Doi:10.2306/scienceasia1513-1874.2013.39.356
R ESEARCH ARTICLE
The effect of mirtazapine on methotrexate-inducedtoxicity in rat liver
Bunyami Ozogula, Abdullah Kisaoglua, Mehmet Ibrahim Turanb,∗, Durdu Altunerc, Ebru Senerd,Nihal Cetine, Cengiz Ozturke,f
a Department of Surgery, Faculty of Medicine, Ataturk University, 25240, Erzurum, Turkeyb Department of Paediatrics, Faculty of Medicine, Ataturk University, 25240, Erzurum, Turkeyc Department of Pharmacology, Faculty of Medicine, Recep Tayyip Erdogan University, 53100, Rize, Turkeyd Department of Pathology, Erzurum Region Education and Research Hospital, Erzurum, Turkeye Department of Pharmacology-Toxicology, Faculty of Veterinary Medicine, Ataturk University, 25240,
f Department of Family Medicine, Erzurum State Hospital, Erzurum, Turkey
∗Corresponding author, e-mail:
Received 18 Dec 2012
Accepted 20 Mar 2013
ABSTRACT: Methotrexate is used as a chemotherapeutic agent and its anti-oxidant activity is used to treat many cancertypes. This study conducts a biochemical and histopathological investigation into whether mirtazapine has a protective effecton methotrexate-induced hepatotoxicity in rats. Distilled water was given to a healthy group intraperitoneally. Methotrexatealone was injected in the control group, again intraperitoneally. Mirtazapine and, 1 h later, methotrexate were given tothe rats in the final group. This procedure was repeated over 7 days. In the control group rats receiving methotrexate,blood AST, ALT, and LDH levels were 227 ± 3 µmol/l, 85 ± 2 µmol/l, and 357 ± 13 µmol/l, respectively. In the ratsreceiving mirtazapine and methotrexate, these values were 152 ± 3 µmol/l, 25 ± 1 µmol/l, and 141 ± 15 µmol/l. In thehealthy rat group, AST, ALT, and LDH levels were 136 µmol/l, 20 µmol/l, and 133 µmol/l, respectively. Histopathologically,apoptotic bodies with condensed cytoplasm, peripheral, and pyknotic nuclei in the hepatocytes, focal necrosis and intenseinflammation in the interstitial areas were present in the control group. In the methotrexate and mirtazapine group, therewere no apoptotic bodies or inflammation, only isolated necrosis in the hepatocytes. In conclusion, mirtazapine protectedthe liver against methotrexate toxicity.
KEYWORDS: drug, hepatotoxicity, oxidant/anti-oxidant
terious toxic effects of methotrexate can theoreticallybe reduced or prevented with the addition of folic acid
Methotrexate, a folic acid anti-metabolite, is used
to the treatment, there is as yet no consensus on the
as a chemotherapeutic agent for many cancer types
subject. On the contrary, there are studies showing
(leukaemia, lymphoma, osteosarcoma, head and neck
that with the addition of folic acid, the therapeutic
tumours, lung cancer, breast cancer, etc.). Methotrex-
effectiveness of methotrexate decreases . This indi-
ate is also used for the treatment of multiple scle-
cates that the mechanism of action of methotrexate
rosis, dermatomyositis, sarcoidosis, psoriasis, and
hepatotoxicity has not yet been fully explained .
rheumatoid arthritis, disorders causing inflammation.
Toxicity studies with methotrexate highlight the
However, the use of high-dose methotrexate (as in
role of oxidative stress in causing toxicity on the liver
leukaemia) or prolonged use may result in hepato-
and other organs. Levels of both enzymatic and non-
toxicity that may lead to progressive fibrosis and
enzymatic anti-oxidants are inhibited and the levels
cirrhosis Clinically, hepatotoxicity, which occurs
of oxidants increase in the liver, kidney, and gut
in long-term use of methotrexate, remains one of the
tissues of laboratory animals given methotrexate It
significant restrictions on its use in the doses desired .
