Layout
A Guide to Asthma Medications
and Delivery Devices
February 20, 2009
This program was made possible through an unrestricted grant from: Monaghan
Medical Corporation, Lupin Pharmaceuticals Inc., and Forest Laboratories Inc.
American Association for Respiratory Care
9425 N. MacArthur Blvd., Suite 100
Irving, Texas 75063
Phone: (972) 243-2272
Fax: (972) 484-2720
Email:
[email protected]
Asthma Medications
Because each student's asthma symptoms and triggers are different, the med-ications required to manage asthma will vary. The student's health care providerwill develop a medication treatment plan based upon the student's age, fre-quency of asthma episodes, results of peak flow monitoring, and the severity ofthe asthma episodes.
In general, there are three categories of asthma medications:
Quick-relief medications (short-acting bronchodilators)
Long-term-control medications (inhaled corticosteroids and long-actingbronchodilators)
Nonsteroid anti-asthma agents (leukotriene modifiers, mast cell stabilizers,anti-IgE agents)
Quick-Relief Medications
These medications are medically known as short-acting bronchodilators sincethey can stop the symptoms of an asthma episode and provide "quick relief" inminutes. These medications are taken as needed to provide relief when a stu-dent begins to experience asthma symptoms, such as coughing, wheezing,chest tightness or shortness of breath. They are also beneficial when taken priorto exposure to a student's known asthma trigger or following a drop in peakflow meter readings.
Most quick-relief medications last between four to six hours and are considered"short-acting" bronchodilators. The most commonly prescribed short-actingmedication for asthma is albuterol. Most students with asthma will be pre-scribed an albuterol metered dose inhaler (MDI) or an albuterol nebulizer solu-tion.
Student access to prescribed quick-relief medications varies by school policy andstate law. Students must be familiar with how to access their "quick- relief"bronchodilator throughout the school day as well as during special school activi-ties such as field trips.
The following is a partial list of short-acting bronchodilators:Generic Name
ProAir® HFA, Proventil® HFA, Ventolin® HFA, Accuneb®inhalation solution
MaxAir® Autohaler®
Xopenex® HFA, Xopenex® inhalation solution
During asthma episodes when the short-acting bronchodilator doesn't providerelief, the student's health care provider may prescribe additional medicationsto reduce or eliminate the symptoms. These medications may include oral corti-
costeroids such as prednisone, methylprednisolone, or prednisolone or alterna-tive bronchodilators such as ipratropium.
Long-Term Control Medications
For students with persistent asthma, daily medications are required to controlchronic symptoms and help prevent asthma episodes. Inhaled corticosteroidswith either a long-acting bronchodilator or a leukotriene modifier may be pre-scribed. The student's health care provider will determine which medicationswill be required based upon the student's age and severity of symptoms.
Inhaled Corticosteriods
Inhaled corticosteroids are prescribed to reduce the inflammation in the air-ways. This helps to make the airways less sensitive to triggers and helps preventacute asthma episodes. Depending upon the student's age, the inhaled steroidsmay be in a dry-powder inhaler, a metered-dose inhaler, or an inhalation solutionfor use in a nebulizer.
The following is a partial list of inhaled corticosteroids:Generic Name
Flovent® HFA, Flovent® Diskus®
Pulmicort Respules®, Pulmicort Flexhaler®
Asmanex® Twisthaler®
Aerobid®, Aerospan® HFA
Note: Following administration of an inhaled steroid, the student should be instructed to "rinse andspit": rinse the mouth with water and spit it out without swallowing.
Long-acting bronchodilators can help prevent symptoms and keep the airwaysopen for 12 hours. They are used in the management of moderate to severeasthma and to prevent nighttime symptoms. Long-acting bronchodilators areprescribed in conjunction with inhaled corticosteroids. Depending upon theage of the student, the student's health care provider may prescribe a combina-tion medication that contains a long-acting bronchodilator and inhaled corticos-teroid in a single unit.
The following is a partial list of long-acting bronchodilators and combined in-haled corticosteroids and long-acting bronchodilators:Generic Name
Serevent® Diskus®
Foradil® Aerolizer®
Combined Corticosteroid andLong-Acting Bronchodilator: Fluticasone and Salmeterol
Advair® HFA, Advair® Diskus®
Budesonide and Formoterol
Note: Following administration of an inhaled steroid, the student should be instructed to "rinse andspit": rinse the mouth with water and spit it out without swallowing.
Nonsteroidal Anti-Asthma Agents
All of the drugs in this category work by either blocking the production or re-lease of harmful chemicals following exposure to allergic triggers. The potentchemicals, such as histamine and leukotrienes can cause airway inflammationand asthma symptoms.
