Haloperidol metabolite ii prodrug: asymmetric synthesis and biological evaluation on rat c6 glioma cells



Contents lists available at
European Journal of Medicinal Chemistry
Haloperidol metabolite II prodrug: Asymmetric synthesis andbiological evaluation on rat C6 glioma cells
Piera Sozio , Jole Fiorito , Viviana Di Giacomo Antonio Di Stefano Lisa Marinelli ,Ivana Cacciatore , Amelia Cataldi , Stephanie Pacella Hasan Turkez Carmela Parenti ,Antonio Rescifina , Agostino Marrazzo *a Dipartimento di Farmacia, Universit�a degli Studi di Chieti Gabriele D'Annunzio, Via dei Vestini 31, 66100 Chieti, Italyb Department of Pathology and Cell Biology and Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Columbia University, 630 W 168thSt., New York, NY 10032, USAc Department of Molecular Biology and Genetics, Faculty of Science, Erzurum Technical University, 25240 Erzurum, Turkeyd Dipartimento di Scienze del Farmaco, Universit�a degli Studi di Catania, Viale Andrea Doria 6, 95125 Catania, Italy
In a previous work we reported the antiproliferative effects of (±)-MRJF4, a novel haloperidol metabolite
Received 1 February 2014
II (HP-mII) (a sigma-1 antagonist and sigma-2 agonist) prodrug, obtained through conjugation to 4-
Received in revised form
phenylbutyric acid (PhBA) [a histone deacetylase inhibitor (HDACi)] via an ester bond. As a continua-
tion of this work, here we report the asymmetric synthesis of compounds (R)-(þ)-MRJF4 and (S)-
Accepted 5 November 2014
(�)-MRJF4 and the evaluation of their biological activity on rat C6 glioma cells, derived from glioblas-
Available online 6 November 2014
toma multiforme (GBM), which is the most common and deadliest central nervous system (CNS) invasivemalignancy. Favourable physicochemical properties, high permeability in the parallel artificial membrane
permeability assay (PAMPA), good enzymatic and chemical stability, in vivo anticancer activity, associ-
ated with the capacity to reduce cell viability and to increase cell death by apoptosis, render compound
(R)-(þ)-MRJF4 a promising candidate for the development of a useful therapeutic for gliomas therapy.
2014 Elsevier Masson SAS. All rights reserved.
Medicinal chemistry
signalling pathways relative to either growth or angiogenesis andwere efficient in preclinical models of gliomas . Decreasing the
Malignant gliomas are the most common types of primary brain
level of migration in various cancer cell types, including GBM,
tumours and remain one of the deadliest forms of brain cancer in
commonly restores a certain level of sensitivity to apoptosis and/or
humans. New efficient chemotherapeutics for such malignant gli-
cytotoxic drugs Furthermore, was reported that glioma cells
omas treatment were developed over the years and many are still
tend to display an overexpression of sigma (s) receptors . In this
under investigation. There is evidence that the best treatment
regard, N-(1-benzylpiperidin-4-yl)-4-iodobenzamide (4-IBP), a se-
consists of surgical resection followed by chemotherapy; combi-
lective s1 agonist, demonstrated significant anti-migratory in vitro
nation of prednisone, lomustine and vincristine could increase
activity in different analysed cancer cell lines, including the highly
survival rate in children with gliomas, whereas temozolomide
motile human U373-MG GBM cell line e. On the other hand,
could prolong the survival of adult patients . Despite the fact that
also haloperidol, a potent s1 antagonist used as anti-psychotic drug,
different treatments are available, the prognosis remains poor,
showed antiproliferative effects against glioma cells at low con-
particularly for glioblastoma multiforme (GBM), which has survival
centration (5 mM) .
rate of less than 3% at 3 years
Literature data reported that the prodrug approach was widely
To date, new anticancer compounds that are currently in clinical
used to improve the delivery of anticancer drugs (chlorambucil,
trial for gliomas are inspired from existing molecules selected for
camptothecin, paclitaxel, doxorubicin, and vinblastine) . In our
other types of cancer. Mainly, these molecules target intracellular
previous work, using this strategy, we synthesized (±)-MRJF4, anovel ester prodrug of haloperidol metabolite II (HP-mII) for thetreatment of prostate cancer ). HP-mII e endowed with
* Corresponding author.
s1 antagonist and s2 agonist properties e resulted to be more
E-mail address: (A. Marrazzo).
lipophilic than the parent drug following the esteri
These authors contribute equally to this work.
0223-5234/ 2014 Elsevier Masson SAS. All rights reserved.

P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
The obtained compounds (1R)-(þ)-4-chloro-1-(4-fluorophenyl)butan-1-ol (R)-(þ)-2 and (1S)-(�)-4-chloro-1-(4-fluorophenyl)butan-1-ol (S)-(�)-2 were condensed with 4-(4-methylphenyl)piperidin-4-ol, which is also reported in literatureand then esterified with 4-phenylbutanoyl chloride, accordingto the procedure already used by us to give (R)-(þ)-MRJF4 and(S)-(�)-MRJF4, respectively. All compounds were characterized bytheir 1H and 13C NMR spectroscopic data that resulted superim-posable with the literature ones .
Moreover, the reduction of compound 1 resulted highly enan-
tioselective and the target compounds (R)-(þ)-MRJF4 and (S)-(�)-MRJF4 were isolated, after two recrystallization from ethylacetate/diisopropyl ether, almost enantiomerically pure (98% eeand 99% ee, respectively) as ascertained by HPLC utilising a Chir-alcel OJ[-RH] column
Major hurdle in the treatment of malignant glioma with sys-
temic chemotherapy is the restricted delivery, due to the presence
Fig. 1. Chemical structures of MRJF4 enantiomers.
of BBB, of systemically administered agents for the therapy of braintumours.
