Engaging workplaces in tb care and control

Multidrug-resistant Tuberculosis
Charles L. Daley, MD
National Jewish Health
Chair, Global GLC, WHO and Stop TB Partnership
Disclosures
• World Health Organization – Chair, Global GLC • Otsuka – Chair, Data Monitoring Committee
for clinical trials of delaminid
Multidrug-resistant Tuberculosis
• Pathogenesis and Transmission of Drug
• Epidemiology of MDR-TB
• Diagnosis of MDR-TB
• Treatment of MDR-TB
• New Drugs for MDR-TB
Definitions for Multidrug and
Extensively Drug Resistant TB
9 million TB cases
Drug Susceptible
Resistance
MDR-TB: Resistance to at least isoniazid and rifampin
XDR-TB: MDR plus resistance to fluoroquinolones and
one of the second-line injectable drugs (amikacin,
kanamycin, or capreomycin)
Source: Peter Cegielski
Pathogenesis and Transmission of
Drug-resistant TB
M. tuberculosis
Resistant Mutants
Inadequate treatment
Acquired Resistance
Inadequate infection control Diagnostic delay
Primary Resistance
MDR-TB Notification and Enrollment
Estimated MDR-TB cases among notified TB patients in 2010
No data 0-300 301-3,000 3,001-30,000 30,001-60,000 >60,000
Percentage of Previously Treated
TB Cases with MDR-TB
Countries with at Least One Case
of XDR-TB by end of 2011
WHO Global TB Report, 2012
MDR-TB Notification and Enrollment
MDR cases reported vs estimated among notified TB, 2010
WHO Region
Estimated
Reported
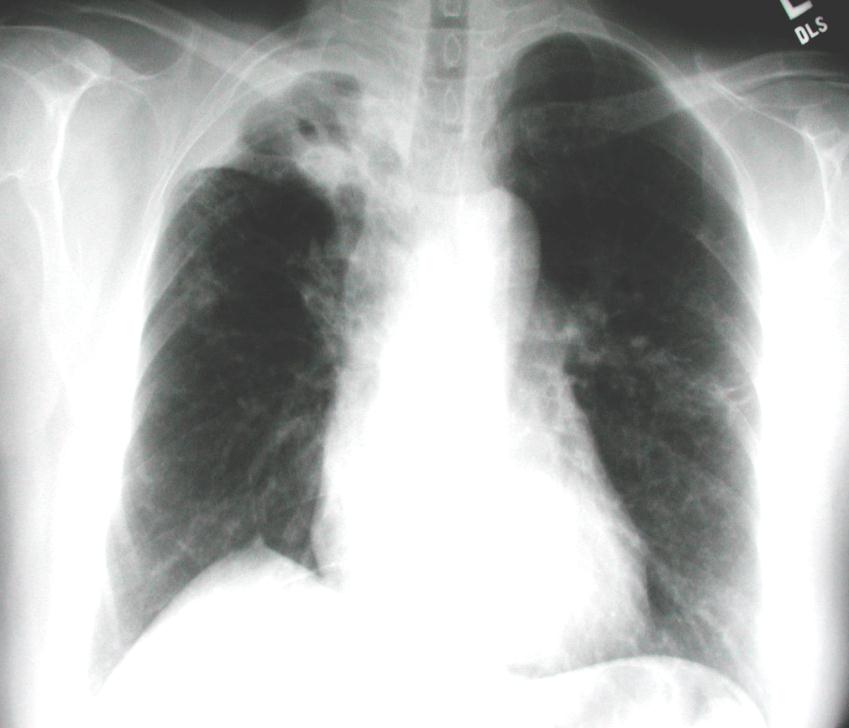
Clinical Case
• 57 year old Filipina
woman seen in TB clinic for immigration screening 7 days after arrival to U.S.
