Diapositiva


Prof. Francesco Castelli
Clinica di Malattie Infettive e Tropicali
Centro Interuniversitario Ricerca sulla Malaria (CIRM)
Università di Brescia
2° U.O. di Malattie Infettive
Azienda Ospedaliera Spedali Civili di Brescia
WHO Collaborating Center
for TB/HIV co-infection
Malaria:
nuove prospettive terapeutiche
Conflicts of interest
Related to this presentation:
PI in a company-sponsored (Sigma-tau) clinical trial on DHA-PQ
PI in the Sigma-tau surveillance register for DHA-PQ in pregnancy
Unrelated to this presentation:
I suffered from malaria in february 1987 in Mali
Principal investigator of multiple company-sponsored or company-
supported clinical trials on:
a) HIV infection (BMS, Medestea, VIIV, Abbott)
b) viral hepatitis (Janssen, BMS, Roche)
c) antibiotics/antifungals (Novartis)
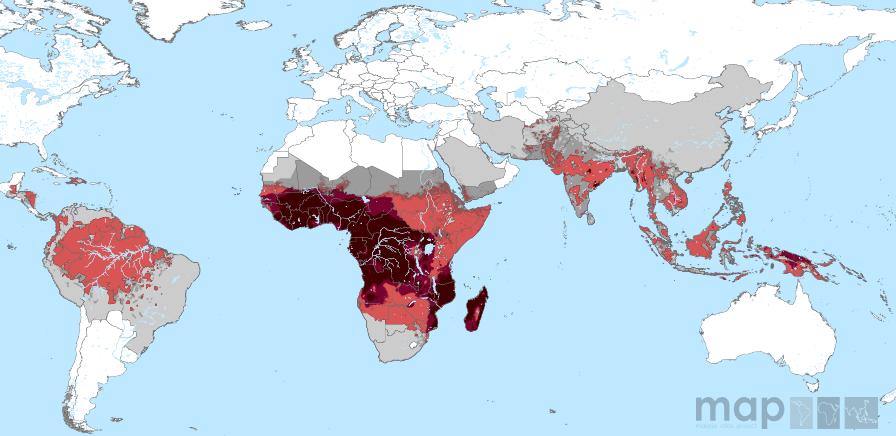
www.map.ox.ac.uk
Roll-Back Malaria (RBM) & World Health Assembly (WHA) target
To reduce malaria cases
Vector control:
- impregnated bednets
- indoor residual spraying
compared to 2000 level
- insecticide resistance
Chemoprevention:
- intermittent preventive therapy
a) pregnant women
- seasonal malaria chemoprophylaxis
Diagnostic test:
- universal diagnostic testing
Treatment:
- artemisinin-based combination
104 endemic countries:
80% :17 countries
- control phase: 79
40%: RDC, Nigeria, India
- pre-elimination phase: 10
- elimination phase: 10
- prevention of reintroduction phase: 5
80%: 14 countries 40%: RDC, Nigeria
Source: WHO World Malaria Report, 2012
WHO/Europe 2011,
Centralized information
system for infectious
diseases (CISID).
• EuroTravNet 2010: significant increase in reported P.falciparum malaria
observed in 2010 as compared to 2008 and 2009.
Stockholm Liverpool
Porto Saint Mandé
Munich Marseille
Madrid London Hamburg
Geneva Cambridge
Euro Surveill. 2012;17(26):pii=20205. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20205
Imported Malaria by years in Brescia, Italy
Castelli, unpublished data
Imported P. vivax malaria by years in
Brescia, Italy
3 patients died = 5.3% (3/56)
2 patients died = 20% (2/10)
Best practice = 0.5%
Complications in children (UK and Ireland)
In a logistic regression model,
thrombocytopenia (OR 3.9, 95% CI 1.6 –9.2, P = 0.002),
age < 5 years (OR 2.9, 95% CI 1.3– 6.8, P 0.01), and
diagnosis outside London (OR 2.8, 95% CI 1.3– 6.1, P = 0.01)
remained independently associated with severe malaria.