PhBA (an HDACi) thus facilitating its entrance into CNS. MTT cell
The physicochemical properties of drugs influence the diffusion
viability assays have highlighted a notable increase of anti-
through the biological membranes; therefore, their evaluation may
proliferative activity of (±)-MRJF4 compared to PhBA, HP-mII, and
be useful to understand the pharmacokinetics profile of drugs
respective equimolar pharmacological association. (±)-MRJF4 has
employed as antineoplastic agents in CNS tumours. Indeed, the
also been used in combination with s
apparent partition coefficient (log P) may be used to predict the
1 agonist (þ)-pentazocine and
distribution of a drug in a biological system and can be correlated to
2 antagonist AC927 to evaluate the role of s receptor subtypes in
prostate cancer cell death
its adsorption, distribution, and CNS penetration. For both MRJF4
In this study we report the asymmetric synthesis of prodrugs
enantiomers water solubility and chemical stability were deter-
(R)-(þ)-MRJF4 and (S)-(�)-MRJF4 (and the evaluation of
mined, while CLogP was theoretically calculated (they
their biological activity on rat C6 glioma cells, derived from GBM,
displayed low water solubility (1.2 mg mL�1) and relatively high
which is the most common CNS invasive malignancy Taken
into account that the blood brain barrier (BBB) restricts the delivery
The chemical stability of (±)-MRJF4 was evaluated at pH 1.3 and
of systemically administered agents for treating brain tumours, we
pH 7.4 using a 0.02 M phosphate buffer, containing 0.1% (v/v) of
evaluated the pharmaceutical profiles of our new agents to deter-
Cremophor ELP at 37 �C The compounds showed good
mine their stability and the potential BBB permeability. Therefore,the present study included the evaluation of chemical and enzy-matic stability of (R)-(þ)-MRJF4 and (S)-(�)-MRJF4, their solubilityin Fasted State Simulated Intestinal Fluid (FASSIF), and theirrespective permeability coefficient as measured by PAMPA assay.
Furthermore, we also investigated the effect of MRJF4 racemic
mixture and its two enantiomers on the molecular mechanisms,which drive malignant C6 glioma cells proliferation and migrationsince gliomas constitute nearly 60% of primary brain tumours byinducing angiogenesis and infiltrating in the normal brain paren-chyma. We also evaluated the ability of our prodrug ant its enan-tiomers to inhibit HDAC3, a member of HDACs upregulated in solidbrain tumours; these prodrugs, being sensitive to esterases hy-drolysis, can release PhBA, which is a well-known HDACi eIn fact, HDAC inhibitors (HDACis), alone or in combination withother drugs, are emerging as a new class of anticancer agents andwere demonstrated to exert antitumour effects such as growtharrest, differentiation, and apoptosis .
2. Results and discussion
The synthesis of both enantiomers of the potential antineo-
plastic MRJF4 (4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butyl 4-phenylbutanoate) was achieved via the
Scheme 1. Synthesis of MRJF4 enantiomers. (i) (þ)-Ipc2BCl or (�)-Ipc2BCl, THF, 25 �C,
chiral reduction of 4-chloro-1-(4-fluorophenyl)-1-butanone (1)
(HOCH2CH2)2NH, diethyl ether; (ii) 4-(4-methylphenyl)piperidin-4-ol, NaHCO3, DMF,
with (þ)- or (�)-DIP-chloride, as also reported in literature
80 �C; (iii) 4-phenylbutanoyl chloride, THF, DMAP, r.t.
P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
General characteristics of (±)-MRJF4 and its two enantiomers.
s1, s2, D2 and D3 binding assays.
Ki (nM) ± S.E.M.
Water solubility (mg/mL)
PAMPA BBB Peff (10�6 cm s�1)
a Values are means SD of three experiments.
b n-Octanol/water partition coefficients were theoretically calculated by the
program ClogP for windows, version 2.0 (Biobyte Corp., Claremont, CA), according to
the methods based on the atom-additive and fragmental approaches.
c "CNSþ" (high BBB permeation predicted), Peff � 4.0 � 10�6 cm s�1; "CNS" (BBB
uncertain permeation), Peff from 4.0 to 2.0 � 10�6 cm s�1; "CNSe" (low BBB
permeation predicted), Peff < 2.0 � 10�6 cm s�1 .
b Not determined.
stability both at pH 1.3 and 7.4 (about 5 days). The degradationproducts were not characterized.
in the presence and absence of co-solvents, indicating that the
The enzymatic stability of both MRJF4 enantiomers was also
presence of Cremophor ELP did not alter the capacity of the phos-
studied at 37 �C in rat and human plasma (). As could be
pholipid layer to act as a barrier
deduced from the rate of hydrolysis (kobs) both compounds un-
Our results indicate that (±)-MRJF4 showed good permeability
dergo a fast hydrolysis in rat plasma, whereas in human plasma
(Peff �4 � 10�6 cm s�1) and, consequently, both (R)-
they were fairly stable. Taken together, these results point out that
(þ)-MRJF4 and (S)-(�)-MRJF4 enantiomers seem to be very
(R)-(þ)- and (S)-(�)-MRJF4 are sufficiently stable in the acidic
promising in BBB penetration .
environment of the stomach and are potentially absorbed by the
Considering these results, binding affinity and selectivity were
intestine after oral administration.
measured for both enantiomers of MRJF4 and HP-mII, by con-
Moreover, to study the stability of (±)-MRJF4 in the presence of
ducting competitive binding assays In the s1, s2, dopamine
glioma C6 cells we adopted a mass spectrometric approach. Glioma
D2 and D3 assays, guinea pig membrane (for s1 and s2), rat striatum
C6 cells were incubated with (±)-MRJF4 (5 mM) for 24 h at 37 �C,
(for D2), and rat olfactory tubercle (for D3) tissue membranes were
and then the cell lysates were analysed to verify the potential
used as receptor sources. Moreover, [3H]-(þ)-pentazocine, [3H]-1,3-
generation of metabolites within cells; in particular, a signal at m/z
di(2-tolyl)guanidine ([3H]-DTG) with unlabelled (þ)-pentazocine,
214 was observed in the control cell lysates (We iden-
[3H]-spiperone and [3H]-7-OH-DPAT were used as radioactive
tified additional prominent ions at m/z 524, 360, and 212 in the
(±)-MRJF4-treated cell lysates (The peak at m/z 524 re-
displays s1, s2, D2, and D3 receptor affinities of
fers to MRFJ4 (while the peak at m/z 360 was generated
(þ)-MRJF4, (�)-MRJF4, (þ)-HP-mII, and (�)-HP-mII relative to
from hydrolyses of ester bond followed by dehydration. We also
respective racemic mixtures. The (þ)- and (�)-enantiomers of HP-
identified a minor product ion at m/z 212 corresponding to 4-(4-
mII exhibited high s1 binding affinity (Ki ¼ 2.0 ± 0.4 and
chlorophenyl)-4-hydroxypiperidine. At 24 h after (±)-MRJF4
3.0 ± 0.8 nM, respectively). Moreover, (þ)-HP-mII showed lower
treatment, we observed two main peaks originated from hydrolysis
affinity to s2 receptor (Ki ¼ 32.0 ± 2.0 nM) if compared with its
of ester prodrug and one metabolite showing a reduced meta-
opposite isomer (�)-HP-mII (Ki ¼ 9.8 ± 1.3 nM) and haloperidol
bolism within the glioma C6 cells at physiological pH.
