxide response in POMCneurons (unpublished observations).
One of the classical feeding centers of the hypothalamus
is the ventromedial nucleus in which resides glucose-respon-sive neurons, i.e. neurons that increase their firing in re-sponse to elevations in blood glucose levels (48 –50). Based onrecent single cell RT-PCR experiments, ventromedial glu-cose-responsive neurons express Kir6.2 plus SUR1 subunits(27). Therefore, it appears that glucose-responsive neuronscan transduce, via the KATP channel, changes in extracellularglucose levels to changes in neuronal excitability. Based onour single cell RT-PCR results, arcuate guinea pig POMCneurons also express this same compliment of KATP channelsubunits. In preliminary experiments with specific primersto mouse Kir6.2, we have found that the majority of dis-persed mouse POMC-EGFP neurons express Kir6.2. How-ever, the expression of Kir6.2 plus SUR1 is necessary but notsufficient for sensing changes in glucose (41). Therefore, it is
FIG. 9. POMC neurons are glucose responsive. A, Reduction in ex-
important that we have found that a small reduction in
tracellular concentrations of glucose from 10 mM to 5 mM significantly
extracelluar concentrations of glucose significantly inhibited
decreased the firing rate of a POMC neuron recorded in the cell-
POMC cell firing, whereas adjacent non-POMC neurons
attached mode. The cell fully recovered to its original baseline firing
were not affected even though some of these may express
rate. B, Expansion of chart record in A shows that the spontaneousfiring rate decreased from 6 Hz (bracket 1) to about 3 Hz (bracket 2)
Kir6.2 and SUR1 mRNA. It is also known that POMC neurons
or a 50% reduction in firing. C, Summary of the decrease in the
express glucokinase, which is a necessary enzyme for glu-
spontaneous firing rate in POMC vs. unidentified, adjacent arcuate
cose-sensing cells (51). So, in addition to activation by leptin
neurons following a reduction in the glucose concentration from 10 to
(31, 52), POMC neurons appear to be glucose responsive.
5 mM. Bars represent the mean, and lines 1 SEM of the baseline firingrate. Eighty percent (10 of 12) of the POMC neurons were inhibited
It is not surprising that we have identified three subpopu-
by a reduction in glucose concentration.
lations of POMC neurons based on their reponse to -opioidagonists and KATP channel openers. In addition to their role
guinea pig POMC neurons (35), and we have found a similar
in energy homeostasis (43, 53, 54), POMC neurons have
coupling in mouse POMC neurons. This indicates that GABA
multiple other functions including regulating reproduction
input from local arcuate GABA/NPY neurons would also
(12, 55), parturition (13), fluid balance (56), stress responses
provide a powerful inhibitory tone onto these POMC neu-
(4, 6), and natural reward (5, 30, 57). Because of their in-
rons via GABAB receptors (18, 31, 36), which is thought to
volvement in all these different functions, POMC neurons are
play an important role in inhibiting POMC neurons during
thought to be the "command" neurons of the hypothalamus.
activation of feeding circuits (43).
Perhaps part of the diversity in the POMC neurons lies in the
On the other hand, KATP channels couple membrane ex-
differential processing of POMC to ␣-MSH, which is prom-
citability to cellular metabolism by directly sensing and in-
inent in feeding circuits, and to -END, which is involved in
tegrating intracellular concentration changes of nucleotides
modulating reproduction, stress and natural rewards. In ad-
such as ATP and ADP (20). Sulfonylurea binding and elec-
dition, POMC neurons are located in the arcuate and peri-
trophysiological studies have characterized neuronal KATP
arcuate region including the median eminence, which is out-
channels with different properties in a variety of cell types,
side the blood brain barrier. Therefore, they are in a strategic
including hippocampal and midbrain neurons. Kir6.2 is
location for sensing humoral signals and translating these
widely distributed in rat brain, and is present in neurons
signals into neural activity.