Eleven children (6% of all cases, 7% of P. falciparum cases and 24% of severe
P. falciparum malaria cases) were admitted to a pediatric intensive care unit
for coma (n=5), convulsions (n=3), and 1 case each of hyper-parasitemia,
circulatory shock requiring inotropic drugs and cardiac monitoring.
Ladhani et al. Pediatr Infect Dis J 2010;29:343-8
Treatment of children with malaria in the UK and Ireland
Quinine Only 45 (30.4%)
Oral only 25 (16.9%)
Intravenous only 50 (33.7%)
Both 15 (10.1%)
Quinine with PMT-SDX 44 (29.7%)
Quinine with PMT-SDX only 38 (25.7%)
Quinine + PMT-SDX + Clindamycin 3 (2.0%)
Quinine + PMT-SDX + ATV-PGN 2 (1.4%)
Quinine + PMT-SDX + MFQ 1 (0.7%)
Quinine and another antimalarial 30 (20.3%)
Quinine + ATV-PGN 16 (10.8%)
Quinine + Clindamycin 11 7.4%)
Quinine + AMT-LMF 2 (1.4%)
Quinine + doxycycline 1 (0.7%)
Another antimalarial 29 (19.6%)
ATV-PGN 25 (16.9%)
AMT-LMF 2 (1.4%)
There is a need for standardization
Mefloquine 1 (0.7%)
of diagnosis and management of
PMT-SDX 1 (0.7%)
malaria in Europe
P. vivax (n=12)
Chloroquine + primaquine 10 (83.3%)
Chloroquine + quinine 1 (8.3%)
Chloroquine only 1 (8.3%)
P. ovale (n=5)
Quinine only 2 (40.0%)
Chloroquine + quinine 2 (40.0%)
Chloroquine + primaquine 1 (20.0%)
Ladhani et al. Pediatr Infect Dis J 2010;29:343-8

Revised April 2011
Adapt to European
Study Group
on Clinical Parasitology
ESCMID Study Group on Clinical Parasitology
Helena H Askling (Sweden) Fabrice Bruneel (France) Gerd Buchard (Germany) Francesco Castelli (Italy) Peter L Chiodini (United Kingdom) Martin P Grobusch (The Netherlands) Rogelio Lopez-Vélez (Spain) Margaret Paul (Poland) Eskild Petersen (Denmark) * Corneliu Popescu (Roumania) Michael Ramharter (Austria) Patricia Schlagenhauf (Switzerland)
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Who should malaria be suspected?
Diagnostic tests for malaria should be performed in
Any ill patient who has a history of exposure, i.e. patients with a history of travel to
malaria-endemic areas, whether or not they are febrile at presentation.
Rare cases of airport malaria, transfusion malaria, sharing needles (IVDU), congenital
transmission and organ transplants.
A high proportion of migrants may be asymptomatic or present long after arrival in the
host country, with periods of months up to more than 14 years recorded .
The travel history is essential and mandatory
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Microscopy and limitations of rapid tests
Centers managing patints with malaria must be able to provide round the clock
malaria microscopy of thick and thin blood films and parasite density calculations.