(Ki ¼ 18.0 ± 2.2 nM), these data are in accord to literature ones .
In order to better predict the ability of drugs to diffuse through
The apparently anomalous higher affinity of (±)-HP-mII, for s2 re-
the biological membranes, PAMPA was used as a non-cell-based
ceptors, with respect to its enantiomers, could be related to a
assay for measuring passive permeability of the investigated
positive allosteric modulation of the two enantiomers, as previ-
compounds . Depending on the phospholipid type, PAMPA can
ously reported in literature for sigma receptors . Conversely
mimic different adsorption/permeation environments. In partic-
to haloperidol, (þ)-HP-mII and (�)-HP-mII showed a reduced af-
ular, porcine polar brain lipid is used for BBB permeation assays
finity for dopamine D2 and D3 receptors. In particular, (þ)-HP-mII
(PAMPA-BBB) . Since our derivatives were not thoroughly sol-
displayed a 111-fold lower affinity and a 145-fold lower affinity for
uble in the conventional buffer used for PAMPA, the permeability
� D2 ¼ 256 ± 7.4 nM;
tests of new compounds were examined in the presence of co-
Ki � D3 ¼ 1278 ± 22 nM) with respect to haloperidol
solvents (0.1% (v/v) Cremophor ELP). To demonstrate that co-
(Ki � D2 ¼ 2.3 ± 0.7 nM; Ki � D3 ¼ 8.8 ± 1.5 nM), while (�)-HP-mII
solvents do not change the permeability of the phospholipid layer
displayed a Ki value of 71.0 ± 3.5 nM and 353 ± 6.7 nM for D2 and D3
at the investigated concentrations, we evaluated their effect on the
receptors, respectively. According to our previously reported data
permeability of Dopamine (DA), used as reference compound, due
on (±)-MRJF4 , the esterification of the hydroxyl group on the
to its inability to cross the membrane passively. After 18 h of in-
(þ)- and (�)-HP-mII enantiomers with PhBA decreased the affinity
cubation, the effective permeability (Peff) of DA was irrelevant, both
Table 2Chemical and enzymatic stabilities of (±)-MRJF4 and its two enantiomers.
a Values are means SD of three experiments.


P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
for both s receptor subtypes. Nevertheless, good s1 and s2 affinities
Annexin-V/PI staining which allows the detection of apoptotic
were found in (þ)-MRJF4 (Ki � s1 ¼ 87.5 ± 4.5 nM;
features by detecting phosphatidylserine exposure at the mem-
� s2 ¼ 52.7 ± 3.8 nM) compared to (�)-MRJF4
brane level. Under treatment conditions, the amounts of Annexin
(Ki � s1 ¼ 230 ± 8.9 nM; Ki � s2 ¼ 118 ± 7.3 nM) and (±)-MRJF4
Vpos/PIpos late apoptotic cells and Annexin Vneg/PIpos necrotic ones
(Compounds (þ)-MRJF4 and (�)-MRJF4, analogously to
were significantly increased by (±)-MRJF4 and both enantiomers
racemate MRJF4, showed insignificantly affinity for dopamine D2
this increase was more significant after 48 h of treatment
and D3 receptors (Ki > 5000 nM).
compared to 24 and 72 h samples. In particular, (R)-(þ)-MRJF4 was
In the present study, we also investigated the effect of both
the most effective compound (about 40% of late/necro) followed by
enantiomers of MRJF4 on the molecular mechanisms of glioma cell
(±)-MRJF4 and (S)-(�)-MRJF4 (35% and 25%, respectively).
migration and invasion. To assess the effects of (R)-(þ)-MRJF4, (S)-
The effect of all five compounds on C6 cell proliferation was
(�)-MRJF4, and their racemic mixture on C6 cells, in terms of
studied after 24, 48, and 72 h of treatment at different concentra-
apoptosis and cell death, we performed flow cytometry analysis of
tions ranging from 0 to 5 mM as time and dose response experimentCell proliferation was inhibited in a concentration-
Fig. 2. Annexin V/PI detection of early apoptotic and apoptotic necrotic/late cells in C6cells treated with 5 mM compounds (±)-MRJF4, (R)-(þ)-MRJF4, (S)-(�)-MRJF4, PhBAand (±)-HP-mII for 24, 48, and 72 h. Early apoptotic cell populations (Annexin-Vpos/
Fig. 3. Effects of compounds (±)-MRJF4, (R)-(þ)-MRJF4, (S)-(�)-MRJF4, PhBA and
PIneg) can be discriminated from late apoptotic (Annexin-Vpos/PIpos)/necrotic cells
(±)-HP-mII on C6 cell proliferation. Graphs show results of MTT assay after 24, 48, and
(AnnexinVneg/PIpos) according to their fluorescence emission. *p < 0.05; **p < 0.01
72 h of treatment with increasing concentration of all five agents. *p < 0.05; **p < 0.01
relative to control sample.
relative to control sample.