is therefore thought that anti-oxidant therapy may be
Methotrexate inhibits the formation of tetrahydrofo-
useful in preventing or reducing hepatotoxicity due to
late from folic acid. The inhibition of tetrahydrofolate
methotrexate. Studies have demonstrated that various
formation is responsible for both the therapeutic and
anti-oxidants are protective against methotrexate hep-
toxic effects of methotrexate . Although these dele-
Mirtazapine, which we tested for methotrexate
histopathological and biochemical examinations were
hepatotoxicity in our study, is an anti-depressant drug
performed. Histopathological and biochemical data
used for the treatment of major depression.
from the mirtazapine group were assessed in compar-
tazapine has been shown to inhibit the production of
ison with those obtained from the control and healthy
enzymatic and non-enzymatic oxidant parameters, but
to increase anti-oxidants in gastric tissue . Our reviewof the literature elicited no information or interven-
Biochemical analysis
tions regarding the protective effects of mirtazapine on
AST, ALT, and LDH measurements:
methotrexate-induced oxidative stress in rats. The aim
samples were collected into tubes without anticoag-
of this study was therefore to investigate, biochem-
ulant. Serum was separated by centrifugation after
ically and histopathologically, whether mirtazapine
clotting and stored at −80 °C until assay. Serum AST
has a protective effect against methotrexate-induced
and ALT activities as liver function tests, and LDH
oxidative stress in the rat liver.
activity as a marker of tissue injury, were measured
MATERIALS AND METHODS
spectrophotometrically on a Cobas 8000 (Roche) au-toanalyser using commercially available kits (Roche
Diagnostics, GmBH, Mannheim, Germany).
Thirty male albino Wistar rats weighing between220 g and 235 g, provided by the Ataturk University
MDA, MPO, GSH, and SOD measurements:
Medical Experimental Practice and Research Centre,
lowing macroscopic analyses, glutathione (GSH), su-
were used in the study. Before the experiments, the
peroxide dismutase (SOD), myeloperoxidase (MPO),
animals were housed and fed in groups at room tem-
and malondialdehyde (MDA) enzyme activities and
perature (22 °C). Animal experiments were performed
levels were determined in rat liver tissues.
in accordance with national guidelines for the use and
livers were frozen at −80 °C until biochemical in-
care of laboratory animals and approved by the local
To prepare the tissue homogenates,
animal care committee of Ataturk University (proto-
liver tissues were ground with liquid nitrogen in a
col number: B.30.2.ATA.0.01.02/5388, approval date:
mortar. The ground tissues (0.5 g each) were then
treated with 4.5 ml of buffers (consisting of 0.5%HDTMAB [0.5% hexadecyl tri methyl ammonium
Chemical substances
bromide] pH: 6 potassium phosphate buffer for MPO
Of the chemical substances used for the experi-
analysis, 1.15% potassium chloride solution for MDA
ments, thiopental sodium was provided by IE Ulagay-
analysis, and pH: 7.5 phosphate buffer for the SOD,
Turkey and mirtazapine was obtained from Organon
GSH analysis). The mixtures were homogenized on
ice using an Ultra-Turrax homogenizer for 15 min.
Homogenates were filtered and centrifuged using a
Experimental procedure
refrigerated centrifuge at 4 °C. The supernatants wereused for the determination of the enzymatic activities.
The rats to be used in the experiment were divided
All assays were carried out at room temperature in
into three groups: a control group given methotrexate
(MTXC), a group given mirtazapine and methotrexate(MMTX), and a healthy (H) group.
Total GSH determination
MTXC group rats (n = 10) were injected in-
traperitoneally with 5 mg/kg methotrexate.
The amount of GSH in the hepatic mucosa was mea-
MMTX group (n = 10) was given mirtazapine
sured according to the method described by Sedlak
30 mg/kg by the oral route; 1 h after administration
and Lindsay apoptotic . The mucosal surface of the
of mirtazapine 5 mg/kg methotrexate was injected
liver was collected by scraping, weighed, and then
intraperitoneally. The H group was given an equiv-
homogenized in 2 ml 50 mM Tris-HCl buffer contain-
alent volume of distilled water, using the same tech-
ing 20 mM EDTA and 0.2 mM sucrose, pH 7.5. The
nique, by the oral route.