Rapid tests may yeld false negative in cases with
1) very high P.falciparum density "pro-zone" with HRP2 (Gillet et al., 2009)
HRP2 deletion in South America
2) variant P. ovale
(Tordrup et al. Malaria J 2011;10:15)
3) P.knowlesi
HRP2 based tests will be negative
Rapid tests may yeld false positive results in cases with 1) Rheumatoid factors
Use a rapid test which include pan Plasmodial antigen ie. LDH or Aldolase
Take a blood sample (RDT + films) daily for three days if clinical suspicion persists
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Treatment of uncomplicated malaria in adults
1° line treatment
Artemether/lumefantrine
4 tablets BID for 3 days
Take with fatty food
(20/120 mg tablets)
(reduced efficacy SEA)
3 or 4 (bw) tablets QD for 3
Take in fasting state
Atovaquone/proguanil
4 tablets QD for 3 days
Take with fatty food
(250/100 mg tablets)
2° line treatment
Quinine + doxycycline
10 mg/kg TID quinine + 200 mg
QD doxycycline for 7 days
Quinine + clindamycin
10 mg/kg TID quinine + 10 mg/kg Off label BID clindamycin for 7 days
Mefloquine (250 mg tablets)
3 + 2 + 1 tablet 6-8 hours apart
Take with fatty food (reduced efficay SEA)
ESCMID Study Group on Clinical Parasitology. Malaria J 2012;11:328
ESCMID Study Group on Clinical Parasitology. Malaria J 2012;11:328
Pregnancy
ESCMID Study Group on Clinical Parasitology. Malaria J 2012;11:328
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
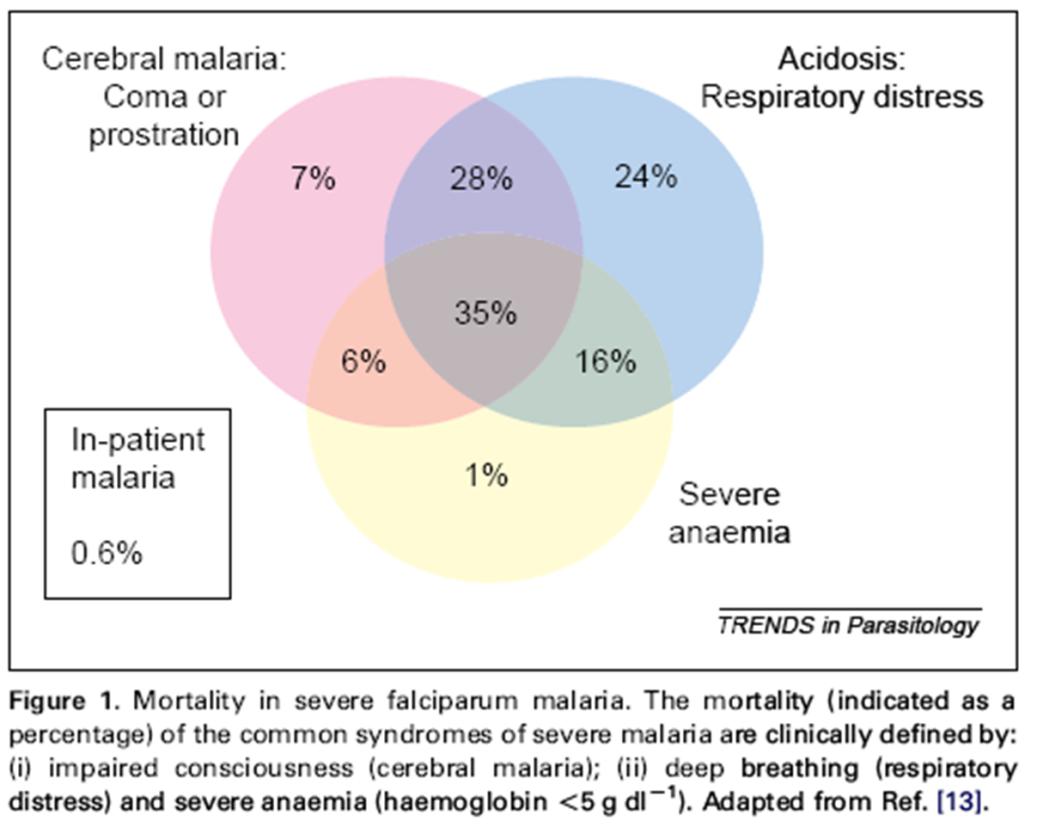
Severe and/or complicated malaria (Pf, Pv, Pk)
Blood films and
parasitaemia should be
assessed at least daily
Ventilator Blood presure support Dialysis
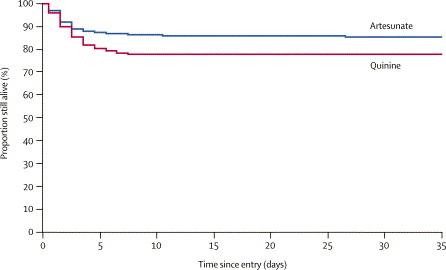
Artesunato vs chinino nel trattamento della malaria grave.