P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
dependent manner. At 24 h, concentrations higher than 0.5 mM
with (±)-HP-mII did not show any significant decrease of cell
induced a significant reduction of cell viability in all treated sam-
viability; PhBA reduced cell viability of about 20%, while the cell
ples, with (R)-(þ)-MRJF4 being the most effective (40% of the
viability of samples treated with the other three compounds was
control sample with respect to about 50% of (S)-(�)-MRJF4 and of
around 60%. At 72 h, only (±)-MRJF4, (R)-(þ)-MRJF4, and (S)-
(±)-MRJF4) with IC50 value of 5 mM. At 48 h, the samples treated
(�)-MRJF4 retained an antiproliferative effect with cell viabilitydecrease of about 40%.
Since (±)-MRJF4 causes a notable increase of antiproliferative
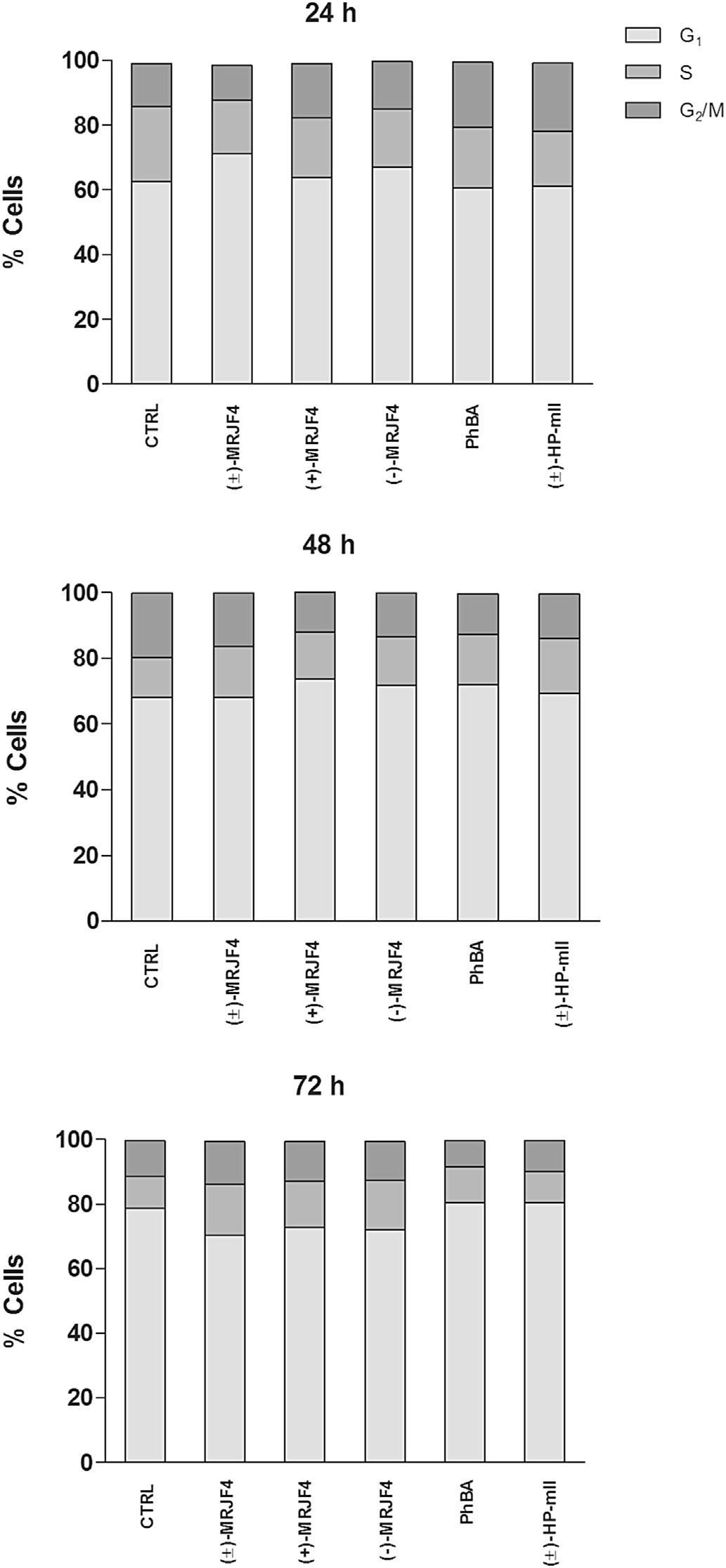
activity in LNCaP and PC3 cell lines flow cytometry cell cycleanalysis on C6 cells was performed. Our analysis showed an in-crease in the S phase when cells were treated with (±)-MRJF4 andboth enantiomers for 72 h (whereas shorter treatments didnot produce any significant changes. Such effects were quiteevident in the histograms events/DNA content of 72 h that showedan increased S phase with (±)-MRJF4 and both enantiomers, whilein samples treated with PhBA and (±)-HP-mII S phase was foundcomparable to controls.
Transwell chamber assay was employed to determine the effect
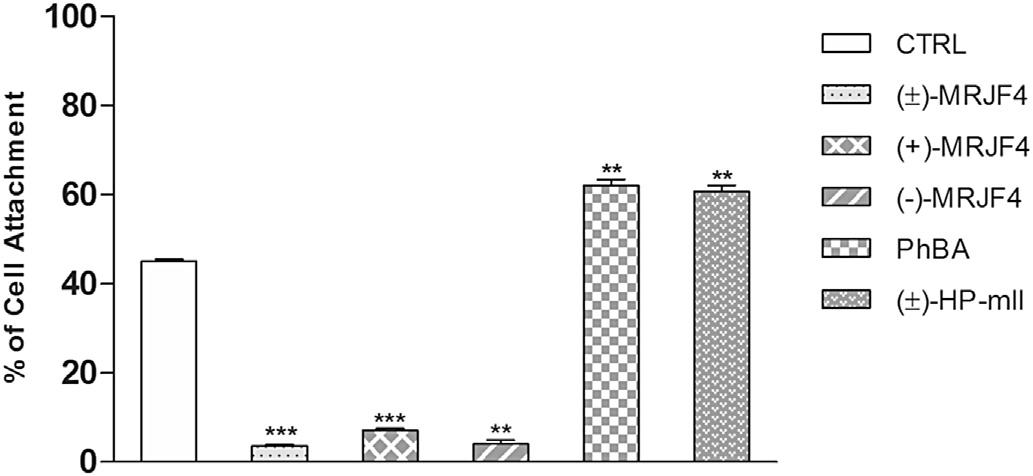
of these agents on the migration capability of C6 glioma cell line. Asshown in the percentage of cells passing through the insertedfilter significantly decreased when cells were treated for 24 h withcompound (±)-MRJF4 and both enantiomers (less than 10% vsalmost 60% in the control sample). On the other hand, PhBA and(±)-HP-mII had no effect on the capability of C6 glioma cellmigration.