Methotrexate was given
homogenate was immediately precipitated with 0.1 ml
in a single dose to the MTXC and MMTX groups.
of 25% trichloroacetic acid, and the precipitate was
The MMTX group was given mirtazapine for 7 days,
removed by centrifugation at 1380g for 40 min at 4 °C.
and the H group distilled water for 7 days. At the
The supernatant was used to determine GSH using
end of this period, all animals were sacrificed using
5,50-dithiobis(2-nitrobenzoic acid). Absorbance was
high-dose anaesthesia, their livers were removed and
measured at 412 nm using a spectrophotometer. GSH
levels in the mucosa were expressed as nanomoles permilligram of tissue (nmol/mg tissue).
GSH nmol/g protein
MPO activity was measured according to the modified
method described by Bradley et al . The homoge-nized samples were frozen and thawed three times and
centrifuged at 1500g for 10 min at 4 °C. MPO activityin the supernatant was determined by adding 100 ml of
the supernatant to 1.9 ml of 10 mM phosphate buffer(pH 6.0) and 1 ml of 1.5 mM o-dianisidine hydrochlo-
ride containing 0.0005% (wt/vol) hydrogen peroxide.
The changes in absorbance at 450 nm of each samplewere recorded on a UV-Vis spectrophotometer. MPO
Fig. 1 The GSH level and SOD activity in MMTX, MTXC,
activity in liver tissues was expressed as millimoles
and SG rat groups. Results expressed as mean ± standard
per minute per milligram of tissue (mmol min−1 (mg
error of the mean (n = 10).
Statistical analysis
Determination of lipid peroxidation or MDAformation
All data were analysed by one-way ANOVA usingSPSS 13.0. Differences among groups were calcu-
Concentrations of hepatic mucosal lipid peroxidation
lated using the least significant difference option, and
were determined by estimating MDA using the thio-
significance was set at p < 0.05.
barbituric acid test .
Briefly, the rat livers were
promptly excised and rinsed with cold saline. To mini-
mize the possibility of haemoglobin interference withfree radicals, any blood adhering to the mucosa was
Biochemical results
carefully removed. The corpus mucosa was scraped,
MDA, MPO, GSH, SOD results:
weighed and homogenized in 10 ml of 100 g/l KCl.
and MDA, MPO, GSH, and SOD levels
The homogenate (0.5 ml) was added to a solution con-
in the liver tissues of the rats receiving methotrex-
taining 0.2 ml of 80 g/l sodium lauryl sulphate, 1.5 ml
ate in the MTXC group were 7.5 ± 0.1 µmol/g pro-
of 200 g/l acetic acid, 1.5 ml of 8 g/l 2-thiobarbiturate,
tein, 3.4 ± 0.1 µmol/g protein, 2.4 ± 0.1 nmol/g pro-
and 0.3 ml distilled water.
The mixture was then
tein, and 5.5 ± 0.2 µmol/g protein, respectively. In
incubated at 98 °C for 1 h. Upon cooling, 5 ml of
the MMTX group, MDA, MPO, GSH, and SOD
n-butanol:pyridine (15:1) was added.
values were 4.1 ± 0.2 µmol/g protein (p < 0.001),
was vortexed for 1 min and centrifuged for 30 min
1.7 ± 0.1 µmol/g protein (p < 0.001), 4.6 ± 0.1 nmol/g
at 4000 rpm. The absorbance of the supernatant was
protein (p < 0.001), and 6.4 ± 0.1 µmol/g protein
measured at 532 nm. A standard curve was generated
(p < 0.001), respectively.
In the H group, MDA,
using 1,1,3,3-tetramethoxypropane. The recovery was
MPO, GSH, and SOD levels were 1.9 ± 0.1 µmol/g
over 90%. The results were expressed as nanomoles
protein (p < 0.001), 1.5 ± 0.1 U/g protein (p <
MDA per milligram wet tissue (nmol/mg tissue).