Lo studio SEAQUAMAT
Lo studio AQUAMAT
In Africa
(prevalentemente adulti)
107 deaths out of 730 (15 %)
164 deaths out of 731 (22%)
Reduction of 34.7% (18.5 – 46.6) p=0.002
SEAQUAMAT, The Lancet, 2005; 366: 7171-25
Treatment of severe malaria
I.v. Artemisinin hemisuccinate, (IVA) is superior to i.v. quinine (IVQ) in overall
survival, and IVA should be the drug of choice for treatment of severe imported
malaria in Europe.
I.v. Quinine hydrochloride, (IVQ), is the drug of choice if IVA is not available.
Oral follow up treatment: ACT as soon as the parasite density has decreased adequately (< 2%). If ACT is not available: doxycycline (adults only) (or clindamycin during pregnancy) should be used combined with quinine. Mefloquine should be avoided in patients with cerebral malaria even in the recovery phase.
ESCMID Study Group on Clinical Parasitology. Malaria J 2012;11:328
Treatment of severe malaria
Not available in Italy
I.v. Artemisinin hemisuccinate, (IVA) is superior to i.v. quinine (IVQ) in overall
survival, and IVA should be the drug of choice for treatment of severe imported
malaria in Europe.
Available in Italy
I.v. Quinine hydrochloride, (IVQ), is the drug of choice if IVA is not available.
Oral follow up treatment: ACT as soon as the parasite density has decreased adequately (< 2%). If ACT is not available: doxycycline (adults only) (or clindamycin during pregnancy) should be used combined with quinine. Mefloquine should be avoided in patients with cerebral malaria even in the recovery phase.
ESCMID Study Group on Clinical Parasitology. Malaria J 2012;11:328
aria Journa
11:
328
Haemolytic anaemia after Artesunate™
Haemolytic anemia have been reported in six out of 25 patients treated with IVA for
severe malaria diagnosed 14–31 days after the first dose of IVA .
Zoller T et al. Emerg Infect Dis. 2011;17:771-7.
A study including 55 patients with severe malaria reported late onset haemolytic
anaemia in six patients (9%) between 7 and 31 days after start of IVA.
Kreeftmeijer-Vegter AR et al. Mal J 2012, 11:102.
Three more cases have just been reported.
Rolling T et al. Malar J 2012;11:169.
Until further data are available, patients should be monitored for haemolytic anaemia
and leukopenia for 4 weeks following IVA .
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Management of Imported Malaria in Europe
Malaria Journal 2012, 11:328 doi:10.1186/1475-2875-11-328
Intensive care
Fluid management is very important.
Monitoring of plasma lactate is mandatory.
For children, the FEAST trial provided high quality evidence for paediatric malaria
and showed that fluid bolus significantly increases mortality.
Maitland K et al. N Engl J Med 2011, 364:2483-95.
In the shocked and/or acidotic patient with severe malaria, bacterial co-infection
should be sought by blood culture and antibiotic treatment started urgently if
Bruneel F et al. Am J Respir Crit Care Med 2003,167:684-9.
Cerebral malaria: corticosteroids as well as mannitol should not be given.
There is no consensus on the indications, benefits and dangers involved in
exchange blood transfusion, so it should not be used
Guidelines for the treatment of malaria. WHO, Geneva, 2011
Recommendations I
Travel history
Microscopy of thick and thin Giemsa stained blood films.
Use of a rapid diagnostic test does not replace the need for microscopy.
Monitoring after diagnosis
Microscopy of thick and thin Giemsa stained blood films to count parasites.
Managing uncomplicated malaria
Patients with P. falciparum malaria should preferably be managed as inpatients.
Under certain circumstances may outpatient management be acceptable
provided there are daily assessments and daily blood films for parasitaemia .
Managing complicated malaria
Intravenous artesunate (or i.v. Quinine) should be available
Recommendations II.
Centres managing patients with malaria should:
Be competent in microscopy of thick and thin blood films and enumeration
of malaria parasites in blood films.
Centres with access to rapid diagnostic tests only should not manage
patients with malaria.
The Center must have access to i.v. Artesunate / Quinine and ACTs.
The Center must have adequate intensive care facilities.