• Patient had chronic
cough, fatigue, anorexia, and fever for months
– Treated for TB in PI for the
– Hx of diabetes mellitus
Diagnostic Work-Up of
TB Suspect
Sputum Specimen (x2-3) AFB smear
Mycobacterial Culture
Solid media (4-6 wks) Liquid media (2-4 wks)
Species Identification
Biochemical tests (days) Molecular tests (hours)
Drug Susceptibility Testing
Solid media (3-4 wks) Liquid media (5-10 days) Rapid Molecular (2 hrs to 1 day)

Time to DST Results by Method
MDR-TB diagnosis
after 9 to 12 weeks
MDR-TB diagnosis
after 3 to 5 weeks
MDR-TB diagnosis
after 1 to 2 days
MDR-TB diagnosis
after 1 day
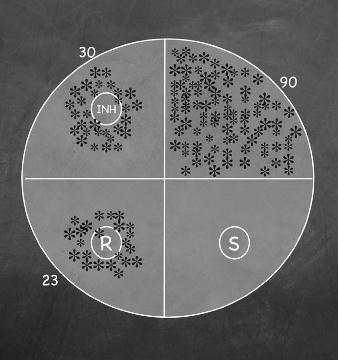
Proportion Method
• Agar plate is inoculated with either 30/90 = 33% resistant
clinical specimen (direct) or suspension of M. tuberculosis (indirect)
• Anti-TB drugs added to the media
as stock solutions (INH, RIF, OFL, KAN)
• Isolate is resistant if > 1% of the
number of colonies on the control agar grow on a given drug agar plate
• Poor reproducibility with
ethambutol and streptomycin
23/90 = 24% resistant
Drug-Resistant Tuberculosis. A Survival Guide for Clinicians
Mutations Associated with Drug
Resistance
Anti-TB drug
Gene mutation
katG, inhA, ahpC,
rpsL, rrs
Flouroquinolones
gyrA, gyrB
inhA, etaA/ethA
alrA, Ddl
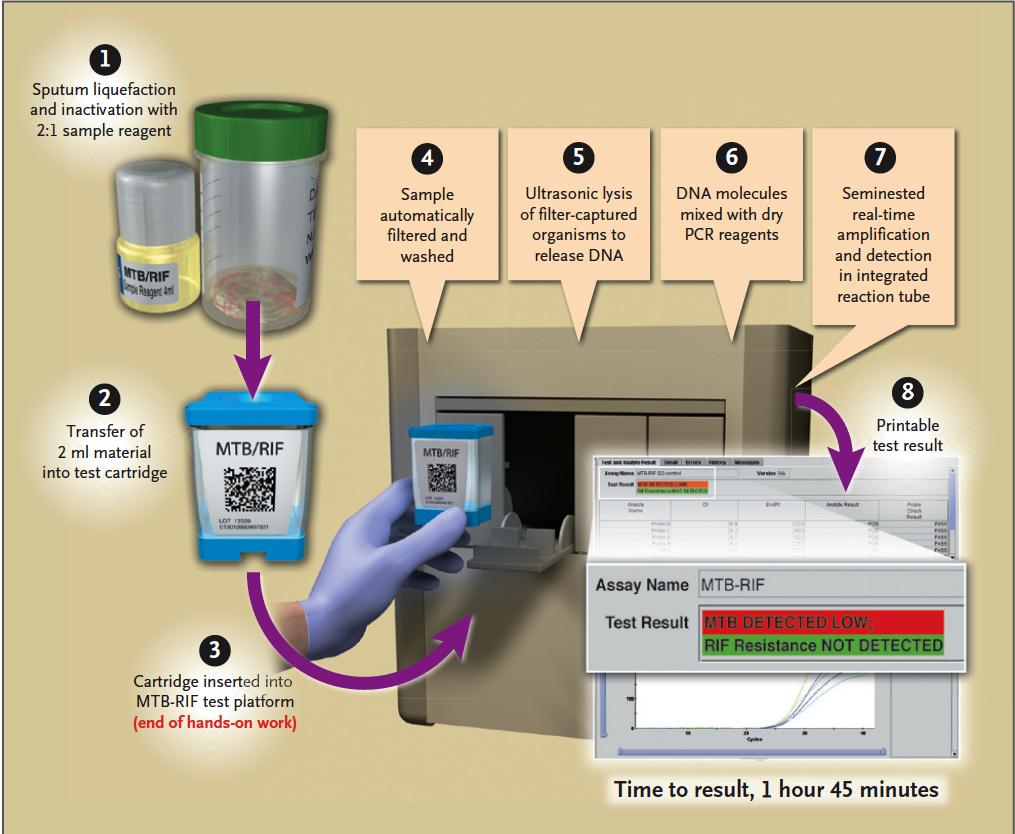
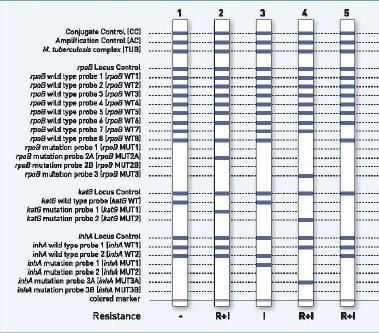
Gene Xpert
Sputum Specimen (1-2)
Sensitivity in AFB sm +: 98%
Sensitivity in AFB sm –: 72% Specificity: 99.2%
Boehme CC, et al. NEJM 2010;363:1005
Sensitivity and Specificity of
Genotype MTBDR Assays
Source: Hain Lifescience
Rifampin
Isoniazid
Sensitivity Specificity Sensitivity
Ling DI, Zwerling AA, Pai M. Eur Respir J 2008;32:1165
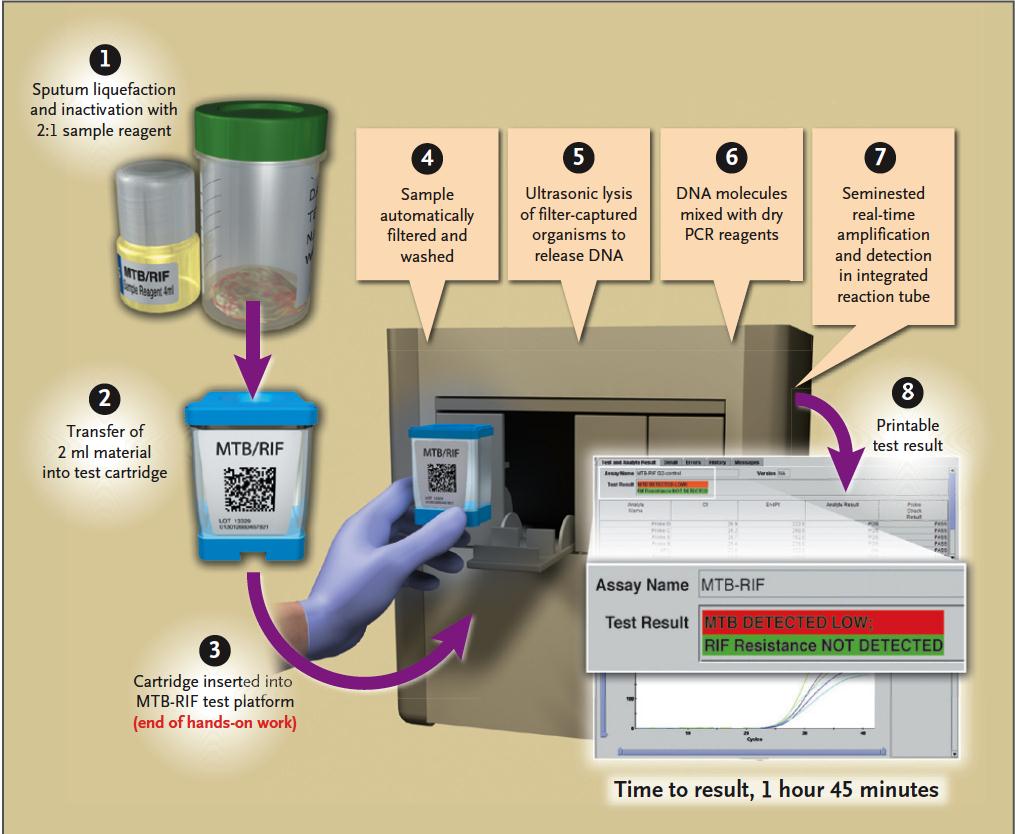
Laboratory Diagnosis of TB with
Xpert MTB/RIF
TB suspect
Sensitivity AFB + 98% AFB - 72%
Xpert MTB/RIF
Specificity – 99%
TB +, RIF +
TB +, RIF -
TB -, RIF -
Designing a Treatment Regimen
General Principles
• Regimens should be based on:
• the history of drugs taken by the patient • drugs and regimens used in the country and • the prevalence of resistance
• When possible, once daily dosing is recommended –
however Ethionamide/Prothionamide, PAS and Cycloserine are often split dosed
• Drug dosages should be determined by body weight
WHO. Guidelines for the programmatic management of drug-resistant TB, 2008
Designing a Treatment Regimen
General Principles
• Treatment of adverse drug effects should be
immediate and adequate
• Each dose should be given by DOT and preferably by