0.001), 4.9 ± 0.1 nmol/g protein (p < 0.001), and8.3 ± 0.2 U/g (p < 0.001), respectively.
AST, ALT, LDH results:
The livers removed from the rats were fixed in
blood AST, ALT, and LDH levels in the
10% formaldehyde. After routine tissue preparation,
MTXC group were 227 ± 3 U/l, 84.8 ± 2.3 U/l, and
haematoxylin and eosin sections 5 µm in thickness
357 ± 13 U/l, respectively, compared to 153 ± 3 U/l
were obtained. Masson's trichrome and reticulin were
(p < 0.0001), 25.1 ± 0.8 U/l (p < 0.0001), and
applied to all sections. Liver lobules and portal areas
142 ± 15 U/l (p < 0.0001) in the MMTX group.
were examined histopathologically.
In the H group, AST, ALT, and LDH levels were
photomicrographs demonstrate the liver histopathol-
135.8 ± 2.1 U/l (p < 0.001), 20.4 ± 0.9 U/l (p <
ogy of test animals.
0.001), and 131.4 ± 1.6 U/l (p < 0.001), respectively.
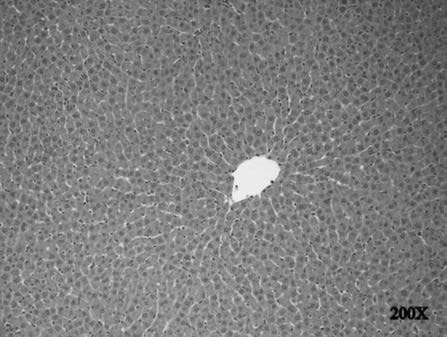
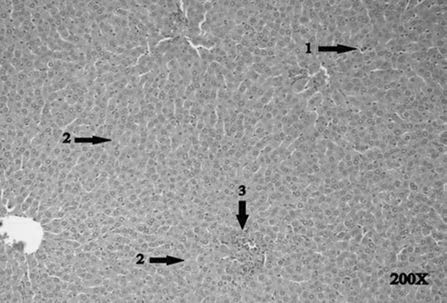
Table 1 The MDA level and MPO activity in mirtazapine + methotrexate, methotrexate, and intact rat groups.
MDA (nmol/mg tissue)
MPO (mmol min−1 (mg tissue)−1)
Mirtazapine (30 mg/kg)
Results are mean ± standard error of the mean (n = 10). p < 0.05 referred as significant versus methotrexate group.
Fig. 2 The AST and ALT activities in MMTX, MTXC and
Fig. 4 The histo-pathological examination in the SG group.
SG rat groups. Results expressed as mean ± standard errorof the mean (n = 10).
Fig. 5 The histo-pathological examination in the MTXC
group. There are apoptotic bodies with condensed cyto-plasm, peripheral, and pyknotic nuclei in the hepatocytes
The LDH levels in MMTX, MTXC and SG rat
(arrow 1), focal necrosis (arrow 2), and intense inflammation
groups. Results expressed as mean ± standard error of the
in the interstitial areas (arrow 3).
mean (n = 10).
MTXC liver tissue:
As seen in in the MTXC
Pathological findings
group given methotrexate, we observed apoptotic bod-
H group liver tissue:
Normal hepatic histology was
ies with condensed cytoplasm, peripheral, and py-
observed in the healthy rat group.
knotic nuclei in the liver cells (arrow 1), focal necrosis
pathological signs in the liver lobules or portal areas
(arrow 2), and intense inflammation in the interstitial
areas (arrow 3).
ous experimental studies with rats have reported thatmethotrexate increased levels of MPO, a marker ofneutrophil infiltration . Another study showed thatmethotrexate increased MPO activity, a marker ofinflammatory response in live and other tissues, inrats . Free radicals lead to activation of neutrophils.
Activated neutrophils release extensive amounts ofMPO in the tissue areas damaged by free radicals.