To evaluate the inhibitory activity of our compounds, histone3
(H3) acetylation was also investigated at 6, 15, and 24 h aftertreatment After 6 h the inhibition was especiallyaugmented by (R)-(þ)-MRJF4 (16.1% vs 4.5% of the DMSO);(±)-MRJF4 had the same activity as PhBA (about 14%) while theother compounds did not produce any significant effects onacetylation. After 15 h of treatment, there were no changes in allsamples with respect to the vehicle alone, whereas after 24 h (R)-(þ)-MRJF4 showed an increase in H3 acetylation (7.2 vs 4.5 of thecontrol sample); this effect could probably be due to the meta-bolic pathways involving both enantiomers. Specifically, first hy-drolysis of ester generates HP-mII and the PhBA responsible foracetylation of H3 at 6 h. Subsequently, as already reported inliterature, HP-mII could undergo additional metabolism gener-ating 4-(p-fluorophenyl)-4-hydroxybutyric acid and/or haloper-idol metabolite III able to interfere with HDAC activity only after24 h
Glioma is an aggressive cancer characterized by high mortality,
especially in children. It is known that glioma cells tend to displayan overexpression of s receptors. This study was aimed to explorethe potential effects of haloperidol metabolite II prodrugs as useful
Fig. 4. Effects of a 5 mM concentration of compounds (±)-MRJF4, (R)-(þ)-MRJF4, (S)-(�)-MRJF4, PhBA, and (±)-HP-mII on cell cycle progression of C6 cells. The amount ofcells in G1, S, and G2/M phase can be detected correlating the number of events with
Fig. 5. Effects of a 5 mM concentration of compounds (±)-MRJF4, (R)-(þ)-MRJF4, (S)-
the fluorescence emission on FL3. Graphs show the percentage of cells found in G1, S
(�)-MRJF4, PhBA and (±)-HP-mII on C6 cell migration after 24 h. *p < 0.05; **p < 0.01;
and G2/M phase.
***p < 0.001 relative to control sample.
P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
Analytical HPLC measurements were run on a Waters 600 HPLC
pump (Waters Corporation, Milford, MA, USA), equipped with aWaters 2996 photodiode array detector, a 20 mL Rheodyne injectorand a computer-integrating apparatus. HPLC was performed usinga Waters Symmetry RP-C18 column (150 � 4.6 mm, 5 mm); themobile phase consisted in a mixture of acetonitrile, water, andformic acid. Two channels were used: channel A with acetonitrile/water 5/95 and 0.1% v/v of formic acid; channel B with acetonitrileand 0.1% v/v of formic acid. The gradient used was from 100% A to100% B over 20 min, 100% B was maintained for 5 min and in the lastminute, we came back to 100% of A. The flow rate was 1 mL min.
The UV-detector was set at a length of 264 nm. EnantioselectiveHPLC analyses were carried out using the same above describedapparatus and mobile phase utilising a Chiralcel OJ[-RH] column(150 � 4.6 mm, 5 mm).
Fig. 6. Effects of a 5 mM concentration of compounds (±)-MRJF4, (R)-(þ)-MRJF4, (S)-(�)-MRJF4, PhBA and (±)-HP-mII on H3 acetylation in C6 cells. Graph shows the
percentage of acetylated H3. *p < 0.05; **p < 0.01 relative to control sample.
4.3.1. (1R)-(þ)- and (1S)-(�)-4-chloro-1-(4-fluorophenyl)-butan-
therapeutic tools for gliomas therapy. Taken together our results
1-ol, (R)-(þ)-2 and (S)-(�)-2
indicate that the racemic mixture and the two enantiomers exhibit
Both compounds were synthesized as already reported in
good anticancer activity; they are able to reduce cell viability
literature and all analytical and spectral data are consistent
(measured by MTT assay), and to increase cell death by apoptosis.
with the reported ones.
The obtained data indicate that cell proliferation is inhibited inconcentration- but not in a time-dependent manner. The amounts
4.3.2. (1R)-(þ)- and (1S)-(�)-4-(4-chlorophenyl)-1-[4-(4-
of Annexin Vpos/PIpos late apoptotic cells and Annexin Vneg/PIpos
necrotic ones were significantly increased with all compounds; in
particular, results obtained with (R)-(þ)-MRJF4 were more
Both compounds were synthesized as already reported in
literature and all analytical and spectral data are consistentwith the reported ones. Here we report only the data inherent topurity, obtained after two recrystallization from ethyl acetate/dii-
4. Experimental section
sopropyl ether.
(R)-(þ)-HP-mII, white solid, mp: 131e132 �C; 98% ee,
4.1. Material and methods
D ¼ þ66.2 (c ¼ 1.5 in CHCl3); HRMS-FAB: m/z [M þ H]þ calcd for
C21H26ClFNO2: 378.1636, found: 378.1633; Anal. calcd for
C21H25ClFNO2: (C, H, N, O).
(S)-(�)-HP-mII, white solid, mp: 132e133 �C; 99% ee,
phenylbutanoyl chloride were purchased from Sigma Aldrich
D ¼ �67.7 (c ¼ 1.5 in CHCl3); HRMS-FAB: m/z [M þ H]þ calcd for
(Milan, Italy). Cremophor® ELP was obtained from BASF-The
C21H26ClFNO2: 378.1636, found: 378.1639; Anal. calcd for
chemical Company. All other chemicals were of the highest purity
C21H25ClFNO2: (C, H, N, O).