a health care worker
• DST, when available and from a reliable laboratory,
should be used to guide therapy (INH, Rifampin, injectables, fluoroquinolone)
WHO. Guidelines for the programmatic management of drug-resistant TB, 2008
Antituberculosis Drugs
Rifampin/Rifabutin
Levofloxacin Moxifloxacin
P-aminosalicylic acid
Clofazimine Imipenem
Thioacetazone Amoxacillin/Clavulanate
Macrolides Linezolid High-dose INH
Building a Treatment Regimen
≥4 likely effective
Ethambutol Pyrazinamide
Group 2 Streptomycin Kanamycin Amikacin Capreomycin
Group 3 Levofloxacin Moxifloxacin Ofloxacin
Group 4 Ethionamide Protionamide Cycloserine Terizidone P-aminosalicylic acid
Group 5 Clofazimine Imipemen Amoxacillin/Clavulanate Macrolides Linezolid Thioacetzone High-dose INH
2011 Updated PMDT Guidelines
• The Guidelines were based on
the results of a meta-analysis that included 32 studies and >9000 patients (XDR-TB patients were excluded)
• For each recommendation, the
"strength" of the recommendation and "quality" of the evidence were provided
Designing a Treatment Regimen
Composition of the Regimen
• Recommendations
– Four second-line drugs likely to be effective
(including a parenteral agent) as well as PZA, should
be used in the intensive phase (conditional
recommendation/very low quality of evidence)
– Regimens should include at least PZA, a
fluoroquinolone, a parenteral agent, ethionamide (or prothionamide) and either cycloserine or PAS, if cycloserine cannot be used (conditional recommendation/very low quality of evidence)
Duration of Therapy
Duration of Therapy
Recommended in 2011 Update
• Recommendations:
– An intensive phase of at least 8 months' duration is
recommended (conditional recommendation, very low quality of evidence)
– A total treatment duration of at least 20 months is
recommended in patients without any previous MDR-TB treatment (conditional recommendation, very low quality of evidence)
WHO 2011 Update
• A 66 year old man presents with chronic cough and
• He has been on and off antituberculosis therapy for
the past 10 years
– Isoniazid, rifampicin, ethambutol, pyrazinamide,
streptomycin and kanamycin
– He has diabetes mellitus but no other co-morbidities
• AFB smear is positive, culture is growing M.
tuberculosis and DST demonstrates in vitro resistance to INH, rifampin, and kanamycin and susceptible to ofloxacin
Building a Treatment Regimen
≥4 likely effective
Ethambutol
Pyrazinamide
Group 2
Streptomycin Kanamycin
Amikacin Capreomycin