This situation leads to further exacerbation of tissuedamage .
If the delicate balance between oxidants and
anti-oxidants cannot be maintained in tissues, manypathological changes extending to cellular damageoccur.
GSH is an endogenous anti-oxidant, which
exists within many cells of the body and protects the
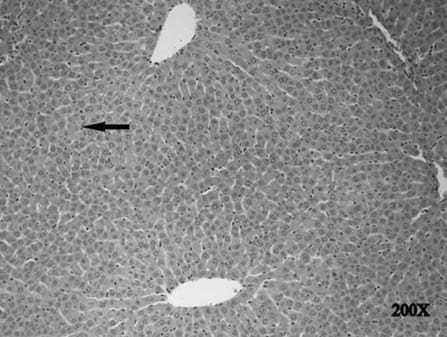
Fig. 6 The histo-pathological examination in the MMTX
functional proteins of the cell against oxidant agents.
group. There was isolated necrosis (arrow).
In our study, mirtazapine significantly eliminatedthe inhibitor effect of methotrexate on GSH produc-
MMTX liver tissue:
No apoptotic bodies or inflam-
tion. Studies in the literature have demonstrated that
mation were observed in the MMTX group, only
methotrexate reduces the levels of glutathione in liver
isolated necrosis
cells . A methotrexate-associated fall in glutathionelevels leads to hepatocyte injury.
In the present study, SOD activity was signifi-
This study was a biochemical and histopatholog-
cantly lower in the MTXC group in comparison with
ical investigation of the effect of mirtazapine on
the MMTX and H groups.
The function of SOD
methotrexate hepatotoxicity in rats. The results of
is to catalyse the dismutation of O2 and to protect
biochemical tests showed a significant increase in the
the tissue against the harmful effects of toxic oxygen
liver tissue of the MTXC group in the levels of oxidant
radicals Yagmurca et al demonstrated that SOD
parameters, e.g., MDA and MPO, and a decrease in
activity reduced doxorubicin hepatotoxicity in
anti-oxidant parameters, e.g., GSH and SOD. Oxida-
If oxidative damage is involved in the beginning
tive stress is implicated in methotrexate toxicity in the
or pathology of the disease, successful anti-oxidant
livOxidative stress is an indicator of the damage
therapy may prevent the disease occurring or delay its
that results from a change in the balance between
oxidants and anti-oxidants in favour of oxidants. Mir-
Our study also ascertained the effects of
tazapine, which we used in our experiment, has been
methotrexate on liver enzymes, e.g., AST, ALT, and
observed to significantly inhibit MDA increase due
LDH. These are associated with hepatocellular injury.
to methotrexate in the liver.
MDA is a 3-carbon
ALT and AST are of particular importance in the
aldehyde, the end-product of lipid peroxidation, and is
determination of liver damage. AST, ALT and LDH
used as a marker of oxidative stress . MDA released
levels were higher in the MTXC group blood samples
after peroxidation of lipids causes irreversible damage
in comparison with the MMTX and H groups. Previ-
to cells and organelle contents . Increased levels of
ous studies have showed that methotrexate increases
plasma MDA may be attributed to overproduction of
liver enzymes. The serum transaminases have high
reactive oxygen species or a deficiency of antioxidant
sensitivity in demonstrating hepatocyte injury. One
There are studies showing that MDA
of the most reliable parameters showing cell destruc-
levels increase in hepatic injury caused by oxidative
tion in the liver is ALT level .
stress . The high level of MDA in the MTXC group
the highest ALT level in this study was the MTXC
liver tissue indicates that methotrexate gives rise to
group, while the MMTX group had the lowest level.
oxidative stress in hepatic tissue.