expressing tyrosine hydroxylase, NPY and glutamic acid
For example, we have found that POMC neurons respond
decarboxylase (24, 27, 39). Presently, our electrophysiological
rapidly to estrogen, which serves to inhibit GnRH neurons
findings together with the single cell RT-PCR indicate that
during the negative feedback phase of the ovulatory cycle
POMC neurons also express Kir6.2. In fact, the EC50 for
(12, 58). Perhaps another subset of POMC neurons respond
diazoxide is similar to what has been reported for pancreatic
rapidly to changing levels of leptin and/or glucose to trans-
-cells (44) and rat hippocampal pyramidal neurons (51 m;
late these metabolic cues into neural signals. Interestingly,
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Ibrahim et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
Endocrinology, April 2003, 144(4):1331–1340
earlier studies in the female rat demonstrated that glucose-
13. Russell JA, Leng G, Bicknell RJ 1995 Opioid tolerance and dependence in the
responsive neurons in the ventromedial nucleus were also
magnocellular oxytocin system: a physiological mechanism. Exp Physiol80:307–340
sensitive to the acute actions of estrogen, which depolarized
14. Cowley MA, Pronchuk N, Fan W, Inulescu DM, Colmers WF, Cone RD 1999
these cells via a cAMP-dependent pathway (59). Because we
Integration of NPY, AGRP, and melanocortin signals in the paraventricular
know that estrogen is anorexic (60) and activates POMC
nucleus of the hypothalamus: evidence of a cellular basis for the adipostat.
Neuron 24:155–163
neurons in the female guinea pig via protein kinase A (34),
15. North RA 1993 Opioid actions on membrane ion channels. In: Herz A, ed.
the steroid may synergize with leptin and glucose to inhibit
Handbook of experimental pharmacolgy: opioid I. New York: Springer-Verlag;773–797
feeding in the female. Electrophysiology experiments are
16. Karschin C, Dissmann E, Stuhmer W, Karschin A 1996 IRK(1–3) and
currently underway to measure the response of mouse
GIRK(1– 4) inwardly rectifying K⫹ channel mRNAs are differentially ex-
POMC neurons to estrogen since there are profound gender
pressed in the adult rat brain. J Neurosci 16:3559 –3570
17. Kelly MJ, Loose MD, Rønnekleiv OK 1990 Opioids hyperpolarize -endor-
differences in the control of metabolic homeostasis (60).
phin neurons via -receptor activation of a potassium conductance. Neuroen-
In summary, we have found that POMC neurons express
docrinology 52:268 –275
a unique complement of inwardly rectifying K⫹ channels
18. Loose MD, Rønnekleiv OK, Kelly MJ 1991 Neurons in the rat arcuate nucleus
are hyperpolarized by GABAB and -opioid receptor agonists: evidence for
(GIRKs, Kir6.2) and SUR proteins that allow them to respond
convergence at a ligand-gated potassium conductance. Neuroendocrinology
to metabolic (e.g. glucose) changes and synaptic input (e.g.
19. Slugg RM, Hayward MD, Rønnekleiv OK, Low MJ, Kelly MJ 2000 Effect of
GABA/NPY neurons). In addition, we know that the syn-
the -opioid agonist DAMGO on medial basal hypothalamic neurons in -
aptic input to POMC neurons can be modulated by gonadal
endorphin knockout mice. Neuroendocrinology 72:208 –217
steroids and that leptin can directly excite these cells through
20. Reimann F, Ashcroft FM 1999 Inwardly rectifying potassium channels. Curr
Opin Cell Biol 11:503–508
activation of a cation-selective current. Therefore, these
21. Clement IV JP, Kunjilwar K, Gonzalez G, Schwanstecher M, Paten U, Agui-
POMC neurons are uniquely situated to respond to ascend-
lar-Bryan L, Bryan J 1997 Association and stoichiometry of KATP channel
ing sensory input, metabolic changes and hormonal fluctu-
subunits. Neuron 18:827– 838
22. Ashcroft FM, Gribble FM 1998 Correlating structure and function in ATP-
ations that enable them to integrate multiple inputs and serve
sensitive K⫹ channels. Trends Neurosci 21:288 –294
as the command neurons of the hypothalamus to maintain
23. Ashcroft FM, Gribble FM 2000 New windows on the mechanism of action of
homeostasis in the mammal.
KATP channel openers. Trends Pharmacol Sci 21:439 – 445
24. Dunn-Meynell AA, Rawson NE, Levin BE 1998 Distribution and phenotype
of neurons containing the ATP-sensitive K⫹ channel in rat brain. Brain Res
25. Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford MLJ 1997 Leptin
inhibits hypothalamic neurons by activation of ATP-sensitive potassium chan-
Received October 7, 2002. Accepted December 20, 2002.
nels. Nature 390:521–525
Address all correspondence and requests for reprints to: Martin J.
26. Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML 2000 Insulin
Kelly, Ph.D., Department of Physiology and Pharmacology, L334, Or-
activates ATP-sensitive K⫹ channels in hypothalamic neurons of lean, but not
egon Health Sciences University, 3181 Southwest Sam Jackson Park
obese rats. Nat Neurosci 3:757–758
Road, Portland, Oregon 97239-3098. E-mail: [email protected].
27. Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M,
Ashcroft F, Minokoshi Y, Roeper J, Seino S 2001 ATP-sensitive K⫹ channels
This work was supported by Public Health Service Grants DA-05158,
in the hypothalamus are essential for the maintenance of glucose homeostasis.
DA-00192 (Research Scientist Development Award, to M.J.K.),
Nat Neurosci 4:507–512
NS-38809, NS-35944, DK-55819, HG-00201, and the International Scholar
28. Roeper J, Hainsworth AH, Ashcroft FM 1990 Tolbutamide reverses mem-
Program of the Howard Hughes Medical Institute.
brane hyperpolarisation induced by activation of D2 receptors and GABABreceptors in isolated substantia nigra neurones. Pflugers Arch 416:473– 475