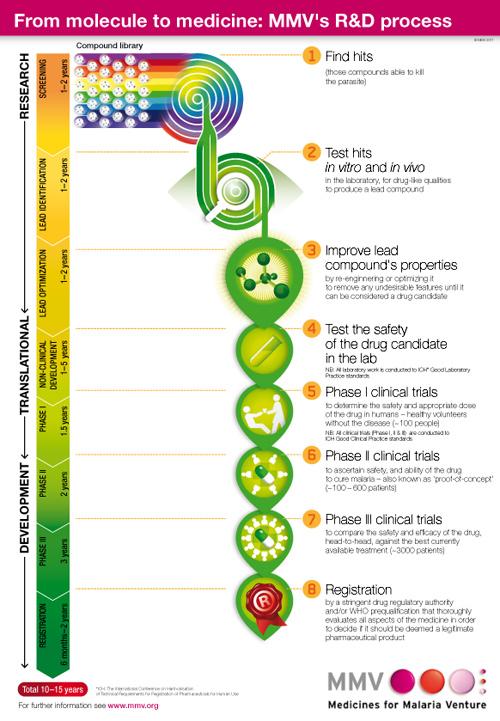
Global Malaria Portfolio, 3Q 2012
Research
Development
AMT-LMF®-D
Farmaguinhos/DNDi
Biotec/Monash/LSHTM
University of Iowa
Jomaa Pharma GmbH
University of Iowa
Imidazolidinediones
N-tert butyl isoquine
Liverpool School of
Tropical Hygeine/GSK
dUTP´ase inhibitors
Institut of Tropical
pobeguinii
Biocryst/Albert Einstein
College of Medicine
Included in MMV portfolio post registration
Medicines for Malaria Venture http://www.mmv.org/research-development/rd-portfolio
Le nuove "indicazioni" per la profilassi antimalarica
della Società Italiana di Medicina Tropicale (SIMET)
con la collaborazione di SIMIT, SIMM, SIMVIM, SoIPA
Geographical prophylaxis of malaria is based on the A-E scale
A: Awareness B: Bite prevention C: Chemoprophylaxis D: Diagnosis E: Emergency stant-by treatment
Recommendations for antimalarial chemoprophylaxis have been based on:
1) Local incidence data
- n. cases of yearly imported malaria / 100.000 travellers - API (Annual Parasite Incidence: yearly n. cases / 1.000 inhabitants
2) Presence of P. falciparum
- when only P. vivax is present, no chemoprophylaxis is recommended;
3) Stand-by treatment is considered under specific circumstances
Le linee-guida italiane per la profilassi antimalarica
-Riportati casi sporadici di malaria: rischio minimo
Egitto, Algeria, Capo Verde
AFRICA SUB-SAHARIANA
-Eritrea (rischio nelle aree < 2000 m, ad Asmara non vi è
Angola, Benin, Burkina Faso, Burundi,
Camerun, Ciad, Comore, Congo, Costa
-Etiopia (rischio nelle aree < 2000 m, ad Addis Abeba non vi
d'Avorio, Eritrea, Etiopia, Gabon, Gambia,
Ghana, Guinea, Guinea Bissau, Guinea
-Mauritania (rischio soltanto nella parte meridionale del
Equatoriale, Kenya, Liberia, Madagascar,
Malawi, Mali, Mozambico, Mauritania, Niger,
-Kenya (a Nairobi il rischio è minimo, la malaria non è
Nigeria, Rep. Centroafricana, Rep. Dem.
presente nelle aree > intorno al monte Kenya)
Congo, Rwanda, Sao Tomé e Principe,
-Senegal (rischio più basso durante la stagione secca,
Senegal, Sierra Leone, Somalia, Sudan, Sud gennaio-maggio) Sudan, Tanzania (eccetto Zanzibar) , Togo,
-Zimbabwe (malaria presente nelle aree < , soprattutto da
Uganda, Zambia, Zimbabwe
novembre a giugno, rischio minimo a Harare e Bulawayo)
AFRICA SUB-SAHARIANA
-nessun rischio nella capitale a Gibuti
Zanzibar, Mafia, Gibuti
-rischio più elevato da ottobre a maggio nel resto di Gibuti
-stagionalità della malaria: la chemioprofilassi è di prima
Botswana (regioni settentrionali), Namibia
scelta solo tra novembre e aprile
(regioni settentrionali e lungo i fiumi Kavango e Kunene), Sud Africa (Kruger e zone limitrofe), Swaziland
Botswana (escluse le regioni settentrionali), Namibia (escluse le regioni settentrionali e lungo i fiumi Kavango e Kunene), Sud Africa (escluso Kruger e zone limitrofe)
Le linee-guida italiane per la profilassi antimalarica
ASIA CENTRALE E PENISOLA ARABICA
- Armenia, Azerbaigian, Kazakistan,
Afghanistan, Arabia Saudita, Azerbaigian, Bhutan, Iran, Iraq, Kirgizistan, Yemen Siria, Turchia, Iran, Irak 100% P.