(þ)-(R)-MRJF4 and (�)-(S)-MRJF4)
To a solution of (R)-(þ)-HP-mII or (S)-(�)-HP-mII (400 mg,
The identity of all new compounds was confirmed by NMR data.
1.058 mmol) in anhydrous THF (10 mL) 4-phenylbutanoyl chloride
Homogeneity was confirmed by TLC on silica gel Merck 60 F254 and
(181 mL, 1.095 mmol) was added at 0 �C and under stirring. The
their purities (>98%) were quantified by HPLC and HR-MS. Solu-
reaction was left for 24 h at r.t. under a nitrogen atmosphere.
tions were routinely dried over anhydrous sodium sulphate prior to
Subsequently, a NaHCO3 saturated solution (20 mL) was added and
evaporation. Chromatographic purifications were performed by
the organic solvent was evaporated. After extraction with CH2Cl2
Merck 60 70e230 mesh ASTM silica gel column.
and purification by flash chromatography the final compound (R)-
NMR spectra were recorded on a Varian VXR 300 MHz spec-
(þ)- or (S)-(�)-MRJF4 was obtained as a colourless oil (250 mg
trometer. Chemical shifts are reported in parts per million (d)
45%): Rf ¼ 0.33 (CHCl3/MeOH 95:5); 1H NMR (300 MHz, CDCl3):
downfield from the internal standard tetramethylsilane (Me4Si).
d 7.37e6.92 (m, 13H, ArH), 5.67 (t, J ¼ 6 Hz, 1H, CH), 3.65 (bs, 1H, OH)
The LC-MS/MS system used consisted of an LCQ (Thermo Finnigan)
2.70e2.66 (m, 2H, CH2), 2.56e2.51 (m, 2H, CH2), 2.35e2.24 (m, 6H,
ion trap mass spectrometer (San Jose, CA, USA) equipped with an
3CH2), 2.06e1.60 (m, 10H, 5CH2); 13C NMR (75 MHz, CDCl3):
electrospray ionization (ESI) source. The capillary temperature was
d 172.71 (s, 1C, CO), 159.86 (s, J ¼ 330.2 Hz, 1C, Ar), 148.74 (s, 1C, Ar),
set at 300 �C and the spray voltage at 4.25 kV. The fluid was
141.28 (s, 1C, Ar), 136.39 (s, 1C, Ar), 134.12 (s, J ¼ 21 Hz, 1C, Ar),
nebulized using nitrogen (N2) as both the sheath and the auxiliary
128.44 (s, J ¼ 54 Hz, 2C, Ar), 128.38 (s, 2C, Ar), 128.19 (s, 2C, Ar),
gas. Melting points were determined on a Büchi B-450 apparatus
126.06 (s, 2C, Ar), 125.98 (s, 1C, Ar), 115.58 (s, J ¼ 38 Hz, 2C, Ar),
and are uncorrected. Optical rotations were taken at 20 �C with a
75.08 (s, 1C, CH), 71.00 (s, 1C, C), 58.12 (s, 1C, CH2), 49.36, (s, 2C,
PerkineElmer 241 polarimeter. Microanalyses were performed on a
CH2), 38.28 (s, 2C, CH2), 35.03 (s, 1C, CH2), 34.18 (s, 1C, CH2), 33.81 (s,
EA1106 Carlo Erba CHN analyser; analyses indicated by the symbols
1C, CH2), 26.47 (s, 1C, CH2), 22.90 (s, 1C, CH2).
of the elements or functions were within ±0.4% of the theoretical
(R)-(þ)-MRJF4, 97.98% ee, R
t 3.21 min, [a]D ¼ þ65.4 (c ¼ 1.2 in
CHCl3); HRMS-FAB: m/z [M þ H]þ calcd for C31H36ClFNO3:
P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
524.2368, found: 524.2372; Anal. calcd for C31H35ClFNO3: (C, H, N,
4.8. Stability studies of MRJF4 in glioma C6 cells
(S)-(�)-MRJF4, 98.89% ee, R
t 3.48 min, [a]D ¼ �66.9 (c ¼ 1.2 in
Glioma C6 cell lysates (2 mg) were prepared for the LC-MS
CHCl3); HRMS-FAB: m/z [M þ H]þ calcd for C31H36ClFNO3:
analysis as reported by Kim et al. The stability of MRJF4 in
524.2368, found: 524.2371; Anal. calcd for C31H35ClFNO3: (C, H, N,
presence and in absence of glioma C6 cells (at 24 h) was assayed
using a LCQ™ Deca XP Plus LC/MSn spectrometer (Thermo Fin-nigan, San Jose, CA, USA). The potential at the nanospray needle wasset at 4400 V. The orifice potential was 46 V, and the curtain gas
4.3.4. (R)-(þ)-MRJF4 and (S)-(�)-MRJF4) oxalates
was 15 psi (pound-force per square inch).
Both enantiomers were transformed into oxalate salts to best
preserve them for biological tests. All spectral data are consistentwith the reported ones for (±)-MRJF4
4.9. PAMPA method
(R)-(þ)-MRJF4 oxalate, white solid, mp: 111e113 �C; 98% ee,
HRMS-FAB: m/z [M þ H]þ calcd for C31H36ClFNO3: 524.2368, found:
The following protocol was applied to measure the Peff through
524.2371; Anal. calcd for C33H37ClFNO7: (C, H, N, O).
the artificial membrane to predict oral absorption and BBB
(S)-(�)-MRJF4 oxalate, white solid, mp: 112e114 �C; 99% ee,
permeation. The effective permeability of BBB was measured using
HRMS-FAB: m/z [M þ H]þ calcd for C31H36ClFNO3: 524.2368, found:
phospholipid mixture from porcine polar brain lipid extract,
524.2365; Anal. calcd for C33H37ClFNO7: (C, H, N, O).
composed by phosphatidylcholine (PC) 12.6%, phosphatidyletha-nolamine (PE) 33.1%, phosphatidylserine (PS) 18.5%, phosphatidy-linositol (PI) 4.1%, phospatidic acid (PA) 0.8% and 30.9% of other
4.4. Kinetics of chemical hydrolysis
compounds (purchased from Avantis Polar Lipids-Alabaster, AL).