29. Smith PA, Sellers LA, Humphrey PP 2001 Somatostatin activates two types
of inwardly rectifying K⫹ channels in MIN-6 cells. J Physiol 532:127–142
1. Elde RP, Ho¨kfelt T 1979 Localization of hypophysiotropic peptides and other
30. Hayward MD, Pintar JE, Low MJ 2002 Selective reward deficit in mice lacking
biologically active peptides within the brain. Annu Rev Physiol 41:587– 602
-endorphin and enkephalin. J Neurosci 22:8251–8258
2. Khachaturian H, Lewis ME, Haber SN, Akil H, Watson SJ 1984 Proopio-
31. Cowley MA, Smart JL, Rubinstein M, Cerda´n MG, Diano S, Horvath TL,
melanocortin peptide immunocytochemistry in rhesus monkey brain. Brain
Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through
Res Bull 13:785– 800
a neural network in arcuate nucleus. Nature 411:480 – 484
3. Khachaturian H, Lewis ME, Shafer MKH, Watson SJ 1985 Anatomy of the
32. Wagner EJ, Rønnekleiv OK, Kelly MJ 2001 The noradrenergic inhibition of
CNS opioid systems. Trends Neurosci 8:111–119
an apamin-sensitive, small conductance Ca2⫹-activated K⫹ channel in hypo-
4. Millan MJ, Herz A 1985 The endocrinology of the opioids. Int Rev Neurobiol
thalamic ␥-aminobutyric acid neurons: pharmacology, estrogen sensitivity and
relevance to the control of the reproductive axis. J Pharmacol Exp Ther
5. Di Chiara G, North RA 1992 Neurobiology of opiate abuse. Trends Pharmacol
Sci 131:185–193
33. Williams JT, North RA 1984 Opiate-receptor interactions on single locus
6. Rubinstein M, Mogil JS, Japo´n M, Chan EC, Allen RG, Low MJ 1996 Absence
coeruleus neurones. Mol Pharmacol 26:489 – 497
of opioid stress-induced analgesia in mice lacking -endorphin by site-directed
34. Lagrange AH, Rønnekleiv OK, Kelly MJ 1997 Modulation of G protein-
mutagenesis. Proc Natl Acad Sci USA 93:3995– 4000
coupled receptors by an estrogen receptor that activates protein kinase A. Mol
7. Loose MD, Rønnekleiv OK, Kelly MJ 1990 Membrane properties and re-
Pharmacol 51:605– 612
sponse to opioids of identified dopamine neurons in the guinea pig hypo-
35. Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ 1996 Estrogen rapidly
thalamus. J Neurosci 10:3627–3634
attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology
8. Horvath TL, Naftolin F, Leranth C 1992 -Endorphin innervation of dopamine
neurons in the rat hypothalamus: a light and electron microscopic double
36. Kelly MJ, Loose MD, Rønnekleiv OK 1992 Estrogen suppresses -opioid and
immunostaining study. Endocrinology 131:1547–1555
GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neu-
9. Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, Ashcroft FM
rosci 12:2745–2750
2000 A novel method for measurement of submembrane ATP concentration.
37. Wagner EJ, Bosch MA, Kelly MJ, Rønnekleiv OK 1999 A powerful GABAB
J Biol Chem 275:30046 –30049
receptor-mediated inhibition of GABAergic neurons in the arcuate nucleus.
10. Thind KK, Goldsmith PC 1988 Infundibular gonadotropin-releasing hormone
neurons are inhibited by direct opioid and autoregulatory synapses in juvenile
38. Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ 2001 Estrogen biphasically
monkeys. Neuroendocrinology 47:203–216
modifies hypothalamic GABAergic function concomitantly with negative and
11. Goldsmith PC, Boggan JE, Thind KK 1991 Opioid synapses on vasopressin
positive control of luteinizing hormone release. J Neurosci 21:2085–2093
neurons in the paraventricular and supraoptic nuclei of juvenile monkeys.
39. Bosch MA, Qiu J, Ibrahim N, Kelly MJ, Rønnekleiv OK 2001 Whole-cell
Neuroscience 45:709 –719
patch-clamp recording and single-cell RT-PCR analysis of K-ATP channel
12. Lagrange AH, Rønnekleiv OK, Kelly MJ 1995 Estradiol-17 and -opioid
expression in guinea pig hypothalamic arcuate neurons. Soc Neurosci Abstr
peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of neg-
ative feedback? Endocrinology 136:2341–2344
40. Lagrange AH, Rønnekleiv OK, Kelly MJ 1994 The potency of -opioid hy-
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Endocrinology, April 2003, 144(4):1331–1340
Ibrahim et al. • POMC Neurons Respond to Opioids, Metabolic Inhibition
perpolarization of hypothalamic arcuate neurons is rapidly attenuated by
50. Oomura Y, Nishino H, Karadi Z, Aou S, Scott TR 1991 Taste and olfactory
17-estradiol. J Neurosci 14:6196 – 6204
modulation of feeding related neurons in behaving monkey. Physiol Behav
41. Levin BE 2002 Metabolic sensors. Viewing glucosensing neurons from a
broader perspective. Physiol Behav 76:397– 401
51. Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE 2002 Glucoki-
42. Silver IA, Erecinska M 1994 Extracellular glucose concentration in mamma-
nase is the likely mediator of glucosensing in both glucose-excited and glucose-
lian brain: continuous monitoring of changes during increased neuronal ac-
inhibited central neurons. Diabetes 51:2056 –2065
tivity and upon limitation in oxygen supply in normo-, hypo-, and hypergly-
52. Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbæk C, Flier JS, Saper
cemic animals. J Neurosci 14:5068 –5076
CB, Elmquist JK 1999 Leptin differentially regulates NPY and POMC neurons
43. Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH 2001 The hypo-
projecting to the lateral hypothalamic area. Neuron 23:775–786
thalamus and the control of energy homeostasis: different circuits, different
53. Elmquist JK 2001 Hypothalamic pathways underlying the endocrine, auto-
purposes. Physiol Behav 74:683–701
nomic, and behavioral effects of leptin. Physiol Behav 74:703–708
44. Babenko AP, Aguilar-Bryan L, Bryan J 1998 A view of SUR/KIR6. X, KATP
54. Spiegelman BM, Flier JS 2001 Obesity and the regulation of energy balance.
channels. Annu Rev Physiol 60:667– 687
Cell 104:531–543
45. Matsumoto N, Komiyama S, Akaike N 2002 Pre- and postsynaptic ATP-
55. Ferin M, Van Vugt D, Wardlaw S 1984 The hypothalamic control of the
sensitive potassium channels during metabolic inhibition of rat hippocampal
menstrual cycle and the role of endogenous opioid peptides. Recent Prog
CA1 neurons. J Physiol 541:511–520
Horm Res 40:441– 485
46. Liss B, Bruns R, Roeper J 1999 Alternative sulfonylurea receptor expression
56. Bicknell RJ 1985 Endogenous opioid peptides and hypothalamic neuroendo-
defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain
crine neurones. J Endocrinol 107:437– 446
neurons. EMBO J 18:833– 846
57. Spanagel R, Weiss F 1999 The dopamine hypothesis of reward: past and
47. Liss B, Roeper J 2001 A role for neuronal KATP channels in metabolic control
current status. Trends Neurosci 22:521–527
of the seizure gate. Trends Pharmacol Sci 22:599 – 601
58. Kelly MJ, Lagrange AH 1998 Nontranscriptional effects of estradiol in neu-
48. Oomura Y, Ono T, Ooyama H, Wayner MJ 1969 Glucose and osmosensitive
ropeptide neurons. Curr Opin Endocrinol Diabetes 5:66 –72
neurones of the rat hypothalamus. Nature 222:282–284
59. Minami T, Oomura Y, Nabekura J, Fukuda A 1990 17-Estradiol depolar-
49. Minami T, Oomura Y, Sugimori M 1986 Electrophysiological properties and
ization of hypothalamic neurons is mediated by cyclic AMP. Brain Res
glucose responsiveness of guinea-pig ventromedial hypothalamic neurones in
vitro. J Physiol (London) 380:127–143
60. Geary N 2001 Estradiol, CCK and satiation. Peptides 22:1251–1263
The Endocrine Society. Downloaded from press.endocrine.org by [${individualUser.displayName}] on 09 April 2015. at 10:24 For personal use only. No other uses without permission. All rights reserved.
Source: http://digitalcommons.georgefox.edu/cgi/viewcontent.cgi?article=1064&context=bio_fac
CONVOCATORIA PARA LA INVITACIÓN A CUANDO MENOS TRES PERSONAS DE CARÁCTER NACIONAL NUM. INP-007-13 INSTITUTO NACIONAL DE PSIQUIATRÍA "RAMÓN DE LA FUENTE MUÑÍZ" SUBDIRECCIÓN DE RECURSOS MATERIALES De conformidad con la Ley de Adquisiciones, Arrendamientos y Servicios del Sector Público, se convoca a los interesados a participar en la Invitación a cuando menos tres personas número INP-007-13, cuya convocatoria que contiene las bases de participación disponibles para consulta en Calzada México-Xochimilco No. 101, Colonia san Lorenzo Huipulco, C.P. 14370, Tlalpan, distrito Federal, Teléfono 4160-5012, 4160-5014 y fax 4160-5292 los días lunes a viernes del año en curso de las 09:30 a 14.00 horas.
Marilyn Herie, Ph.D., TSI Tim Godden, M.S.S., TSI Joanne Shenfeld, M.S.S. Colleen Kelly, M.S.S., TSI Guide d'information Guide à l'intention des personnes aux prises avec une toxicomanie et de leur famille Marilyn Herie, Ph.D, TSI Tim Godden, M.S.S., TSI Joanne Shenfeld, M.S.S. Colleen Kelly, M.S.S., TSI Un Centre collaborateur de l'Organisation panaméricaine de la santé et de