vivax -Afghanistan 90% P. vivax
-nelle altre regioni della Cina la
Cina (Hainan, Yunnan, Anhui, Henan, Hubei, Ghuizhou, Jinagsu)
malaria non è presente
ASIA SUBCONTINENTE INDIANO
Bangladesh (regione di Chittagong), India (Assam e Orissa, in particolare nella stagione dei monsoni)
ASIA SUBCONTINENTE INDIANO
-India (nelle zone centrali il rischio è
Bangladesh (eccetto regione di Chittagong), India (eccetto Assam e Orissa),
lievemente più alto rispetto a quelle
Nepal (Terai), Pakistan, Sri Lanka.
settentrionali e meridionali) -Sri Lanka 96% P.vivax -Pakistan 70% P.vivax
ASIA SUBCONTINENTE INDIANO
Nessun rischio oltre i 2000 m
Nepal (Kathmandu, Pokhara)
SUD-EST ASIATICO
-Segnalata la presenza di P.vivax
Myanmar, Cambogia (eccetto Phnom Penh, Angkor Wat e Tonle Sap), Indonesia resistente alla clorochina e di (Lombok, Sumba, Sumbaya, Timor, Flores, Molucche, Irian Jaya), Laos (parte
P.knowlesi
meridionale), Thailandia (regioni al confine con Myanmar e Cambogia),
SUD-EST ASIATICO
-Segnalata la presenza di P.vivax
Brunei, Cambogia (Phnom Penh, Angkor Wat e Tonle Sap), Filippine, Laos (parte resistente alla clorochina e di settentrionale), Malesia, Vietnam, Indonesia (eccetto Lombok, Sumba, Sumbaya, P.knowlesi Timor, Flores, Molucche, Irian Jaya)
SUD-EST ASIATICO
Singapore, Thailandia (escluse regioni al confine con Myanmar e Cambogia)
Conclusioni
1. L'infezione malarica è ancora una priorità di sanità pubblica
2. La malaria di importazione, dopo un periodo di calo, è oggi
in aumento in alcune aree del Paese
3. Le combinazioni ACT rappresentano oggi lo standard of
care (malaria grave e/o non complicata)
4. Necessità di uniformare le linee guida di management e
profilassi malaria di importazione
- Position paper ESCMID
- Indicazioni SIMET/SIMIT/SIMVIM/SIMM
Source: http://www.m2teamsoftware.it/infezmed3/phocadownload/D-inetpubwebsinfezmeditApp_RivisteCastelli.pdf
Musculoskelet Surg (2010) 94:59–61 Primary hydatid cyst of the biceps femoris M. F. Hamdi • B. Touati • A. Abid Received: 14 September 2009 / Accepted: 18 January 2010 / Published online: 4 February 2010Ó Springer-Verlag 2010 which generally involves the liver and the lungs. Primarymuscle hydatidosis is an uncommon finding. The authors A 25-year-old woman living in a rural area consulted for a
Physical Therapy Treatment Effectiveness forOsteoarthritis of the Knee: A Randomized Comparison of Supervised Clinical Exercise and Manual Therapy Procedures Versus a Home ProgramGail D Deyle, Stephen C Allison, Robert L Matekel,Michael G Ryder, John M Stang, David D Gohdes, Jeremy P Hutton, Nancy E Henderson and Matthew BGarberPHYS THER. 2005; 85:1301-1317.