Group 3
Levofloxacin Moxifloxacin
Ofloxacin
Group 4
Ethionamide Protionamide
Cycloserine Terizidone P-aminosalicylic acid
Group 5 Clofazimine Imipemen Amoxacillin/Clavulanate Macrolides Linezolid Thioacetzone High-dose INH
Odds Ratios of Treatment Success by Duration of
Intensive Phase and Total Treatment
WHO 2011 Update
Treatment Outcomes of MDR-TB: 2
Systematic Reviews and Meta-analyses
• Orenstein et al, Lancet Infect Dis 2009 – 34 studies
– Pooled treatment success of 62% (individualized 64% vs
standardized 54%, p = NS)
– Treatment outcomes were better when:
• Duration of treatment was at least 18 months • Patients received DOT
• Johnston et al PLoS ONE 2009 – 36 studies
– Pooled treatment success of 62% – Treatment outcomes were better when:
• surgical resection • no previous treatment • fluoroquinolone use
Weighted Proportion of Favorable
Outcomes in XDR-TB Studies (N=13)
Later generation FQN used in: at least 50% of patients – 59% less than 50% of patients – 31% P = 0.012
Jacobson KR et al. Clin Infect Dis 2010;51:5-14
History of Thoracic Surgery in TB
• Hippocrates is credited with
performing the first drainage of a tuberculous empyema
Plombage and Thoracoplasty
Oleothorax
Lucite balls
Sputum negative in 30-60%
Closure of cavity in 80%; mortality 10%
Surgical Resection
• Indications:
– Persistently positive smear or sputum culture for AFB
despite aggressive chemotherapy
– High risk for failure/relapse based on drug resistance
profile and extent of disease
– Complications of TB including, empyema, bronchopleural
fistula, hemoptysis
• Contraindications
– Pulmonary hypertension – Predicted inadequate post-operative pulmonary reserves – Other severe morbidities
Effect of Surgery in 8 Cohort Studies in
Patients with MDR/XDR-TB
Effect of Surgical
Resection- MV Odds (CI)
S. Korea 2001-05
0.18 (0.04-0.78)*
S. Korea 1995-04
11.35 (3.02-42.74)
S. Korea 2000-02
3.87 (1.69-8.88)
S. Korea 1996-05
4.23 (1.28-13.93)
*predictor of unfavorable outcome
Kemplar RR, et al. Lancet Infect Dis 2012;12;157-166
18 Retrospective Case Series of
Surgery for MDR-XDR-TB
• 964 patients (895 MDR, 69 XDR-TB) • > 95% treatment failure or high likelihood of failure/ relapse • At least 2 months of culture negativity required in all but one study (-0.6-240 mos) • Pre-operative culture status ranged: 11-100% (median 53%)
Outcomes
Range (median)
Postoperative culture conversion
Perioperative morbidity
Perioperative mortality
Kemplar RR, et al. Lancet Infect Dis 2012;12;157-166