The student's health care provider will determine whether any of the medica-tions in this category are indicated based upon the student's age, current med-ication plan, and severity of symptoms. Several medications in this category maybe prescribed to prevent asthma symptoms triggered by exercise.
The following is a partial list of nonsteroidal anti-asthma agents:
Leukotriene Modifier
Leukotriene Modifier
Mast Cell Stabilizer
Change in Propellants
Effective in 2009, albuterol metered-dose inhalers (MDIs) using chlorofluorocar-bons (CFCs) as a propellant have been discontinued in the United States becauseof their negative effects on the ozone layer. They have been replaced by al-buterol MDIs that use hydrofluoroalkane (HFA) as the propellant, a safe and ef-fective chemical that does not harm the environment. The medicationdelivered from the HFA inhaler remains the same.
MDIs containing CFC propellant may continue to be used until empty or untilthe expiration date has been reached. The prescription will be refilled with an al-buterol MDI containing HFA. These units will be marked with "HFA" following themedication's trade name on the MDI canister and the medication packaging.
The student's health care provider or pharmacist can answer questions about
the new device, including how and when to prime the MDI, proper breathing in-structions, and how to clean and store the MDI.
Similarities Between HFA and CFC MDIs •
The medicine in the inhaler is the same.
The shape of the device is similar.
The size of the device is similar.
Both devices are convenient to use.
Differences in the New HFA MDIs
The mist from the HFA MDI is less forceful and warmer.
There may be a slightly different smell and taste with HFA MDIs.
The care and cleaning of an HFA MDI is different. HFA MDIs should not getwet.
The device is now ozone-friendly to the environment.
Patient Assistance Programs (PAP)
There are many groups, organizations, and companies that may be able to helpindividuals who lack prescription coverage to get free prescription medications.
In fact, there are more than 475 public and private patient assistance programs,including more than 180 programs available from pharmaceutical companies.
The eligibility criteria and application process varies among the organizationsand companies. Many of the programs require that the applicant be a U.S. citi-zen or legal resident, have a household income below 200% of the FederalPoverty Level, and have no prescription coverage.
There are numerous websites and online assistance programs dedicated to help-ing qualifying individuals receive medication at reduced rates. Many of the web-sites offer coupons and discount programs.
If you have questions about the reliability of a particular service, check with thestudent's health care provider or pharmacist.
Here are two examples of programs that may be able to offer prescription assis-tance to qualifying individuals:
Free Medicine Program (www.freemedicineprogram.org)
Partnership for Prescription Assistance (www.pparx.org)
A metered dose inhaler (MDI) consists of a pressurized canister of medicine in-serted into a plastic actuator. MDIs deliver medication that is inhaled directlyinto the lungs. Some MDIs are designed to be inserted directly into the mouth.
Others require an open mouth technique with the MDI held 1-2 inches away. Forothers, a valved holding chamber (VHC) or spacer might be recommended. De-pending on the age of the patient and the prescribed medication, the student'shealth care provider will determine which of the three techniques shown belowfor the student to use and will instruct the student on proper breathing tech-nique for the prescribed medication.
Open Mouth Technique
Closed Mouth Technique
(use this method with CFC inhaler only)
Before using an MDI, remove the cap and check that there are no foreignobjects inside the mouthpiece.
Most MDIs require shaking. Check whether the student's prescribed MDImust be shaken and follow the manufacturer's instructions.
Each new MDI must be primed before using. Release the specified numberof test sprays into the air away from the face. If the MDI has been idle andnot used for awhile, additional priming may be required. Follow the manu-facturer's instructions regarding when and how to re-prime the MDI for use.
Following a "puff" from the MDI, the student should be instructed to holdthe breath for 10 seconds to allow the medication to reach deep into thelungs.
If more than one puff is prescribed, the student should wait 30-60 secondsbetween doses.
Valved holding chambers (VHC) are often prescribed to help increase theamount of medication delivered to the lungs. They may be used to adminis-ter medications from MDIs to young children or to those who may have diffi-culty using the MDI .
VHCs may be prescribed to reduce the incidence of possible local effects inthe mouth from inhaled corticosteroids. In addition, the student is in-structed to rinse the mouth or brush the teeth after receiving an inhaledcorticosteroid.
For more information on VHCs, refer to page 9.
Students should use only their own medications and devices prescribed bytheir health care providers.
Determining How Much Medicine Is in the MDI Canister
The number of doses contained in an MDI will vary depending upon the medica-tion. The medication packaging and insert will identify the number of actuations(puffs) in the canister.
Some of the new MDIs have built-in dose counters and indicate the number ofremaining doses. If the student's prescribed MDI does not have a dose counter,record the number of doses in a full canister and subtract the number of puffsused. Be sure to subtract the number of test primes.
Never float an MDI in water to determine the contents.

Using the Valved Holding Chamber (VHC)
Remove the cap from the MDI and check for foreign objects.
After shaking the MDI (if required), insert the mouthpiece of the MDI intothe open end of the holding chamber.
Exhale gently and place the mouthpiece of the holding chamber in themouth. (If using a mask, fit the mask over the student's nose and mouth.)
Push down on the MDI canister to release the medication. Inhale slowly anddeeply.
Hold the breath and count to 10 before exhaling.
If more than one puff of a quick relief medicine is prescribed, wait 60 sec-onds between actuations.
If using an inhaled steroid, be sure to rinse mouth or brush teeth after eachuse.
VHC should be cleaned regularly following the manufacturer's cleaning instruc-tions.
If powder is visible around the MDI mouthpiece, remove the MDI from the VHC.
Remove the metal canister from the actuator and set it aside. Rinse only themouthpiece and cap in warm water. Air dry. Reassemble by inserting the med-ication canister into the actuator and replacing the cap on the mouthpiece.
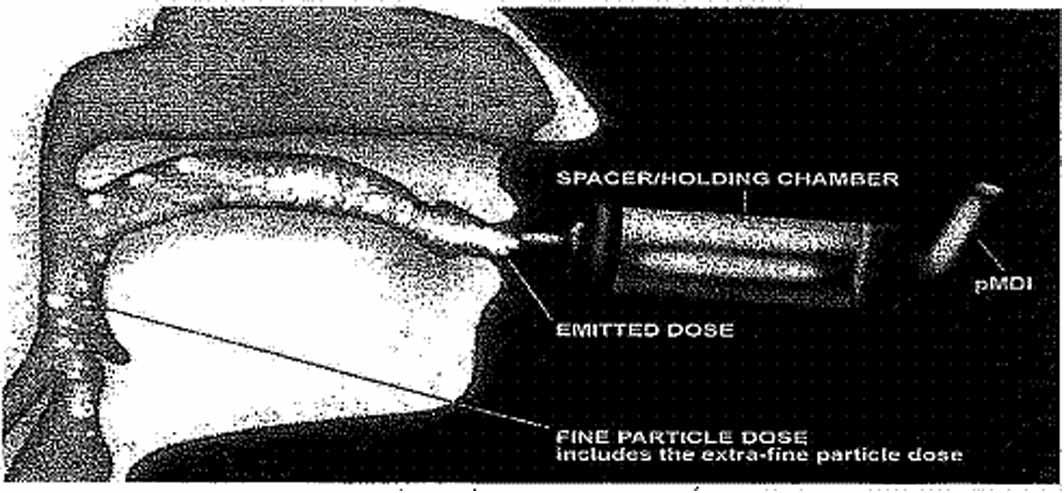
Why Use a Valved Holding Chamber (VHC) with a pMDI?
1. Why Use a Valved Holding Chamber (VHC)?
Only one in five people use their pMDI properly1
Valved holding chambers play a significant role in ensuring the effective delivery of fine particles to the lung by helping overcome the challenges of PMDI technique
c. The National Heart Lung and Blood Institute (NHLBI) – 2007
recommends a VHC for anyone with asthma2i. who cannot effectively use pMDI aloneii. who is prescribed an inhaled corticosteroid
d. Global Initiative for Asthma (GINA)—2006 recommends that children
younger than 4 years of age use a VHC with a face mask and children 4 to 6 years of age use a VHC and a mouthpiece3
2. Most Common Mistakes with a pMDI
a. Poor coordination of actuation and inhalation of pMDIb. Breathing too quickly, too shallow, or through the nose during inhalationc. Incomplete inhalation and/or not holding their breath after inhalationd. Not understanding the VHC product instruction
3. Particle Deposition—Size Distribution
a. A typical pMDI delivers a single dose at over 60 miles/hourb. Without a VHC, 60-80% of the released dose is deposited in the upper
c. VHCs minimize oropharayngeal (mouth & throat) deposits by reducing
the speed of pMDI and removing larger non-respirable particles in the pMDI
Larger particles are
impacted on the interiorsurfaces of the VHC
Fine particles are
typically < 5 μm diameter –these are typically inhaledand reach the lower respiratory tract efficiently,where the drug receptorsare located using a VHC
1. P. Barnes, J.C. Virchow, J. Sanchis, T. Welte and S. Pedersen. Asthma Management: Important Is-sues. European Respiratory Review, Volume 14, Number 97: 147-151.