AST and ALT have been confirmed as markers of
MPO activity in our study was higher in com-
hepatic cell injury . Antioxidants have been shown
parison with the H and MMTX groups in the liver
to prevent severe increases in LDH, AST, ALT and
tissues of the animals receiving methotrexate. MPO
antioxidant parameters in liver injury. In the MTXC
is released from activated neutrophils and is generally
group, in which LDH, AST, ALT activities and ox-
used as a major marker of inflammation .
idant parameters were higher, there were apoptotic
bodies, focal necrosis and intense inflammation in the
5. Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken
interstitial areas. In contrast, in the MMTX group
B (2006) N-acetylcysteine ameliorates methotrexate-
there were only a few necrotic cells. Previous studies
induced oxidative liver damage in rats. Med Sci Monit
have also shown that methotrexate intensifies apopto-
12, 274–8.
sis . Caspase is a gene that controls cell apoptosis.
Lipid peroxidation established by free radicals has
been shown to increase apoptosis by stimulating the
caspase gene. Cell apoptosis intensified in hepatic
injury induced with methotrexate in rats has been
reported . Apoptosis and necrosis may be activated
with the same stimuli .
In necrosis, cytoplasmic
and nuclear contents are released into the intercellular
space. Release of cellular contents into intercellu-
lar space leads to inflammation. The distinguishing
characteristic of this phenomenon is that macrophages
and neutrophils migrate to necrotic tissue.
migrating cells cause phagocytosis in necrotic tissues.
Inflammation is therefore a significant indicator of
These findings from the literature are
compatible with our own histopathological findings.
Mirtazapine blocks 5-HT2 and 5-HT3 receptors with
its anti-oxidant property . Stimulation of both 5-HT2
and 5-HT3 receptors is known to be associated with
toxic adverse effects .
Methotrexate led to oxidative stress in the rat liver,
while mirtazapine significantly prevented methotrex-
13. Gutteridge JM (1995) Lipid peroxidation and antioxi-
ate-induced oxidative stress.
This shows that the
dants as biomarkers of tissue damage. Clin Chem 41,1819–28.
desired dose of methotrexate can safely be used with
14. Amirkhizi F, Siassi F, Minaie S, Djalali M, Rahimi
mirtazapine in the treatment of cancer and non-cancer
A, M, C (2008) Assessment of lipid peroxidation
and activities of erythrocyte cytoprotective enzymes inwomen with iron deficiency anemia. J Res Med Sci 13,
This study was supported by the
Scientific Research Project of Ataturk University-BAP
2005/160-2008/126. We thank Prof. Fatih Akcay for his
contributions to this study.
17. Sullivan GW, Sarembock IJ, Linden J (2000) The role
of inflammation in vascular diseases. J Leukoc Biol 67,
22. Desai SP, Isa-Pratt S (2004) Clinician's Guide to
Laboratory Medicine. Chapter 66. Lexi-Comp Inc.
pp 612–3.
23. Horie T, Li T, Ito K, Sumi S, Fuwa T (2006) Aged garlic
extract protects against methotrexate-induced apoptoticcell injury of IEC-6 cells. J Nutr 136, 861S–3S.
27. A˘garg¨un MY, Ebrinc¸ S (1998) Mirtazapine: a review.
Bull Clin Psychopharmacol 8, 59–68.
Source: http://tarjomefa.com/wp-content/uploads/2015/12/4227-English.pdf
Treatment of Patients who decline transfusion of Blood Components and/or Blood Products November 2008 Page 1 of 23Page 1 of 23 Title: Treatment of Patients who decline transfusion of Blood Components and/or Blood Products Reference Number: Corp09/003 Implementation Date: This policy will be implemented after being signed off by the Chief Executive Review date: This policy will be reviewed one year after the effective date and thereafter every two years Responsible Officer: The officer responsible for reviewing this policy is the Haemovigilance Practitioner on behalf of the Hospital Transfusion Committee This policy has been developed within the context of Equality and Human Rights statutory obligations and requirements.
A list of Australia's most dangerous pesticides July 2010 Jo Immig, Coordinator, National Toxics Network organic pollutant on the basis of its persistence, transported long distances. It found endosulfan Over 8000 pesticide and veterinary products are was "likely, as a result of its long-range registered for use in Australian agriculture, environmental transport, to lead to significant