Common Drug Side Effects
Ethionamide PAS Quinolones
Clofazimine Aminoglycosides Rifabutin
Quinolones INH Cycloserine
Ethambutol Ethionamide
Clofazimine Cycloserine INH
Rifabutin PAS Ethionamide
Photosensitivity Clofazimine Quinolones
INH Pyrazinamide Rifabutin
Ethionamide PAS Quinolones
Cycloserine INH Quinolones
Common Drug Side Effects
Musculoskeletal/joint
Pyrazinamide Quinolones Rifabutin
Visual changes, eye
Ethambutol Rifabutin Clofazimine
INH (high-dose) Linezolid
Hearing loss, tinnitus,
Aminoglycosides Capreomycin
Peripheral neuropathy
INH Ethionamide Cycloserine
Ethambutol
Ethionamide PAS
Hypokalemia/hypomag Aminoglycoside/capreomycin
Bedaquiline (TMC207) for MDR-TB
• Phase 2, randomized,
controlled trial
• 47 patients with MDR-TB
randomized to TMC207 or placebo plus standard five-drug regimen
– Reduced time to conversion – Increased proportion that
converted (48% vs 9%)
– Mild to moderate AEs with
nausea more common with TMC207 (26% vs4%)
Diacon AH, et al. NEJM 2009;360:2397
Delamanid for MDR-TB
• Phase 2 randomized,
placebo-controlled trial
• 481 MDR-TB patients were
randomized to delamanid or placebo plus WHO regimen
– Increased proportion that
converted by 2 months
• 100 mg 45.4% • 200 mg 41.9% • Placebo 29.6%
– AEs evenly distributed
• QT prolongation more common
Gler MT, et al. NEJM 2012;366:2151
• MDR-TB should be treated with at least 4
drugs likely to be effective plus PZA
• The regimen should include an injectable
agent, late-generation fluoroquinolone, ethionamide, PZA plus cycloserine (or PAS)
• The intensive phase should last at least 8
months and the continuation phase at least 20 months
• Treatment outcomes remain suboptimal
Source: https://panelphysician.org/wp-content/uploads/2014/10/Multidrug-resistant-Tuberculosis_Daley-2013.pdf
IEEE TRANSACTIONS ON COMPUTER-AIDED DESIGN OF INTEGRATED CIRCUITS AND SYSTEMS, VOL. 28, NO. 7, JULY 2009 The Tire as an Intelligent Sensor Sinem Coleri Ergen, Member, IEEE, Alberto Sangiovanni-Vincentelli, Fellow, IEEE, Xuening Sun, Member, IEEE, Riccardo Tebano, Sayf Alalusi, Giorgio Audisio, and Marco Sabatini Abstract—Active safety systems are based upon the accurate
INDIAN INSTITUTE OF MANAGEMENT CALCUTTA WORKING PAPER SERIES WPS No. 614/ September 2007 Is Product Patent Protection Necessary in Developing Countries for Innovation? R&D by Indian Pharmaceutical Companies after TRIPS Sudip Chaudhuri Professor, IIM Calcutta, Diamond Harbour Road, Joka P.O., Kolkata 700104 India