Each donor filtration plate well was carefully impregnated with
A 0.02 M phosphate buffer of pH 7.4 or a 0.02 M chloridric buffer
5 mL of this solution and, immediately after, 150 mL of phosphate
of pH 1.3, containing 0.1% (v/v) Cremophor ELP, was used to eval-
buffer (pH 7.4/pH 6.5), containing 500 mM of each compound, and
uate chemical stability at physiological pH. Reaction was initiated
iPrOH 20% as co-solvent was added. Then the drug-filled donor
by adding 1 mL of 10�4 M stock solution (in acetonitrile) of the
plate was placed into the acceptor plate that was prefilled with the
compound to 10 mL of thermostated (37 ± 0.5 �C) aqueous buffer
same buffer (300 mL) as acceptor solution. After plate lid was
solution. At appropriate time intervals (for a total period of one
replaced, the resulting assembled donor-acceptor plates were
week), samples of 20 mL were withdrawn and analysed by HPLC.
incubated at r.t. for 18 h, following which drugs concentration in
Pseudo-first-order rate constants (kobs) for the hydrolysis of the
the acceptor and donor solutions were determined by HPLC
compounds were then calculated from the slopes of the linear plots
Log Peff can be calculated from the equation below:
of log (% residual compound) against time. The experiments were
run in triplicate and the mean values of the rate constants were
4.5. Kinetics of enzymatic hydrolysis
where Peff is the effective permeability coefficient (cm s�1), VD isvolume of donor compartment (0.15 cm3) and VA is volume of
Human and rat plasma were obtained by centrifugation of blood
acceptor compartment (0.30 cm3), A is effective filter area
samples containing 0.3% citric acid at 3000 � g for 15e20 min.
(0.28 cm2), t is incubation time for the assay (s), [drug]acceptor is the
Plasma fractions (4 mL) were diluted with 0.02 m phosphate buffer
concentration of the compound in the acceptor compartment at the
(pH 7.4) to give a final volume of 5 mL (80% plasma). Incubation was
completion of the assay, and [drug]equilibrium is the concentration of
performed at 37 ± 0.5 �C using a shaking water bath. The reaction
compound at theoretical equilibrium.
was initiated by adding 200 mL of a stock solution of drug (1 mg/mLin acetonitrile) to 5 mL of preheated plasma. Aliquots (100 mL) were
4.10. Receptor binding studies
taken at various times and deproteinized by mixing with 200 mL of0.01 M HCl in methanol. After centrifugation for 5 min at 5000 � g,
10 mL of the supernatant layer were analysed by chromatography as
s1, s2, D2 and D3 receptor binding studies were performed
according to literature Briefly, guinea pig brain membranes
described above. The amounts of remaining intact compound were
(500 mg protein) were incubated with 3 nM [3H]-(þ)-pentazocine
plotted as a function of incubation time .
(29 Ci/mM; (Kd) was 14 ± 0.3 nM, n ¼ 3) and six concentrations oftested compounds or sigma ligands (from 10�5 to 10�10 M) in 1 mL
4.6. Water solubility
of 50 mM TriseHCl (pH 7.4). The reaction was performed for150 min at 37 �C and terminated by filtering the solution through
Compounds (R)-(þ)-MRJF4 and (S)-(�)-MRJF4 (50 mg) were
Whatman GF/B glass fibre filters which were presoaked for 1 h in a
placed in deionized water (1 mL), shaken at 25 �C for 1 h to ensure
0.5% poly(ethylenimine) solution. Filters were washed with ice-
the solubility equilibrium and then centrifuged. The supernatant
cold buffer (2 � 4 mL). Nonspecific binding was assessed in the
(20 mL) was analysed by HPLC .
presence of 10 mM of unlabelled haloperidol. s2 binding assays weremade according to the following protocol: Guinea pig brain mem-branes (360 mg protein) were incubated with 3 nM [3H]DTG
4.7. Lipophilicity
(53.3 Ci/mM; Kd ¼ 11 ± 0.8 nM; n ¼ 3) and each test compound(from 10�5 to 10�10 M) in 0.5 mL of 50 mM TriseHCl (pH 8.0) for
n-Octanol/water partition coefficients were theoretically calcu-
120 min at room temperature in the presence of 400 nM
lated by the program ClogP for windows, version 2.0 (Biobyte Corp.,
(þ)-SKF10,047 to mask s1 sites. Nonspecific binding was evaluated
Claremont, CA), according to the methods based on the atom-
with DTG (5 mM). Each sample was filtered through Whatman GF/B
additive and fragmental approaches.
glass fibres filters, which were presoaked for 1 h in a 0.5%
P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
poly(ethylenimine) solution, using a Millipore filter apparatus.
exclusion of aneuploid cells and nuclei doublets from further
Filters were washed twice with 4 mL of ice-cold buffer.
analysis. PI fluorescence gathered at linear FL3 was used to measure
The rat striatum and rat olfactory tubercle were used for D2 and
DNA content.
D3 receptors, respectively. Tissue preparations and binding assayswere carried out according to Mennini et al. After incubation,
4.15. Migration assay
the samples were filtered through Whatman GF/B or GF/C glassfibre filters, which were pre-soaked in a 0.5% poly(ethylenimine)
Cell migration was assayed by means of a transwell chamber
solution, using a Millipore filter apparatus. The filters were washed
containing a polycarbonate insert with 8 mm pores placed between
twice with 4 mL of a suitable ice-cold buffer.
the upper and lower wells (Corning, NY, USA). Cells were cultured
Radioactivity was counted in 4 mL of ‘Ultima Gold MV' in a 1414
to 70e80% of confluency, and then starved for 24 h in serum free
Winspectral PerkinElmer Wallac or Beckman LS6500 scintillation
condition. C6 glioma cells were then trypsinized, centrifuged and
counter. Inhibition constants (Ki values) were calculated using the
resuspended in serum free HAM'S F12 at a concentration of 105
EBDA/LIGAND program purchased from Elsevier/Biosoft.