2. Guidelines for the Diagnosis and Management of Asthma, US Department of Health and HumanServices. National Institute of Health, National Heart, Lung and Blood Institute. NIH publication num-ber 08-5846, October 2007.
3. Global Initiative for Asthma. Pocket Guide for Asthma Management and Prevention in Children. APocket Guide for Physicians and Nurses. Medical Communication Resources, Inc. 2006.
Key Characteristics of a Well-Designed Valved HoldingChamber (VHC)
VHC Characteristics1. Responsive inhalation and exhalation valves2. Ability to ensure a good facemask seal to face3. Constructed from shatter-resistant materials and designed to
prevent dislodgement of small components when subjected
to mechanical shock
4. Use of transparent materials
5. Designs that are specific for infant, child, or adult use6. Facemask with minimal dead volume and comfortable fit with
the minimum of applied force to achieve a seal to the face
7. Exhalation valve in facemask that offers low resistance to
Cap DesignStrong tether
• To indicate improper inhalation• To train patients in proper technique
Inspiratory/Expiratory Valve• Safe and secure
• Low resistance silicone
• Universal – accepts all approved pMDIs
• Exhaustion away
• Clear body chamber
• Clear instructions on the barrel
Dry-Powder Inhalers (DPI)
A dry-powder inhaler (DPI) delivers a small dose of medication from the deviceas the user takes in a deep breath. Unlike MDIs, there is no propellant used todeliver the medication. Once the dose is loaded, the device must be held leveluntil the dose is inhaled.
It is important to avoid exhaling into the unit. A deep, rapid breath is requiredto remove the medication from the DPI. Because the dose is so small, DPI usersmight not feel, taste, or smell the powdered medication.
Some DPIs require the user to load a powdered medication into a capsule eachtime. Other DPIs are pre-filled with multiple doses and contain a counter thatdisplays the number of doses remaining in the unit.
The following are examples of several of the DPIs currently available:
The student should:
1. Hold the Diskus® in a flat level position in one hand.
2. Place the thumb of the other hand in the thumb grip.
3. Open the Diskus® by pushing the thumb grip around until it clicks and the
mouthpiece appears.
4. Slide the lever until it clicks.
5. Breathe out away from the Diskus®.
6. Place the lips around the mouthpiece until a good seal is formed. 7. Breathe in quickly and deeply through the mouth. 8. Remove the Diskus® from the mouth and hold the breath for approxi-
mately 10 seconds
9. Breathe out slowly away from the Diskus®.
10. Slide the thumb grip and click the Diskus® shut.
11. Store in a dry location away from heat and sunlight.
To clean the Diskus®:Wipe the mouthpiece with a clean, dry tissue or cloth.
Note: Remember to rinse the mouth after using an Advair® Diskus® or Flovent® Diskus®.
Using an Aerolizer®
The student should:
1. Lift off the blue cap.
2. Hold the blue base with one hand and grasp the white mouthpiece with
the other. Twist in the direction of the arrow.
3. Remove a medication capsule from the blister pack and place in the slot
inside the white base.
4. Twist the mouthpiece back into place.
5. Hold the Aerolizer® upright and firmly squeeze the two blue buttons on
the side once to pierce the capsule.
6. Breathe out away from the mouthpiece.
7. Tilt head back slightly, hold the Aerolizer® level, and seal lips around the
8. Inhale deeply and rapidly. (The capsule will rattle in the chamber.)9. Remove the mouthpiece and hold the breath for 10 seconds.
10. Breathe out slowly.
11. Open the chamber to see whether any powder remains in the capsule. (If
powder is visible, twist the mouthpiece back into place and repeat steps6-10.)
12. Remove and discard the empty capsule.
13. Replace the blue cap.
Cleaning the Aerolizer®Wipe mouthpiece with a clean, dry tissue or cloth.
Store the Aerolizer® in a dry place away from heat.
Using the Autohaler®
The student should:
1. Release two test sprays according to manufacturer's directions if the MDI is
new or hasn't been used for 48 hours.
2. Remove the mouthpiece cover.
3. Hold the Autohaler® in an upright position without blocking the vents at
4. Lift the gray lever up at the top and shake the device gently.
5. Breathe out normally.
6. Seal the lips around the mouthpiece.
7. Breathe in through the mouth deeply. A click can be heard as the
Autohaler® releases a dose of medication.
8. Remove the Autohaler® from the mouth and hold the breath for 10 sec-
9. Return the gray lever to its original position and replace the mouthpiece
Cleaning the Autohaler®Wipe mouthpiece with a clean, dry tissue or cloth.
Using a Flexhaler™
The student should:
1. Twist and remove the cover.
2. Hold the Flexhaler™ upright (mouthpiece pointed up).
3. Twist the brown grip to the right as far as it will go then twist it fully back in
the opposite direction. A click will be heard during the twisting motion.
4. Breathe out away from the Flexhaler™. 5. Seal the lips around the mouthpiece and breathe in forcefully and deeply. 6. Remove the Flexhaler™ from the mouth and hold the breath for approxi-
mately 10 seconds.
7. Breathe out away from the Flexhaler™. 8. Repeat steps 2-7 if more than one dose is prescribed. 9. Replace the cover.
Cleaning the Flexhaler™Wipe mouthpiece with a clean, dry tissue or cloth.
Store in a dry place away from heat.
Using a Compressor-Driven Nebulizer
Nebulizers are commonly used to deliver medications to infants and small chil-dren as well as individuals who may be unable to effectively use an MDI or DPIduring acute asthma symptoms.
Nebulizers change asthma medications from a liquid to an aerosol mist that caneasily be inhaled into the lungs.
The following supplies are required:•
Portable air compressor
Nebulizer medication cup
Mouthpiece or mask
Instructions for Using the Nebulizer:
Place the air compressor on a sturdy surface.
Connect the air compressor to the appropriate power source (3-prongelectrical outlet or battery source).
Wash hands with soap and water and thoroughly dry them.
Insert the prescribed medication dose into the nebulizer medication cup.
Connect the nebulizer cup, the top of the nebulizer, and the mouthpieceor mask.
Connect the tubing to the air compressor and to the nebulizer.
Turn the air compressor on. A light mist should be visible.
Sit upright and place the mouthpiece between the teeth or place themask over the nose and mouth and adjust the head strap for a comfort-able fit.
Take slow, deep breaths. Try to hold the breaths for 2-3 seconds beforeexhaling. This helps the medication settle out in the airways.
Continue the treatment until the nebulizer makes sputtering noises. Thetreatment will take approximately 10 minutes. A small amount of medica-tion will stick to the sides of the nebulizer cup and may be gently shakento loosen the droplets.
When the treatment is complete, turn the air compressor off.
Disconnect the tubing from the nebulizer and store with the compressor.
Clean the rest of the nebulizer unit as directed.
If dizziness or jitteriness occurs during a treatment, stop and rest for 5minutes. Resume the treatment and breathe slowly. If dizziness or jitteri-ness continues to be a problem, inform the student's health care provider.
Cleaning and Disinfecting NebulizersNebulizers, mouthpieces, and masks should be cleaned and disinfected accord-ing to the manufacturer's directions. Use the recommended solutions to cleanthe nebulizer components. The nebulizer tubing does not require rinsing or dis-infection. Replace the nebulizer as recommended by the manufacturer.
Keep the compressor clean and dry. Call your medical equipment provider to re-port any problems. Do not attempt to repair it yourself. Replace the equipmentas recommended by the manufacturer.
Source: http://www.peakperformanceusa.info/asthma_management_guide/medication_guide.pdf
Advanced Software Protection Now Diego Bendersky1,2, Ariel Futoransky1, Luciano Notarfrancesco1, Carlos Sarraute1,3, and Ariel Waissbein1,3 1 Corelabs, Core Security Technologies; 2 Departamento de Computaci´on, FCEyN, Universidad de Buenos Aires (UBA), 3 Departamento de Matem´atica, FCEyN, UBA Argentina. Abstract. We introduce a novel software-protection method, which canbe fully implemented with today's technologies, that provides traitortracing and license-enforcement functionalities, and requires no addi-tional hardware nor inter-connectivity other than those needed to ex-ecute the respective protected software.In [1] authors introduce the secure triggers and show that it is secure,modulo the existence of an ind-cpa secure block cipher. In this work,we present a framework for license enforcement and fingerprinting ofcomputer programs in which these capabilities are coupled with securetriggers to produce a secure software protection system.
Nummer 23, März 2007 P.b.b. Verlagspostamt 1010 Wien - Erscheinungsort Wien Zeitschrift der Misrachi Österreich Von Purim bis Pessach Purim in Kürze von Raw Pardess B u c h t i p p Teddy Kollek s.A. Aus der Bewegung VON PURIM BIS PESSACH Zu Purim feiern wir den Sieg über Von Purim bis Pessach