cells mL�1. 100 mL of such suspension was added to the upperchamber of the transwell and 600 mL of HAM'S F12 with 5% FCS was
4.11. Cell culture
added in the lower chamber. Compounds, at a final concentration of5 mM, were added 2 h later in order to allow cells to adhere to
C6 rat glioma cell line was obtained from the American Type
membrane. After 24 h of incubation at 37 �C, cells on the upper side
Collection (ATCC) and maintained in HAM'S F12 supplemented
of the filter were removed with a cotton swab, while cells that
with 2 mM Glutamine, penicillin-streptomycin (100 mg mL�1) and
migrated through the pores to the lower side of the membrane
10% FBS. Cells were grown at 37 �C in a humidified atmosphere of
were fixed with absolute methanol and stained with DAPI. The filter
was then cut out with a scalpel, mounted on a slide and nuclei werecounted under a microscope in five random fields whose area were
4.12. Cell viability assay
To calculate migration, the total number of cells was determined
Cell viability was measured by MTT (3[4,5-dimethylthiazol-2-
counting and averaging the total number of cells in each of the
yl]-2,5-diphenyl tetrazolium bromide) growth assay, according to
random fields. The number obtained was divided by area of the
manufacturer's instruction (Sigma Aldrich, St Louis, USA). In this
microscope viewing field and multiplied by the entire area of the
assay the cell number was quantified by the amount of tetrazolium
transwell insert. Percentage of migration was calculated by dividing
reduction in viable mitochondria. Cultured cells were incubated
the total number of cells by the number of cells seeded and
into 24-well plate at 5 � 104 cells/well and exposed to various
multiplying this value by 100 to get percent.
concentrations of all the five agents (0.1e5 mM). After 24, 48, and72 h cells were processed and the absorbance of each well was
4.16. Flow cytometry detection of acetylated histone H3
detected at 570 nm. Percentage of viable cells was calculated usingthe equation As/A0 � 100 where As is the absorbance value obtained
C6 cells were stained for acetylated H3 as previously described
for a sample containing cells in the presence of a given concen-
Briefly, after incubation, cell culture medium was removed
tration of agent, and A0 is the absorbance value of vehicle treated
and cells were fixed for 15 min in 1% p-formaldehyde on ice. Then
control. Four independent experiments were repeated under the
cells were trypsinized and pellets were washed with 1 mL of PBS/
same experimental conditions.
BSA 1% and centrifuged at 130 g for 10 min at 4 �C. Cell was thenpermeabilized in 200 mL of 0.1% Triton-X in PBS for 10 min at room
4.13. Annexin-V/PI detection of apoptotic and necrotic cells in flow
temperature. After washing, each pellet was resuspended in 100 mL
of a saturation solution (PBS without calcium and magnesiumcontaining 10% of goat serum) and incubated for 20 min on ice.
To assess apoptosis, a commercial Annexin-V-FITC/PI Kit
Anti-Acetyl H3 (Lys 9) rabbit monoclonal antibody (Thermo Sci-
(Bender Med System, Vienna, Austria) was used according to the
entific, OH, USA) was added diluted 1:100 in the saturation solu-
manufacturer instructions. Briefly, 2.5 � 105 cells were gently
tion. Samples were incubated for 1 h on ice. Primary antibody was
resuspended in binding buffer and incubated for 10 min at room
removed and secondary FITC goat anti-rabbit IgG antibody (Milli-
temperature in the dark with Annexin-V-FITC. Samples were then
pore, MA, USA) was added (20 mg mL�1) and incubated on ice in the
dark for 45 min. Secondary antibody was removed and, prior to
(5 mg mL�1) and analysed on a FC500 flow cytometer with the FL1
running on the FC500, cells were resuspended in 400 mL of PBS.
and FL3 detector in a log mode using the CXP analysis software
About 10000 events were collected for all samples on FC500 using
(Beckmann Coulter, FL, USA). For each sample, at least 104 events
488 nm laser excitation and analysed with CXP software (Bekmann
were collected. Viable cells were Annexin-Vneg/PIneg (unlabelled),
Coulter). Mean fluorescence intensity (MFI) was obtained by his-
early apoptotic cells were Annexin-Vpos/PIneg, late apoptotic and
togram statistics and are provided to quantify the H3 acetylation.
necrotic cells were Annexin-Vpos/PIpos and Annexin-Vneg/PIpos,respectively.
Appendix A. Supplementary data
4.14. Cell cycle analysis
Supplementary data related to this article can be found at
Approximately 3 � 105 cells per experimental condition were
harvested, fixed in 70% (v/v) cold ethanol and kept at 4 �C over-night. Cells were then resuspended in 20 mg mL�1 PI and
100 mg mL�1 RNAse, final concentrations. Cell cycle profiles (104cells) were analysed by a FC500 flow cytometer with the FL3 de-
tector in a linear mode using the CXP software (Beckmann Coulter,
FL, USA). A dual FL3-Area/FL3-Width graph was used for the
P. Sozio et al. / European Journal of Medicinal Chemistry 90 (2015) 1e9
Source: http://openaccess.erzurum.edu.tr/bitstream/handle/123456789/30/Hasan%20%20Turkez.pdf?sequence=1&isAllowed=y
"Farmacología kinésica deportiva" Cátedra Kinesiología Deportiva Encargado de enseñanza Dr. Mastrángelo, Jorge Lic. Spinetta, Daniel Integrantes Balzi, Brenda Bettini, Florencia Ferraris, Juan Manuel Fortuondo, María Emilce Gómez, Vanina Guisasola, Pablo L'Afflitto, Mariana Micó, Gustavo Vazquez, Lorena Vignolo, Florencia
Ramset Chemset Injection Reo 502 ITW Australia Pty Ltd (Ramset) Chemwatch Hazard Alert Code: Issue Date: 09/09/2015 Version No: 3.1.1.1 Print Date: 09/09/2015 Material Safety Data Sheet according to NOHSC and ADG requirements Initial Date: Not Available SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING








